Abstract
While a range of cellular mechanisms have been proposed to underlie control of neurovascular coupling, a comprehensive, reconciliatory model has yet to be determined. To fit with such a model, it is essential that candidate mechanisms exhibit reaction times, spatial ranges and speeds of propagation that are consistent with the vascular manifestations of the ‘hemodynamic response’. Understanding these vascular dynamics is therefore a critical step towards developing a robust model of neurovascular coupling. In this study, we utilize highspeed optical imaging of exposed rodent somatosensory cortex to explore and characterize the spatiotemporal dynamics of surface vessels during functional hyperemia. Our high-speed, high resolution optical imaging approach allows us to study the hemodynamic response independently in individual vessels, and in discrete regions of the parenchyma with enough resolution to precisely characterize subtle spatial and temporal features of the response. Specifically, we explore when and where the first hemodynamic changes occur in response to stimuli, the direction and speed at which these changes propagate in arterioles and regions of the parenchyma, and the relative timing at which each of these compartments returns to its original baseline state. From these results, we are able to conclude that the hemodynamic response is initiated in the parenchyma and then spreads rapidly to surface arterioles. Following the initial onset we find evidence that the response spreads spatially outwards via the dilation of targeted arterioles. This propagation of vasodilation is independent of the direction of blood flow within each arteriole. We also find evidence of a decay phase that acts with a more uniform spatial dependence, rather than along targeted vessels, causing the periphery of the responding region to return to baseline first. We hypothesize that different underlying cellular mechanisms/signaling pathways are responsible for the response initiation and the response decay. Our results advance a fundamental understanding of the hemodynamic response, as well as our ability to evaluate potential cellular mechanisms for their involvement in neurovascular coupling.
Keywords: Neurovascular coupling, optical imaging, vascular dynamics, propagation of vasodilation
I INTRODUCTION
A regulated and localized increase in perfusion accompanies almost all neuronal activation in the brain, and is essential for normal brain function. Orchestration of this hemodynamic response most likely involves cellular signaling mechanisms that communicate to the local vasculature that changes in blood flow are required. Cellular systems capable of actuating and propagating dilations and constrictions must also be present, with distributions and connectivities consistent with the spatiotemporal spread and decay of the hemodynamic response.
A range of candidate cellular mechanisms for neurovascular coupling have been studied in recent years. In vivo and in vitro studies at the cellular level have used high resolution imaging techniques, such as Dodt gradient contrast imaging and two-photon microscopy, with specific attention being paid to astrocytes, interneurons and pericytes (Cauli et al., 2004; Peppiatt et al., 2006; Schummers et al., 2008; Wang et al., 2006; Winship et al., 2007). These studies have focused on isolated cell-vessel interactions, exploring which cell types are capable of eliciting various vasomotor dynamics in neighboring vessel segments. Similarly, hemodynamic changes at the level of individual vessels have been characterized with in-vivo microscopy (Devor et al., 2007; Hillman, 2007; Kleinfeld et al., 1998; Stefanovic et al., 2008; Villringer et al., 1994; Wang et al., 2006). However, while many varied mechanisms have been proposed as a result of these studies, none of them have been incontrovertibly validated as being consistent with the actual behavior of the vascular network during the hemodynamic response. We believe that this confusion is due, in part, to a current lack of detailed knowledge about the vessel-specific behavior that constitutes the hemodynamic response. To date, questions such as the following have not been accurately addressed: When and where does the hemodynamic response start? How, where and at what speed does it propagate? And, how do ‘return to baseline’ dynamics differ from the response initiation dynamics? With proper knowledge of these parameters, it should be possible to more accurately assign potential cellular mechanisms to the different aspects of hemodynamic response orchestration.
Optical intrinsic signal imaging (OISI) of the exposed cortex offers a method of exploring wide-field spatial and temporal characteristics of the hemodynamic response within the superficial layers of the cortex (see Figure S1 for an example). OISI measurements consist of camera images acquired while the cortical surface is illuminated with specific wavelengths of light. Changes in measured light intensity predominantly reflect changes in absorption due to changes in the concentrations of oxy- and deoxy-hemoglobin ([HbO] and [HbR] respectively). OISI images represent a 2D, superficially weighted sum of signals from the superficial vasculature and the deeper microvasculature of the parenchyma. Many OISI studies to date have imaged through thinned skull at relatively slow speeds, and used different wavelengths of light to capture ‘activation maps’ of the hemodynamic response in the cortex. (Berwick et al., 2005; Das and Gilbert, 1999; Grinvald et al., 1986; Sheth et al., 2005). We recently developed an approach to OISI that offers high-resolution, high-speed imaging of exposed cortex, allowing the rapid responses of individual vessels to be observed (Bouchard et al., 2009). Our OISI technique, implemented in rodent somatosensory cortex, allows the study of vascular network changes occurring in almost all superficial vessels in and around the region of activation simultaneously. In order to perform a comprehensive characterization of vascular dynamics during functional hyperemia, we have combined this imaging technique with a new approach to spatiotemporal analysis that overcomes confounds in timing measurements due to response amplitude and finite baseline variance.
II METHODS
II.1 Animal preparation
Sprague Dawley rats (260g ± 40g) were anesthetized with isoflurane (2–3% inhalation in 3:1 air: oxygen mix) during surgical preparation and then switched to intravenous delivery of alphachloralose (40 mg kg−1 h−1) during stimulation and imaging. Surgery consisted of a tracheotomy followed by mechanical ventilation and insertion of femoral arterial and venous cannulae to allow continuous blood pressure monitoring and delivery of intravenous fluids. Core body temperature was measured and maintained at 37 degrees Celsius with a rectal thermometer attached to a homoeothermic heating pad. The rat was then placed in a stereotaxic frame, and a small portion of the skull (4 mm × 6 mm) overlying the left somatosensory cortex was thinned using a dental drill. The IVth ventricle was opened to relieve intra-cerebral pressure just prior to removal of the thinned skull and dura mater. Dental acrylic was then used to seal a glass coverslip with a drop of 1.5 % agarose in artificial cerebrospinal fluid over the exposed region, creating a window for imaging while minimizing brain motion and contamination (ACSF: 125mM NaCl, 5mM KCl, 4.1mM CaCl2, 10mM C6H12O6, 2.8mM MgCl, 10mM C8H12N2O4S). Electrodes were placed on the right hindpaw and forepaw and connected to an electrical stimulus unit (A360, WPI) delivering 3 ms pulses at 3 Hz with 1.0 ± 0.1 mA amplitude. Throughout data collection, systemic blood pressure and depth of anesthesia were monitored to ensure normal physiology, and maintained by slight adjustments to ventilation parameters and anesthesia. All animal procedures were reviewed and approved by the Columbia University Institutional Animal Care and Use Committee.
II.2 Optical imaging of exposed cortex
A ~3 × 3 mm region of the exposed cortex was imaged using our high-speed OISI system. The system utilizes a Dalsa 1M60 CCD camera configured to acquire in synchrony with strobing blue and green light emitting diodes (LEDs) mounted with 470 ± 5 nm and 530 ± 5 nm filters respectively (MBLED, MGLED, FB470, FB530, Thorlabs). Dual wavelength data were acquired either at 60 or 50 frames per second (equivalent to 30 fps or 25 fps for each LED) with a 5 ms exposure time and 256 × 256 pixel resolution. Details of the system are described in (Bouchard et al., 2009). Each imaging run consisted of 6 seconds of pre-stimulation, 4 seconds of stimulation, and 12 seconds of post-stimulation. Forepaw and hindpaw stimulation were presented in sets of 10 runs. A total of 5 rats were used in this study.
II.3 Analysis methods
General Methodology
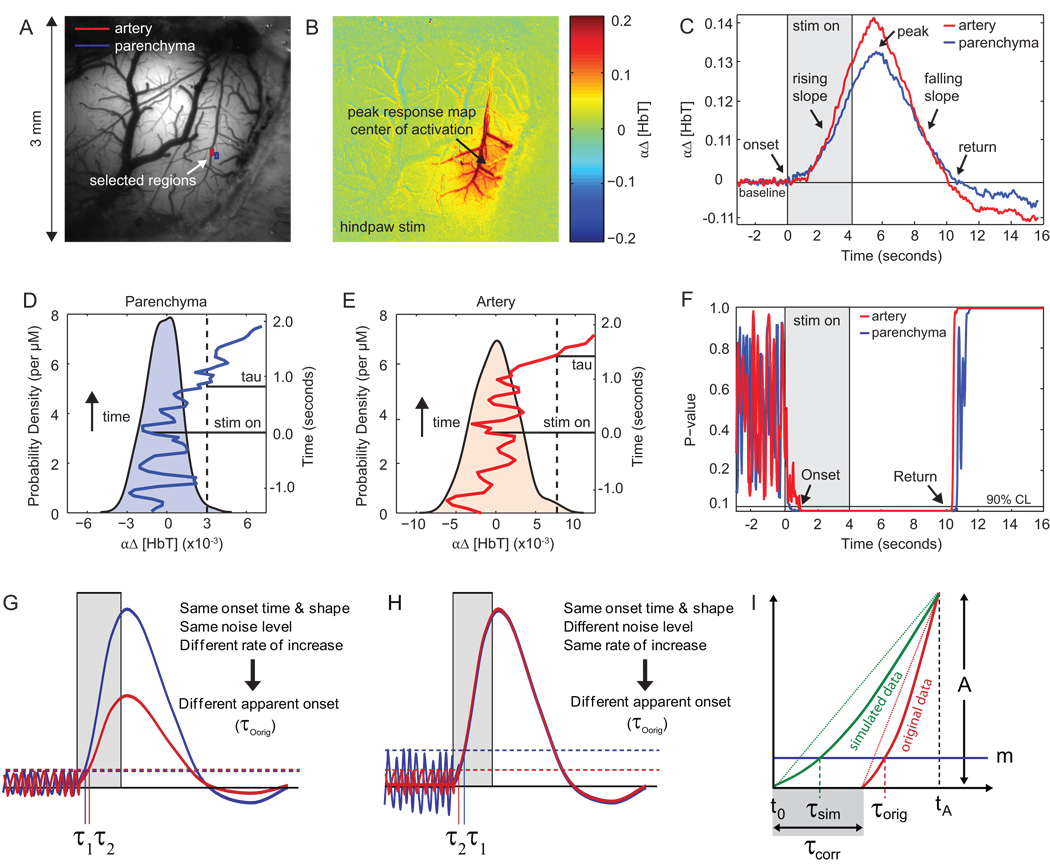
We developed a method of analysis specifically to study the timing characteristics of the hemodynamic response to functional stimulus. Typical time courses of total hemoglobin concentration changes Δ[HbT] following somatosensory stimulation are shown in Figure 1C. The baseline is recorded prior to stimulation, followed by the onset, rising slope, peak, falling slope, and return-to-baseline of the response.
Figure 1. Methods.
(A) Image of exposed cortical surface. (B) Functional map showing region of largest signal change in response to electrical hindpaw stimulation. (C) Δ[HbT] response from selected artery (red) and parenchyma (blue) regions in (A) obtained using 530 nm illumination (single trial). Duration of stimulation is shown in gray and arrows indicate features of interest in the Δ[HbT] signal. (D–E) Baseline PDFs constructed from 3 seconds of natural baseline signal for selected artery and parenchyma regions respectively. The dotted vertical lines indicate the 90th percentile of the baseline distributions. A segment of the Δ[HbT] signal is overlaid on the PDFs, with time on the vertical axis. The tissue is no longer in the baseline state with 90% confidence when the Δ[HbT] signal crosses the dotted 90th percentile line. (F) Plot of p-values (p-value = 1-cdf) computed for each of the time courses shown in (C). (G–H) Schematic showing τ computation methodology. Noise level thresholds are denoted by m. (I) Schematic showing derivation of correction factor. τsim and τorig are the calculated τ values for the simulated and original data sets respectively, τcorr is the estimated true onset time of the original data relative to to, the true onset of the simulated data, and tA is the time at which the simulated data and the original data are normalized to the same amplitude A.
To understand where and when the hemodyamic response begins, we need a method of quantifying the “onset time” of the Δ[HbT] response. Many studies to date have attempted to characterize the overall timing of the hemodynamic response pattern by estimating the time-to-peak (TTP, or to some % of the peak) of the response within different regions or compartments (Berwick et al., 2005; Blood et al., 2002; Sheth et al., 2005; Vanzetta et al., 2005). However, as illustrated in supplemental Figure S3, the TTP or other threshold-based measures may or may not be related to the time at which the response is initiated. For example, if two regions were to exhibit identical onset times and rates of increase, but the post-stimulus decay of one began before the other, then the TTP would be completely unrelated to the signals’ true onset times (Figure S3A). The various combinations of onset time, onset slope, peak amplitude and inflection point all influence the TTP and its relation to the true onset time. With so many free parameters describing the structure of these complex responses, a single statistic (eg. TTP) cannot be used as a reliable measure to characterize all the variations of interest.
In our approach, we estimate when the response signal in each pixel deviates from its baseline state. This allows us to determine when the signal in each region has ‘onset’, i.e. transitioned out of its baseline state, up to a specified level of confidence. An analogous calculation is used to determine the time at which the signal returns to its baseline state. Our technique estimates the earliest moment at which we can confidently observe a transition out of (or into) the baseline state. However, this method may still be affected by the rate at which signals rise, if baseline signals exhibit nonzero variance. For example, two signals with identical onset times and identical noise levels but different rates of increase will yield different apparent onset times (Figure 1G). Alternatively, two signals with identical time-courses but different levels of baseline noise will also yield different apparent onset times (Figure 1H). We therefore apply a second level of processing to compensate for this effect. Details on this technique are provided below.
Pre-processing
All analysis was performed using Matlab™. Data sets from each run were co-registered between consecutive frames and low pass filtered at 5Hz to minimize variance due to motion artifacts related to breathing and heart rate. All analysis was performed on single-run data sets that were mean-normalized by their average baseline value.
Since 530nm is an isobestic point for hemoglobin absorption, the baseline-normalized signal acquired with this wavelength (I/I0) should be independent of changes in oxygenation and thus provide only a measure of Δ[HbT] concentration when converted using the Beer Lambert law:
| (Eq. 1) |
where
| (Eq. 2) |
α is a constant that is expected to be approximately spatially and temporally invariant, and depends on x30 (an estimate of the mean pathlength of 530 nm light in the rat cortex) and εHbT ,530, the mean molar extinction coefficient for HbO and HbR at 530 nm. Since α is dependent on the choice of a light propagation model and the accuracy of estimates of the molar extinction coefficients, we analyzed and display our data in terms of αΔ[HbT], which is linearly proportional to Δ[HbT] (Hillman, 2007). We began by carefully ensuring that our multispectral-converted Δ[HbT] signals (derived from both blue (470 nm) and green (530 nm) data) corresponded accurately to changes in [HbT] calculated using Eq. 1. However, we chose to utilize only data acquired at 530 nm to avoid the potential confounds of inaccurate conversion of spectral data into hemoglobin concentrations (Kohl et al., 2000; Sirotin et al., 2009). While oxygenation dynamics can readily be extracted from our dual-wavelength data, our primary goal here was to understand the timing of vascular responses as indicated by changes in vessel diameter or hematocrit, both of which can be determined from measures of Δ[HbT].
Calculation of response onset and return ‘tau’ (τ)
We model our measured signals as having two states: a baseline state and a stimulated state. We start our analysis by characterizing the signal variability for each pixel under baseline conditions for the 3 seconds prior to stimulation. Figure 1D–E displays the probability distribution function (PDF) of baseline total hemoglobin absorption for parenchyma and artery regions computed using a kernel density estimator. When pixel Δ[HbT] levels are well beyond the PDF observed in the baseline, it is improbable that a pixel is still in the baseline state. At every time point we calculate the probability that a pixel’s signal has departed from the baseline Δ[HbT] PDF, this is computed as the integral of the PDF for values above the observed level (p-value = 1 – cdf, the cumulative distribution function). Figure 1F shows how p-values of the selected pixels change over time. We define onset τ (τO) as the time after stimulation when a pixel is first observed in the stimulated state at the 90% confidence level. A similar analysis is done to estimate the time of the return transition, denoted return τ (τR). We use τ to denote an estimate of the time of events. In practice, τ values are computed by determining the time at which the un-normalized, smoothed (12th order polynomial fit) time course in each pixel crosses a threshold based on that pixel’s baseline variance. Supplemental Figure S4 shows a map of threshold values for the 90% confidence level. We note that while there is heterogeneity across threshold values between vessels and parenchyma, we do not observe any systematic spatial differences across the exposed cortex.
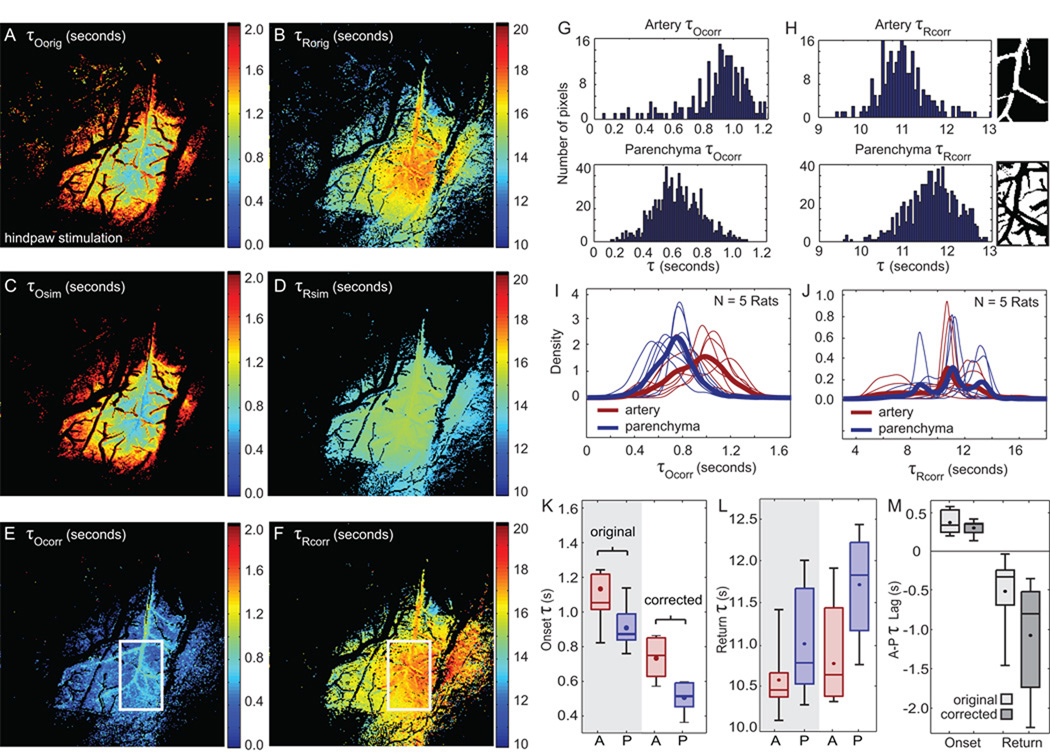
For each run in every rat, we computed the τO and τR of every pixel. Averaging each pixel’s τ values over runs for a single rat generates a “τ map” depicting the average times at which each pixel is observed to transition between baseline and stimulated states (Figure 2A–B). Regions that do not exhibit significant changes in [HbT] are shown in black.
Figure 2. Onset and return τ results.
(A–B) τOorig and τRorig maps. The stimulus is presented at t = 0 s and ends at t = 4 s. (C–D) τOsim and τRsim maps constructed from simulated data set. (E–F) τOcorr and τRcorr maps with white box indicating center region of activity. This masked region is studied in more detail in (G–M). (G) Histograms of τOcorr for arterial (top) and parenchyma (bottom) compartments extracted from the central region of interest. (H) Histograms of τRcorr for arterial (top) and parenchyma (bottom) compartments. Insets show artery (top) and parenchyma (bottom) masks used to differentiate compartments, with veins excluded from both. (I–J) Smoothed histograms of τOcorr and τRcorr for 5 rats are shown. τOcorr distributions are consistent across rats whereas τRcorr distributions are more variable across rats. Bold lines represent mean density functions. (K–L) Median τorig and τcorr values computed from central region indicated by the white box in (E–F). (M) τorig and τcorr lags between artery and parenchyma compartments. For all boxplots, bounding boxes show the inter-quartile range with the solid line indicating the median and the dot indicating the mean value. Whiskers show the extent of data for all 5 rats.
Correcting τ values for initial rate of change
The calculated τO values described above characterize the moment when the first observable hemodynamic response occurs in the tissue. Prior to τO, we cannot claim to observe changes that are distinguishable from baseline noise. However, the calculated τO is not only influenced by the signal’s true onset time, but also by the level of baseline variance and the initial slope of the signal as well (Figure 1G–H, Figure S3).
This effect can be visualized by generating a simulated data set using an average representative time-course from the data for each rat. At every pixel the representative time-course is scaled such that its amplitude matches the amplitude (A) of the original signal at time tA(Figure 1I). Thus, every pixel in the simulated data set has an identical true onset time but varying rates of change, which are prescribed from the original data. If we calculate a map of τ using this simulated data, but with threshold values (m) determined from the baseline variance at every pixel of the original data, we observe a range of apparent onset times that are due only to differences in spatial variations of onset slopes (Figure 1C–D). These simulated (τsim) maps illustrate how much our original estimates of τ are influenced by spatial variations in the rate of signal change. We can thus use these maps as a means to correct our original (τorig) maps, since regions where τorig maps differ from τsim maps are likely to be regions where there are genuine differences in true onset.
More formally, if we assume signals are linear between the true onset time and time tA (Figure 1I), then the relationship between the τorig, the τsim, and the corrected τ (τcorr), highlighted in gray in Figure 1I) can be written as:
| (Eq. 3) |
where t0 is the true onset time of the simulated response curve. The amplitude A of the signal at time tA and the baseline threshold m are both specific to each pixel and are determined by the original data. The value of tA will affect the appropriateness of our linear approximation. For this analysis, we set tA = 2 seconds following the start of stimulation. By applying this correction, we generate τOcorr maps that provide an estimate of “true onset” decoupled from the signal’s rate of change. The same method is applied to obtain a baseline return τRcorr, by using a value of tA = 6 seconds following the start of stimulation.
In summary, the existence of baseline noise places a constraint on what is observable during a single experimental run, if we are not willing to make additional assumptions about the structure of the data below the noise level (τorig). However, the true timing of the signal onset is a meaningful parameter with regards to the physiology of response initiation. Thus, if we are willing to make assumptions regarding the behavior of the signal below the noise level, we can make an estimate what the true onset time would be independently of the signal’s initial rate of change (τcorr).
Distinction of vascular compartments
We use the differing baseline oxygenation properties of the arterial, venous, and parenchymal regions revealed in 470nm and 530 nm baseline images to effectively isolate compartments for further analysis. This method is described in detail in (Bouchard et al., 2009). To explore which compartment has a faster observable onset time, we overlay parenchyma and arterial masks with onset τ and return τ maps to obtain distributions of compartment-specific τ values.
Quantification of signal onset and return τ propagation speed
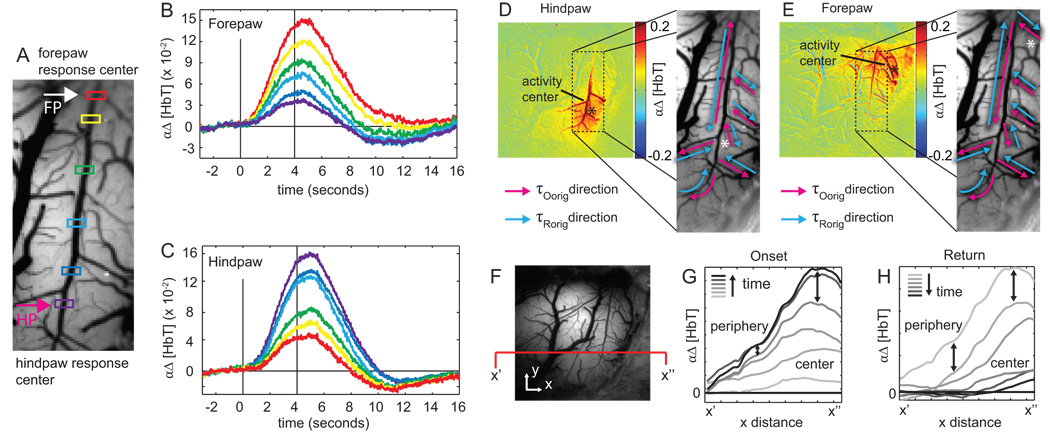
Speed of τOcorr and τRcorr propagation can be quantified by linearly regressing τcorr values on linear distance from the center of the responding region (Figure 3B–E). The inverse slope of the fitted regression line is then the estimated speed of τOcorr and τRcorr in units of mm/s. We also investigate the directions of τOorig and τRorig propagations by taking the sign of the regression slope as a function of distance from the center of the responding region (Figure 4D–E).
Figure 3. τcorr propagation dynamics.
(A) Image of the exposed cortex. Pink and blue arrows indicate vector directions used to compute propagation speeds. (B–E) τOcorr and τRcorr values selected from arterial vector in (A) are plotted as a function of radial distance from the center of activation. (B–C) shows arterial propagation and (D–E) show parenchyma propagation. The inverse slope of the linear regression represents the onset and return propagation speed in mm/s. (F) Summary of propagation speeds computed over 5 rats. Boxes show inter-quartile range with solid line indicating the median and the dot indicating the mean value. Whiskers show the extent of the data. Onset propagation speeds in the parenchyma are excluded due to insignificant p-values (p>0.1). This indicates that any propagation that does occur in the parenchyma is faster than we can reliably measure.
Figure 4. Response amplitude dynamics.
(A) Artery transecting both hindpaw (pink arrow) and forepaw regions (white arrow). (B–C) Δ[HbT] time courses plotted for the color-coded points along the vessel in (A) during forepaw (B) and hindpaw (C) stimulation. Time courses represent the average over 10 runs (unfiltered). (D–E) Directions of τOorig (pink arrows) and τRorig (blue arrows) spatial gradients are shown in the major surface artery and immediate arterial branches during hindpaw (D) and forepaw (E) activation. Arterial blood flow direction is from top to bottom. (F) Gray scale image showing cross section x’-x”. (G–H) Cross sectional signal from x’-x” plotted at 20 time-frame intervals during the onset (G) and the return (H). Signal growth is faster in the center than in the periphery during the onset, but signal decay is more spatially uniform (until baseline is reached) during the return to baseline.
III RESULTS
III.1 Comparison of original, simulated, and corrected τ maps
Figure 2A–F displays τO and τR maps calculated from the original data (A–B), simulated data (C–D), and after applying the rate-of-change correction factor (E–F).
We observe that τOorig maps are very similar to τOsim maps, suggesting that the spatial gradients in the τOorig maps are predominantly due to spatial variations in signals' rates of change (C–D). In contrast to both τOorig and τOsim maps, τOcorr maps exhibit greater spatial uniformity and overall earlier values. Earlier values are to be expected as the true time of onset should occur before we observe it (in the presence of nonzero baseline variance).
τRorig, τRsim, and τRcorr maps all show spatial gradients of varying steepness, with peripheral regions exhibiting earlier returns to baseline than central regions. In contrast to the onset maps, the differences between τRorig and τRsim map gradients suggest that spatial variations in rates of decay are not the primary cause of the spatial gradients observed in the τRorig map. However, removing the effects of decay rates on return-to-baseline, we do note that the regions exhibiting the latest τRorig and τRcorr are slightly shifted from each other.
While applying the correction described in Eq. 3 changes the distribution of parenchyma and artery τO and τR values, the relative order of timings between compartments remains the same for both original and corrected τO and τR values.
III.2 Corrected onset and return τ values of central response
We consistently observe that the central parenchyma region exhibits both the first measureable signal onset and the latest return to baseline, while central arterial regions exhibit later signal onset and earlier baseline return.
Figure 2G–H shows histograms of onset and return τcorr values for masked arterial and parenchyma regions from the central region (see right inset). A greater proportion of parenchyma pixels exhibit earlier onset τcorr and delayed return τcorr compared to arteriolar pixels.
Across rats, the average median τOcorr is 0.537 ± 0.066 seconds for the parenchyma response and 0.748 ± 0.131 seconds for the arteriolar response (Figure 2K). The average onset lag (arterial τOcorr minus parenchymal τOcorr) is 0.211 ± 0.087 seconds (Figure 2M). This analysis was repeated for the τRorig and τRcorr. The arterial region exhibited average median τRcorr of 10.91 ± 0.875 seconds while the central parenchyma exhibited later τRcorr of 11.899 ± 0.825 seconds (Figure 2L). The lag between the τRorr of the arterial and parenchymal regions was −0.989s ± 0.615 seconds (Figure 2M).
Figure 2I–J displays smoothed histograms for artery and parenchyma onset and return τcorr from all 5 rats as well as their average (bold lines). In both compartments, the onset τcorr distributions are remarkably similar across rats. This similarity is what produces the narrow ranges of median onset τcorr in Figure 2K. Histograms for return τcorr in both compartments exhibit greater variability (Figure 2H). As a result, we observe a wider range of median return τcorr in Figure 2L.
We note that the major veins in Figure 2A–B appear black, indicating that no statistically significant changes in Δ[HbT] were detected in these regions under our stimulation paradigm (Bouchard et al., 2009; Hillman, 2007).
The onset and return analysis above was limited to the central region of activation. We now extend our τ analysis to study the spatial spread of the response in both the center and periphery of the responding region.
III.3 Signal propagation speed and direction
The initiation of the response appears to occur simultaneously across all responding locations in the parenchyma. Subsequent propagation in major arterioles occurs rapidly in a direction away from the center of activity and is independent of the direction of blood flow. The return-to-baseline in both compartments is first observed in peripheral regions and occurs last at the center of the responding region.
Arrows in Figure 3A indicate artery and parenchyma regions along which τOcorr and τRcorr are plotted in Figure 3B–E. Linear regression of τOcorr on radial distance and τcorr on radial distance provides measures of propagation speed. All regression fits exhibited significant p-values (p < 0.1) except for onset in the parenchyma (Figure 3D), indicating that any propagation within the parenchyma occurs faster than we can reliably measure. In addition to this constraint, we also discard measured propagation speeds greater than 6.5 mm/s, as this is at the limit of our temporal resolution when imaging ~0.5 mm vessel segments at 25 fps. Eliminating the trials where p > 0.1 or speed > 6.5 mm/s, we estimate average arterial τOcorr propagation speed to be 2.387 ± 1.65.mm/s. We estimate the average rate at which τRcorr returns to baseline along arterioles to be −0.575 ± 0.46 mm/s, and across parenchyma to be −0.659 ± 0.21 mm/s (Figure 3F). Within the artery, τOcorr propagation speeds were consistently found to be faster than τRcorr return rates, and the relationship between these two measures across 5 rats is shown in Supplemental Figure S5. We find that while some rats exhibit fast propagation speeds and others exhibit slow propagation speeds, the ratios between onset propagation speeds and the rates of return to baseline are comparable for different rats. Further investigations across rats did not reveal significant correlations between propagation speed and blood pressure or body weight (Figure S6).
We emphasize here that propagation of τOcorr and τRcorr represent a spatial propagation of a transition between the baseline and stimulated states. The τOcorr and τRcorr maps do not characterize any dynamics occurring between the response initiation and return-to-baseline, and following the return to baseline, and so do not reflect the ultimate amplitude of changes in those regions. Further, τRcorr does not represent the time of initial vasoconstriction.
III.4 Dynamics of the response amplitudes
Following the response initiation, the fastest growing Δ[HbT] signals are observed at central responding regions, while signal decay rates are more uniform across both central and peripheral regions.
The results above indicate that the first hemodynamic change in response to stimulation occurs nearly simultaneously throughout the parenchyma, followed by rapid propagation of vasodilation along the major artery feeding the region. However, despite the spatial uniformity of τOcorr, we nevertheless observe spatial differences in rate of signal growth and peak amplitudes throughout the responding region (Figure 4). These differences are effectively captured in τOorig maps and extracted Δ[HbT] signals, which may provide insights into the location of the “driving force” behind the response evolution.
Figure 4B–C shows time-courses generated by sampling the Δ[HbT] data at different locations along the arteriole transecting both hindpaw and forepaw regions as shown in Figure 4A. Pink and white arrows indicate the locations of the responding forepaw and hindpaw regions respectively, and corresponding functional maps can be seen in Figure 4D–E. These plots demonstrate that peak response amplitudes (and rates of change) are greatest near the center of the responding region and decrease along the artery further away. We also note that the pattern of peak amplitudes as a function of distance from the center of the responding region in a single artery is the same for both forepaw (Figure 4D) and hindpaw (Figure 4E) stimulation, regardless of the absolute location of the responding region along the vessel and the direction of blood flow.
Following the return-to-baseline, regions along the vessel exhibit a brief signal undershoot that corresponds to vasoconstriction. Arterioles at the periphery of the responding region exhibit small peak amplitudes (dilations) and large undershoots (constrictions), while central arterioles exhibit larger peak amplitudes and smaller undershoots (Figure 4F–G).
To further investigate how the response evolves just following τOcorr, we computed the direction of propagation of τOorig and τRorig along branches of the arterial tree. While these measures do not necessarily correspond to the initial onset propagation, they do reflect the spatial gradient of ‘vasodilatory signal strength’ insofar as the end of the branch that exhibits the fastest increase in dilation is likely to be the end that is closest to the source of the signal that is mediating vasodilation (the “driving force”). Figure 4D–E displays functional maps indicating the location of Δ[HbT] increases for each stimulus type. The directions of τOorig (pink arrows) and τRorig (blue arrows) spatial gradients under the two stimulation paradigms are depicted on the magnified images of the major artery and its branches, with white asterisks marking the center of the responding region for each. For hindpaw and forepaw stimulation, the direction of the τOorig gradient is independent of whether the vessel segment is upstream or downstream of the responding region. In many, but not all segments the direction of the τOorig gradient switches when the stimulus is changed from forepaw to hindpaw.
Our τcorr maps show that peripheral branches are the first to return to baseline. In most cases, the τRorig gradient is in the opposite direction to the τOorig gradient for a given stimulus, again showing that these gradients develop irrespective of the direction of blood flow. Two vessel segments can be seen to have τOorig and τRorig gradients in the same direction in Figure 4E, but different directions in Figure 4D.
Finally, because the hemodynamic response is a highly spatiotemporal phenomenon we can also visualize its dynamics from an alternative perspective. The cross-sectional Δ[HbT] signal across x’-x” shown in Figure 4C is plotted at 20 frame intervals of during the signal rise (Figure 4D) and decay (Figure 4E). This plot allows us to look at the differential changes in signal as a function of spatial location relative to the responding region. During the signal rise, we notice that signal amplitude grows much faster in the central regions as compared to signal growth in peripheral regions. However, the decay phase following the signal peak exhibits a relatively constant rate of change in both central and peripheral regions.
III.5 Summary of findings
Our results indicate that the first hemodynamic changes in response to somatosensory stimulation occur as a uniform Δ[HbT] increase in parenchymal regions. This initial response remains parenchyma-specific for no longer than 200 ms before spreading rapidly to major pial arterioles (Figure 3). Following the initial response onset, increasing retrograde dilation of the arteriolar branches feeding the responding region causes the amplitude of the response to grow and spread (Figure 4). At parent arteries, propagation of this vasodilation continues both upstream and downstream along the superficial arterial tree. The direction of this propagation is therefore independent of the direction of blood flow and appears to be governed primarily by the relative location of the activated cortical region.
The return-to-baseline is first observed at the periphery of the responding region. This is true for both arterial and parenchyma compartments. After returning to baseline, peripheral segments of the parent artery and branches feeding non-responding cortical regions undershooting briefly before returning back to their initial baseline state. The last region to return to baseline is the central parenchyma region, which exhibits a sustained Δ[HbT] increase following return-to-baseline of the feeding arteriolar tree. These results are consistent with pictorial evolution of the hemodynamic response shown in supplemental Figure S1.
IV DISCUSSION
In order to properly evaluate hypotheses about the cellular mechanisms governing the hemodynamic response, we must first understand what the hemodynamic response to somatosensory stimulation represents at the vascular level. Combined, our results imply that the cellular mechanism(s) underlying the hemodynamic response must be able to (1) act initially, and rapidly on capillaries and/or pre-capillary arterioles, (2) propagate rapid vasodilation along the arteriolar network at average speeds of ~2.4 mm/s, (3) account for propagation of vasodilation along arterioles in directions that are independent of the direction of blood flow, and (4) mediate vasoconstriction such that vessels at the periphery return to baseline first. We discuss each of these implications below:
IV.1 Initial parenchyma hyperemia
Our results suggest that capillary hyperemia is a major contributor to the ensemble Δ[HbT] response. Although such increases in parenchymal Δ[HbT] have been repeatedly observed in vivo with optical imaging methods (Berwick et al., 2005; Blood et al., 2002; Culver et al., 2005; Devor et al., 2003; Hillman et al., 2007; Jones et al., 2004; Nemoto et al., 2004; Sheth et al., 2005; Sirotin et al., 2009; Vanzetta et al., 2005), the occurrence of capillary hyperemia is often overlooked due to the lack of a known mechanism capable of actuating such an observation (Buxton et al., 1998; Hoge et al., 1999).
Actuation mechanisms for a parenchymal HbT increase
For an increase in parenchymal HbT to occur, it is necessary for there to be an increase in the number of red blood cells (RBCs) per unit volume of tissue at any point in time. One way for this to occur would be for capillaries to increase their diameters, which would allow more plasma, and hence more red blood cells per unit length to be carried within a capillary. Two photon and confocal microscopy studies (Chaigneau et al., 2003; Hutchinson et al., 2006; Kleinfeld et al., 1998; Stefanovic et al., 2008; Villringer et al., 1994) have previously demonstrated that capillaries can exhibit changes in both their diameters and their speed of flow during hypercapnia and functional stimulus. However, these studies were unable to conclude whether this ‘dilation’ occurs passively, as a result of initial upstream arteriolar dilation (Vanzetta et al., 2005) or actively, via physical regulation of microvessel diameters (Iadecola et al., 1997; Sheth et al., 2005).
Our results indicate that initial functional hyperemia of the parenchyma occurs prior to dilation of upstream pial arterioles, suggesting that the initial onset of the hemodynamic response might indeed be directly and actively initiated by the capillaries themselves. While apparent lack of smooth muscle at the capillary level makes this seem unlikely, a recent study suggested that pericytes may be capable of physically modulating the diameters of microvessels (Peppiatt et al., 2006). This hypothesis is also attractive, since it allows for almost arbitrary positioning of initial flow control, and spatially couples sensing, signaling and actuating mechanisms at the site of peak metabolic activity and nutrient exchange (Borowsky and Collins, 1989; Klein et al., 1986).
In order for passive capillary dilation to occur, a part of the vascular network other than the pial arterioles would need to actively decrease its resistance. (Tian et al., 2010) recently reported that deep segments of pre-capillary arterioles can be seen to dilate prior to pial branches, which is consistent with retrograde propagation of dilation. Such changes could feasibly cause increases in capillary flow (and / or diameter) prior to observable dilations of pial vessels, however it is not clear whether changes in capillary tone or HbT precede these changes and therefore whether these deep arteriolar dilations could in fact represent back-propagated signals from capillaries. (Stefanovic et al., 2008) showed that capillary diameters increase during functional stimulus, but used steady-state stimulus and so could not explicitly examine the dynamics of the early parenchymal HbT response that we observe prior to arteriolar dilation.
Besides capillary dilation, another explanation for parenchymal HbT increases might be an increase in capillary hematocrit, for example due to an increase in post-capillary resistance, causing red blood cells to be trapped within the capillary beds while plasma is able to leave. A further possibility might be that changes in capillary hematocrit could be modulated by changes in capillary water permeability. Fluctuations in venous hematocrit that could be consistent with these mechanisms have been previously observed using two-photon microscopy (Hillman et al., 2007).
In addition to a rapid parenchymal HbT increase, our results also revealed sustained elevation of HbT in a localized region in the center of activation after stimulus cessation (Figure 2F, Figure S6, and Figure S1). This sustained increase in HbT following the return of arteriolar dilation to its baseline is consistent with a mechanism that forces extra RBCs into each capillary during the high-flow phase of the response, only to have them get trapped once flow has reduced upon arteriolar constriction. (Berwick et al., 2008) observed a similar sustained localization of increased Δ[HbT] in the stimulated barrel following electrical whisker stimulation. They suggest that this later phase of the response may be a spatially specific and less transient indicator of neuronal activity than very early changes.
Relevance to the initial dip
It is important to note how our findings relate to the ‘initial dip’ (a previously noted initial increase in HbR thought to indicate increase oxygen consumption prior to increases in flow) (Malonek and Grinvald, 1996). A recent study by (Sirotin et al., 2009) demonstrated that traditional OISI measurements at 610 nm to 630 nm can provide misleading time-courses that do not specifically reflect changes in HbR, but are contaminated by early changes in HbT. To summarize; initial decreases in detected signal perceived as increases in HbR can be readily explained by simultaneous (or even faster) increases in HbT. Sirotin et al. demonstrated in awake behaving primates, that multispectral OISI data could be converted to changes in HbO, HbR and HbT that were consistent with our findings, and did not exhibit an initial increase in HbR. As a result, much of the recent literature that has utilized OISI at these wavelengths and identified the ‘initial dip’ spatial extent as the center of the responding region may in fact have been measuring an early, rapid and localized increase in HbT.
If the first changes in the parenchyma in fact corresponded to an increase in HbR in the absence of an active increase in HbT, one would also need to observe a concurrent decrease in HbO. This feature of the hemodynamic response is not routinely observed, and was not a noticeable feature in our 470 nm imaging data which would be sensitive to this oximetric change. This leads us to conclude that if an increase in HbR does occur early during the response to stimulus, it is accompanied by an active increase in HbT in the parenchyma, which robustly precedes changes in the diameter of pial arterioles. Nevertheless, it should be noted that since our analysis of the earliest initial onsets (τocorr) pushes our data to the limits of its statistical power, we cannot unequivocally know that these small rapid changes have no oximetric component at all. Equally, if fast scattering changes were occurring in the cortex during neuronal firing, our analysis would not be able to distinguish between their effects and those of an HbT increase.
IV.2 Retrograde propagation of vasodilation and vascular signaling networks
Following the initial onset of parenchyma hyperemia, we observe retrograde propagation of vasodilation along arterioles at the cortical surface at a speed on the order of 2.4 mm/sec. These observations support the existence of mechanisms that actuate control along vascular paths between capillaries and surface arteries, rather than signaling networks that establish a direct communication between active neurons and surface arteries (Vanzetta et al., 2005) or diffusive molecular signals that act non preferentially on vessels (Roy and Sherrington, 1890).
Previous in vivo imaging studies have generally acquired images at rates that were too slow to detect propagation direction, although several have suggested that their results indicated either retrograde or anterograde vasodilations (Duling and Berne, 1970; Iadecola et al., 1997; Sheth et al., 2005). Our higher frame rate, high-resolution imaging and statistical analysis have allowed us to more clearly characterize and quantify propagation of vasodilation in somatosensory cortex. While retrograde vasodilation was previously described in response to direct stimulation of parallel fibers in the cerebellum (Iadecola et al., 1997) measurements of propagation speeds in hamster cheek pouch vessels suggested likely rates of 200 µm/sec (Duling and Berne, 1970). We have now determined that retrograde propagation in cerebral arterioles in-vivo in response to functional stimulus occurs at over 10 times that rate, explaining why such propagation had not been detected previously at slower imaging speeds.
Interplay between arteriolar and parenchymal propagation dynamics
Just after initial onset of the response in the parenchyma, given by τOcorr, our results seem to indicate the presence of a secondary “wave” of actuation that is responsible for spatial differences in rate and amplitude of vasodilations along arterial vessels and throughout the parenchyma. This is to be expected since as dilation spreads along pial arterioles and their branches, regions fed by these vessels will receive increased flow, on top of any initial increases in Δ[HbT]. Increases in Δ[HbT] in peripheral parenchyma are also consistent with this model, since their patterns appear to more closely follow the contours of the arteriolar branches than being isotropic (Figure 2A–B, Figure 4D–E).
Our results also suggest that the vascular path between capillaries and each surface arteriole may serve as the signaling conduit between the active region and more distant vessels. This vascular path back to parent pial arteries may provide a convenient “road map” indicating which vessels are required to dilate in order to orchestrate a neatly localized hemodynamic response. Mechanistic hypotheses that fit this scheme include signal transduction via endothelial gap junctions in vessel walls (Bartlett and Segal, 2000) and networks of pericytes or glial cells that are closely integrated with vasculature (McCaslin et al., 2010; Peppiatt et al., 2006).
IV.3 Second mechanism responsible for return to baseline
Previous studies have focused mainly on the rising (vasodilation) phase of the response, often neglecting to address the constriction phase that returns blood flow to its baseline state. We find that the response decay does not seem to result simply from a lack of dilatory actuation, but rather from active constriction that causes Δ[HbT] to undershoot the baseline before returning to normal after stimulus cessation.
Furthermore, if we were to simply assume that the agents actuating vasodilation and vasoconstriction originated from the same source at the center of the responding region, then we would expect the response to evolve spatially in the pattern of an expanding ring of increased [HbT], a scenario which we never observe. This leads us to hypothesize that two spatiotemporally distinct mechanisms are responsible for rising and decay phases of the response. Further evidence to support this includes our observation that the response grows the fastest in the central responding region relative to the periphery whereas the response decay occurs at a more spatially uniform rate (Figure 4G–H). Figure 4B–C show that during the onset phase, all responding regions reach their peak amplitudes in approximately the same amount of time. During the decay phase however, the peripheral regions return to baseline in less time than the central regions, leading to the staggered returns to baseline captured in our τR maps. The large variability of τRorig and τRcorr values across rats, compared to the low variability of τOorig and τOcorr values (Figure 2J), suggests that the response initiation is a much more controlled and consistently regulated than the return phase.
Different mechanisms for the “excitatory” and “inhibitory” phases of the response may be required to suit different purposes. Onset signaling along the vascular network may provide the most direct, targeted pathway to allow rapid, localized flow increases by dilating only the most necessary arteriolar branches. However, since return-to-baseline is less time-critical, the inhibitory vasoconstrict signal does not necessarily need to travel along the same network. A wide-ranging inhibitory domain (not organized along the vascular tree) would not directly constrict the specific vessels that had initially dilated, but would guarantee the eventual return to baseline of vessels within the entire cortical region, while also potentially acting to constrain the area over which vasodilate signals are able to spread initially. The presence of two spatiotemporally independent dilate / constrict mechanisms would explain anomalies such as the vessel segments in Figure 4A–B that in one case reverse directions between onset and decay gradients, and in the other exhibit gradients of onset and return in the same direction.
The pattern of dilation and constriction that we observe agrees well with the center-surround pattern of hemodynamics described by Devor et al. and recently supported by Boorman et al. (Boorman et al., 2010; Devor et al., 2007). Both reported that arteriolar constriction and decreases in Δ[HbT] are accompanied by neuronal inhibition, as indicated by voltage sensitive dyes and electrode array recordings. As noted by Boorman et al. and Devor et al, the presence of inhibition in peripheral regions could relate to interneuron involvement. Cauli et al. demonstrated that several species of interneurons are capable of mediating vasoconstriction, such as somatostatin interneurons whose morphology (soma located around layer III/IV with dense, wide-reaching projections to layer I) lends itself well to this hypothesis (Cauli et al., 2004; Karagiannis et al., 2009).
A hemodynamic response which is a balance between two effects; one dilatory and one constrictory, is an attractive model since few physiological systems can achieve homeostasis with only an excitatory mechanism in the absence of an inhibitory one. Natural oscillations in baseline blood flow could also represent the normal settling back and forth between these two effects (Mayhew et al., 1996; Raichle, 2009; White et al., 2009).
IV.4 Methodological conclusions
Previous OISI studies of cortical hemodynamics have focused on studying spatiotemporal dynamics of the timing and amplitude of the signal peak. The peak of the hemodynamic response is an obvious feature in the measured response signal; however the peak time and peak amplitude result from the confluence of numerous effects including the signal onset and rates of signal change before and after the peak (Figure 4,Figure S2). The unknown spatiotemporal dynamics of these very different effects can confound the meaning of the response peak.
Further, each feature of the hemodynamic response, such as onset, rate of change, and peak timing and amplitude may represent distinct physiological mechanisms or events. It is therefore important to treat these measures independently of each other. For example, it is easy to mistake fast signal growth for fast signal onset. Because the response onsets occur on such a rapid time scale, the visual effect of response growth and peak amplitude in a series of images or time courses quickly dominates the subtle differences exhibited in onset transitions between compartments. The fast arteriolar and slow parenchyma signal peak time that we observed may therefore explain why some studies reported initial arteriolar Δ[HbT] responses that subsequently spread to the parenchyma (Nemoto et al., 2004; Vanzetta et al., 2005).
Here we used an analysis methodology that seeks to overcome the influence of peak amplitudes and rates of change on onset determination. Our τOorig measures represent the time at which the effects of the response actuating mechanisms are first observable via OISI given the baseline variability. To better understand the precise physiological correlates underlying response initiation, we make an estimate (τcorr) of what the true onset time would be given what we can observe (τorig) and a structured model of the response (τsim). By using these measures in addition to studies of peak amplitude and timing, we have been able to develop a more complete picture of the hemodynamic response.
In summary, we have conducted a thorough characterization of the hemodynamic response through the use of high-speed multispectral optical imaging. We conclude that the hemodynamic response is initiated at the capillary level and then propagates back along targeted arterioles in the vascular network. We hypothesize that a different underlying mechanism is responsible for the response’s decay back to baseline, and show evidence for a decay phase that acts non-preferentially across the responding region. Our results advance a fundamental understanding of the hemodynamic response as well as our ability to determine the potential cellular neurovascular coupling mechanisms that are responsible for orchestrating the observed vascular changes.
Supplementary Material
Acknowledgments
We thank Bruno Cauli for useful discussions. Funding was provided by NIH (NINDS) grants R21NS053684 and R01NS063226, NIH (NEI) grant R01EY019500, NSF CAREER 0954796, NIH (NCI) U54CA126513, the Human Frontier Science Program and The Rodriguez Family. B. R. Chen receives funding from a National Science Foundation graduate fellowships and M. B. Bouchard is supported by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H604–H612. doi: 10.1152/ajpheart.2000.278.2.H604. [DOI] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Martin C, Kennerley AJ, Redgrave P, Mayhew JEW. Fine Detail of Neurovascular Coupling Revealed by Spatiotemporal Analysis of the Hemodynamic Response to Single Whisker Stimulation in Rat Barrel Cortex. J Neurophysiol. 2008;99:787–798. doi: 10.1152/jn.00658.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JEW. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. Eur. J. Neurosci. 2005;22:1655–1666. doi: 10.1111/j.1460-9568.2005.04347.x. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Pouratian N, Toga AW. Temporally staggered forelimb stimulation modulates barrel cortex optical intrinsic signal responses to whisker stimulation. J Neurophysiol. 2002;88:422–437. doi: 10.1152/jn.2002.88.1.422. [DOI] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J. Negative Blood Oxygen Level Dependence in the Rat:A Model for Investigating the Role of Suppression in Neurovascular Coupling. J. Neurosci. 2010;30:4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989;288:401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- Bouchard MB, Chen BR, Burgess SA, Hillman EMC. Ultra-fast multispectral optical imaging of cortical oxygenation and blood flow dynamics. Optics Express. 2009 doi: 10.1364/OE.17.015670. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of Blood Flow and Oxygenation Changes during Brain Activation: The Balloon Model. Magn Reson Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong X-K, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA Interneurons in Neurovascular Coupling: Relays for Subcortical Vasoactive Pathways. J. Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc. Natl. Acad. Sci. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver JP, Siegel AM, Franceschini MA, Mandeville JB, Boas DA. Evidence that cerebral blood volume can provide brain activation maps with better spatial resolution than deoxyhemoglobin. Neuroimage. 2005;27:947–959. doi: 10.1016/j.neuroimage.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Das A, Gilbert CD. Topography of Contextual Modulations Resulting from Short-range Interactions in Primary Visual Cortex. Nature. 1999;399:655–661. doi: 10.1038/21371. [DOI] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of Total Hemoglobin Concentration, Oxygenation, and Neural Activity in Rat Somatosensory Cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EMC, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative BOLD. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duling BR, Berne RM. Propagated Vasodilation in the Microcirculation of the Hamster Cheek Pouch. Circ Res. 1970;26:163–170. doi: 10.1161/01.res.26.2.163. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EMC. Optical Brain Imaging In-vivo: Techniques and Applications from Animal to Man. J Biomed Opt. 2007;12:051402. doi: 10.1117/1.2789693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD Signal Dependence on Cerebral Blood Flow and Oxygen Consumption: The Deoxyhemoglobin Dilution Model. Mag. Res. Med. 1999;42:849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hutchinson EB, Stefanovic B, Koretsky AP, Silva AC. Spatial Flow-Volume Dissociation of the Cerebral Microcirculatory Response to Mild Hypercapnia. Neuroimage. 2006;32:520–530. doi: 10.1016/j.neuroimage.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Yang G, Ebner TJ, Chen G. Local and Propagated Vascular Responses Evoked by Focal Synaptic Activity in Cerebellar Cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- Jones M, Hewson-Stoate N, Martindale J, Redgrave P, Mayhew J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. Neuroimage. 2004;22:956–965. doi: 10.1016/j.neuroimage.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B, Kuschinsky W, Schrock H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol Heart Circ Physiol. 1986;251:H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl. Acad. Sci. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl M, Lindauer U, Royl G, Kuhl M, Gold L, Villringer A, Dirnagl U. Physical model for the spectroscopic analysis of cortical intrinsic optical signals. Phys Med Biol. 2000;45:3749–3764. doi: 10.1088/0031-9155/45/12/317. [DOI] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions Between Electrical Activity and Cortical Microcirculation Revealed by Imaging Spectroscopy: Implications for Functional Brain Mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Mayhew JEW, Askew S, Zheng Y, Porrill J, Westby GWM, Redgrave P, Rector DM, Harper RM. Cerebral Vasomotion: A 0.1-Hz Oscillation in Reflected Light Imaging of Neural Activity. Neuroimage. 1996;4:183–193. doi: 10.1006/nimg.1996.0069. [DOI] [PubMed] [Google Scholar]
- McCaslin AFH, Chen BR, Radosevich AJ, Cauli B, Hillman EMC. In-vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: Implications for neurovascular coupling. Journal of Cerebral Blood Flow and Metabolism. 2010 doi: 10.1038/jcbfm.2010.204. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto M, Sheth S, Guiou M, Pouratian N, Chen JWY, Toga AW. Functional Signal- and Paradigm-Dependent Linear Relationships between Synaptic Activity and Hemodynamic Responses in Rat Somatosensory Cortex. J. Neurosci. 2004;24:3850–3861. doi: 10.1523/JNEUROSCI.4870-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. PNAS. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. A Paradigm Shift in Functional Brain Imaging. J. Neurosci. 2009;29:12729–12734. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. 1890;11:85–158. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou MW, Walker MA, Toga AW. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. J Cereb Blood Flow Metab. 2005 Mar;2:1–2. doi: 10.1038/sj.jcbfm.9600091. [DOI] [PubMed] [Google Scholar]
- Sirotin YB, Hillman EMC, Bordier C, Das A. Spatiotemporal precision and hemodynamic mechanism of optical point-spreads in alert primates. PNAS under review. 2009 doi: 10.1073/pnas.0905509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, Silva AC. Functional reactivity of cerebral capillaries. 2008;28:961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D'Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci U S A. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzetta I, Hildesheim R, Grinvald A. Compartment-resolved imaging of activity-dependent dynamics of cortical blood volume and oximetry. J Neurosci. 2005;25:2233–2244. doi: 10.1523/JNEUROSCI.3032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer A, Them A, Lindauer U, Einhaupl K, Dirnagl U. Capillary perfusion of the rat brain cortex. An in vivo confocal microscopy study. Circ Res. 1994;75:55–62. doi: 10.1161/01.res.75.1.55. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian G, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- White BR, Snyder AZ, Cohen AL, Petersen SE, Raichle ME, Schlaggar BL, Culver JP. Resting-state functional connectivity in the human brain revealed with diffuse optical tomography. Neuroimage. 2009;47:148–156. doi: 10.1016/j.neuroimage.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid Astrocyte Calcium Signals Correlate with Neuronal Activity and Onset of the Hemodynamic Response In Vivo. J. Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.