Abstract
In experimental models of central nervous system (CNS) aging, injury and disease, administering human umbilical cord blood (HUCB) cells induce recovery, most likely by interacting with multiple cellular processes. The aim of this study was to examine whether the HUCB cells produce trophic factors that may enhance survival and maturation of hippocampal neurons in an in vitro test system. We co-cultured the mononuclear fraction of HUCB cells with hippocampal neurons isolated from either young (7-months of age) or aging (21 month of age) rat brain for 14, 21, 28, 35 and 42 days in vitro (DIV), respectively. Immunocytochemistry was then employed to identify neurons (MAP2+) and glial cells (GFAP+) as well as arborization of neurites. The average number of MAP2+ hippocampal neurons cells in both young and aging neuronal-HUCB co-cultures was significantly higher than in the control cultures (hippocampal mono-cultures). These MAP2+ neurons in co-culture were richly arborized, especially in 21 and 28 DIV co-cultures, and expressed functional enzymes (Synaptophysin, tyrosine hydryoxlase (TH)), gamma amino butyric acid receptor (GABAAr) and glutamate transporter (EAAC1). The majority of hippocampal neurons in both co-culture systems grew very well and survived for up to 42 DIV with an increment of immature neurons which were positive for Nestin and TuJ1. Using a multiplex protein array, a number of secreted proteins that could have trophic effects on the neurons were identified.
Keywords: Cell survival, dendrites, proliferation, adhesion molecules, cytokine, growth factor
As we age, cognitive function tends to decline, although there is significant variability between individuals even across species [1]. These changes in cognitive function are accompanied by changes in the hippocampal milieu. For example, vascular changes within the cerebrovasculature include atherosclerosis of cerebral arteries and increased leakiness of the blood brain barrier (BBB) [2]; this contributes to the increased incidence of stroke and dementias within the aging population. At the level of the neurons, astrocytes, oligodendrocytes and microglia, there are structural, biochemical, metabolic and electrophysiological changes that lead to decreased function and make aging neural cells more susceptible to stressors and disease processes [3]. It is known that cholinergic innervation of the hippocampus and frontal cortex is crucial for maintenance of memory processing and learning in mammals [4–9]. There is a loss of cholinergic neurons with age in rodent models, which in some reports has been correlated with age-related declines in behavior and treatments such as nerve growth factor (NGF) which improve spatial learning tasks also lead to increased numbers of cholinergic neurons [4, 7]. This change in cholinergic neurons may not be the only important change, however, as selective lesions of cholinergic neurons in young animals do not mimic the loss of spatial learning abilities that is observed in aged rats [10]. GABAergic interneurons are also lost with age [11].
Recent research demonstrated that the hippocampus is one of the rare regions in which neurogenesis continues throughout adulthood. The role this neurogenesis plays in the normal function of the hippocampus is unclear, although there is evidence that neurogenesis in the subgranular zone (SGZ) of the dentate gyrus may be linked to learning and memory (see [12] for a recent review of this subject). Neurogenesis in this region decreases with age [13] and this appears to be a function of decreased proliferative capacity of the stem cells themselves [14]. Whether this is an intrinsic change within the stem cells themselves or induced by a change in the neurogenic environment is as yet unclear. Molofsky et al [15] showed that increasing expression of p16INK4a with age is linked to decreased proliferation of subventricular zone (SVZ) stem/progenitor cells. While this gene is not linked to decreased hippocampal neurogenesis in aging animals, a similar gene may control this function in the hippocampus.
In addition to the potential changes within the stem cells themselves, decreased hippocampal neurogenesis may be influenced by environmental factors. For example, environmental enrichment increases neurogenesis [16], as does physical activity [17] and learning and memory tasks. In contrast, stress decreases neurogenesis [18]. Several critical growth factors including basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF), decline dramatically in middle-aged brain [19]. Mice deficient in the FGF receptor 1 in the dentate gyrus have a greater than 80% reduction in cellular proliferation [20]. Similar declines were also observed for IGF-1 and VEGF [19]. IGF mRNA expression decreased in the aging animal [21, 22] while IGF-1 administration increased proliferation in the subventricular zone and subgranular zone and enhanced cognitive function [23]. Increasing VEGF expression in the hippocampus through gene transfer in adult rats, more than doubled neurogenesis [24]. Further, this increased neurogenesis was associated with improved performance in a spatial maze. In contrast to the decrease in growth factor in the hippocampus, pro-inflammatory cytokines may increase. One prominent cytokine, IL-1β, appears to decrease spatial learning [25].
An altered trophic environment may also have effects on the local neurons. For example, there are a number of studies in different species and different brain regions that report pruning of the dendritic tree, including both loss of dendrites and dendritic spines [26], reliably occurs with age (for a review see [27]). This would imply a loss of synapses, and a likely loss of receptors. In contrast, there are also studies that suggest there is dendritic hypertrophy [28], which may be a function of cellular remodeling in response to cell loss. Aging axons may also be impaired compared to young axons. Transport rate of both fast and slow axonal transport decreases with age [29] and there is a reduction in neurofilament protein[30, 31]. These changes in axonal transport and structure can lead to changes in the absolute or relative levels of neurotransmitters [32]. It is likely that by providing trophic support to these aging neurons, it would be possible to enhance proliferation and maintain dendritic and axonal connections. One such avenue is based on human umbilical cord blood (HUCB) cellular therapy.
HUCB cells have gotten considerable attention as an alternative cell source for cellular therapies. HUCB cells can successfully treat patients with life-threatening blood diseases [33, 34]. Experimental models of cardiovascular disease indicate that the cells may be effective at preserving cardiac function [35] and may enhance vasculogenesis [36]. HUCB treatment has also been examined in animal models of neurodegenerative diseases. The mononuclear fraction of HUCB contains populations of hematopoietic and non-hematopoietic stem-like cells [37] that can be partly reprogrammed in laboratory cultures to express neural antigens. We therefore expected that these cells would be useful in cell replacement therapies for neurodegenerative disorders and brain injury. In animal models of amyotrophic lateral sclerosis [38], San Filippo syndrome [39], stroke [40, 41], spinal cord injury [42] as well as in clinical studies where HUCB cells were administered to treat inborn errors of metabolism [43], the HUCB cells had therapeutic efficacy. However, the capacity of the HUCB cells to replace lost neurons was minimal. Even so, evidence suggests that the HUCB have other important mechanisms of action.
In order to effectively develop new therapeutic strategies for central nervous system (CNS) diseases, it is important to understand how these cells may be manipulated to support hippocampal function not only in the severe degenerative disorders, but also in normal aging brain. In this study, we will examine the ability of HUCB cells to enhance proliferation, increase survival and support neuritic outgrowth of hippocampal cells harvested from aging adult rat brain.
MATERIALS AND METHODS
Preparation and culture of HUCB cells
Cryopreserved HUCB cells (supplied by Saneron CCEL Therapeutics, Inc.) were thawed quickly at 37° C and then transferred into 10 ml of complete medium composed of Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco) with 20 % fetal bovine serum (FBS, Gibco) and 0.1 % Gentamicin (Sigma); the cells were centrifuged at 1000 rpm for 7 minutes, the pellet resuspended in fresh complete medium and the cells plated in 100 mm dishes (Nunc) at a density of 106 cells / cm2. The HUCB cells were incubated in 5 % CO2, 95 % O2 at 37°C for 10 or 45 DIV. Initial viability was determined by 0.4 % trypan blue (BioWhittaker) to be between 70 % and 80 %. Fresh complete medium was supplied on the second day and every 5 days thereafter.
Isolation and culture of adult rat hippocampal neurons
Adult and aging rat hippocampi were dissected and prepared according to a procedure originally described by Brewer [60]with some modification. Briefly, four 7 month old and four 21 month old male Fisher 344 rats (Harlan, Indianapolis) were anaesthetized with chloroform and decapitated by guillotine. Hippocampi were rapidly dissected from the fresh brain in 2 ml of Hibernate A (BrainBits) / B27 (Gibco, Invitrogen) / 0.5 mM L-Glutamine (Sigma Aldrich) at 4°C and the meninges and excess white matter removed. Hippocampi were cut perpendicularly into 0.5 mm thick slices which were then transferred to a tube of the same medium. After shaking for 8 minutes at 30°C, the slices were incubated with Hibernate A containing papain (2 mg/ml; Worthington) for 30 minutes in a 37°C water bath on a rotating platform (170 rpm). Slices were transferred to a 15 ml tube with Hibernate A/B27 and triturated with a fire-polished, siliconized 9 inch Pasteur pipette. The cell suspension was added to the top of a gradient of Optiprep (Axis-Shield) 1.15 gms /cm3 in a 15 ml centrifuge tube with four 1.0 ml steps of a gradient 35, 25, 20, and 15 % (v/v) Optiprep in Hibernate A/B27. After centrifuging at 1900 rpm for 15 minutes, four fractions were observed. The third fraction was enriched in neurons. The cells from fraction 3 were centrifuged at 1100 rpm for 1 minute and resuspended in Neurobasal A (Invitrogen)/B27 with 0.5 mM L-glutamine and 0.25 mM Glutamate (Gibco) with a supplement of human basic fibroblast growth factor (hbFGF, 10 ng / ml). The hippocampal neurons were then plated in poly-L-lysine (PLL, 1mg/ml) precoated 4-well or 12-well plates (Nunc) and incubated in 5% CO2, 95% O2 at 37°C. Fresh medium was added on the second day and every 5 days thereafter, although glutamate was excluded from the media at this stage.
Coculture of HUCB and hippocampal cells
For the coculture of HUCB with both young and aging hippocampal neurons, the HUCB cells were precultured for 10 DIV (viability: 95%). The nonadherent HUCB were harvested from this culture in the media; this population is rich in hematopoietic and non-hematopoietic cells [61]. The media and cells were centrifuged at 1000 rpm for 7 minutes and the pellet containing the non-adherent fraction of HUCB cells was resuspended in 1 ml of Neurobasal A/B27 with 0.5 mM L-Glutamine. The viability was determined by 0.4 % Trypan blue dye exclusion.
The hippocampal neurons were precultured for 1 DIV (viability: 50%) prior to co-culturing. To create the co-culture, the media was removed from the neuronal culture and replaced with resuspended HUCB cells at 105 cells/well with the same medium to which hbFGF (10 ng/ml) was added. All control mono-cultures also had hbFGF. The co-cultures of HUCB cells and hippocampal neurons were then incubated in 5% CO2, 95% O2 at 37°C for up to 28 days (young groups) or 42 days (aging groups).
Viability of co-cultured cells
Prior to fixation, the fluorescein diacetate (FDA, Molecular Probes [Invitrogen]) / propidium iodide (PI; Sigma Aldrich) live/dead viability assay was utilized to assess the viability of those cells in aging groups at 1, 7, 14, 21, 28, 35 and 42 DIV. The numbers of living (green) and dead (red) cells were counted in 10 randomly selected fields per well of the 4-well plate system per time point under an inverted fluorescent microscope. Cell viability was calculated as the number of living cells divided by the total number of cells multiplied by 100.
Immunocytochemistry
Cultures were washed with 0.1 M phosphate buffered saline (PBS) and then fixed with 4% paraformaldyhyde (Sigma-Aldrich) in 0.1 M PBS and immunocytochemistry performed for identification of cell-type specific antigens. After rinsing three times in 0.1 M PBS, the cultures were blocked with 10% goat serum and 0.1% Triton X-100 in PBS for 1 hour at room temperature (RT). They were then incubated with the primary antibody: polyclonal rabbit anti-microtubule-associated protein 2 (MAP2) antibody (1:1000, Chemicon), polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:500, Dako), chicken anti-GFAP polyclonal antibody (1:400, Chemicon [Millipore]), mouse anti GFAP antibody (1: 1000), mouse anti-human mitochondria (1:50, Chemicon, [Millipore]), neuronal class III β-Tubulin (TuJ1) polyclonal antibody (1:2000, Covance), mouse anti-Nestin antibody (1:100, BD Biosciences Pharmingen), mouse anti-synaptophysin monoclonal antibody (2μg/ml; Chemicon [Millipore]), mouse anti-Tyrosine hydroxylase (TH, 1:100; Chemicon [Millipore]), mouse anti-GABAA receptor (GABAAr), β-chain monoclonal antibody (20 μg/ml; Chemicon [Millipore]), goat anti-glutamate transporter (EAAC1) polyclonal antibody (1:4,000, Chemicon [Millipore]), rabbit polyclonal antibody to VEGF receptor 1(VEGFR1, 1: 100; Abcam), rabbit polyclonal antibody to VEGF receptor 2 (VEGFR2, 1:50; Abcam) at 4°C for 24 hours. The cultures were washed in 0.1 M PBS three times and then incubated with a species-appropriate secondary antibody (Alexa Fluor 594, 488; Molecular Probes, 1:1000 and 1:500, respectively) for 1 hour at RT. After the final washes in cold 0.1 M PBS, the cultures were coverslipped with 95% glycerol or VECTASHIELD HardSet Mounting Medium with DAPI (Vector). Cell nuclei were visualized by counterstaining with 4′, 6′-diamidino-2-phenylindole hydrochloride (DAPI). All culture slides were examined under the Olympus BX-60 or IX-71 microscopes.
Measures of Neuronal Health
Cultures were fixed with 4% PFA and immunostained for the neuron-specific marker MAP2. Fluorescent photomicrographs were taken using both a 20x and 40x objective. The Image-pro Plus 4.1 software (Media Cybernetics) was used to determine both the percentage of MAP2+ cells in culture and the length of the neurites. The number of MAP2+ and total cells respectively, were counted in 10 randomly selected fields per well. The percentage of MAP2+ cells was calculated as the number of MAP2+ cells / the total number of cells × 100 and presented as mean percentage ± standard error of the mean (SEM).
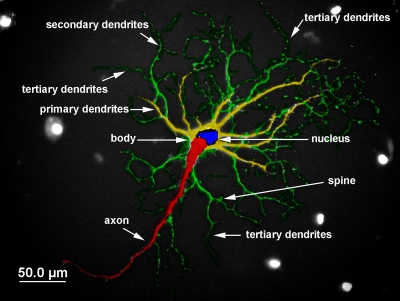
We also measured the mean process length and number of processes from 10 randomly chosen individual MAP2+ cells (Figure 1). The linear distance of primary dendritic shaft as it emerged from the cell body and secondary and tertiary branches were measured. All data (percentage of MAP2+ cells, arbor length and dendritic branch points) were analyzed with a two way analysis of variance (ANOVA) followed by Bonferroni post hoc tests. Significance was set at p < 0.05.
Fig. 1.
Neuronal schematic for counting dendritic branching of hippocampal neurons.
ELISA
To explore the mechanisms by which HUCB cells are able to support the development of adult hippocampal neurons, a series of enzyme-linked immunoadsorbant assays (ELISAs) was performed using the Pierce Endogen multiplex Searchlight system according to manufacturer’s specifications. Briefly, standards and samples (50 μl) were added to the custom array plates and incubated covered at room temperature (RT) for 1 hr on a rotating platform shaking at 200 rpm. The plates were then washed and 50 μl of Biotinylated Antibody Reagent was added to each well. The plates were incubated shaken at 200 rpm for 30 min at RT and then washed again. Streptavidin-HRP (50 μl) was added to the plate for 30 min at RT, shaking continuously (200 rpm). After washing, SUPERSIGNAL Substrate was added to each well and the luminescence read using the SearchLight Imaging System and the density of each spot in the array calculated using ARRAYVISION software. Protein concentrations of the samples were determined based on the standard curve for each protein. The following 21 target proteins were measured in the supernatant collected from the non-adherent HUCB fraction cultured for 45 DIV: (nerve growth factor (β-NGF), brain derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophin-3 (NT3), platelet derived growth factor-BB (PDGF-BB), vascular endothelial growth factor (VEGF), stromal cell-derived factor-1β (SDF-1ß), granulocyte colony-stimulating factor (G-CSF), granulocyte monocyte colony stimulating factor (GM-CSF), interleukin 1alpha (IL-1α), IL-3, IL-6, IL-7, IL-8, IL-11, interferon γ (IFNγ), tumor necrosis factor alpha (TNFα), E-selectin, L-selectin, intercellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1). Nonconditioned media (DMEM with 10% FBS) was used as a control.
RESULTS
To verify the effect of HUCB cells on both of young adult and aging hippocampal neurons, we co-cultured non-adherent HUCB cells and hippocampal neurons isolated from 7 and 21 month old Fisher 344 rat brain. Hippocampal cultures without the addition of HUCB cells served as controls. Long-term survival, proliferation, phenotypic maturation and dendritic arborization were examined over time. Since the isolated adult hippocampal neurons did not survive well in mono-culture without growth factor support, hbFGF (10 ng / ml) was added in all cultures.
1. The effect of HUCB cells on hippocampal cells derived from young adult rat brain
Prior to investigating the effect of HUCB cells on aging hippocampal neurons, we first examined whether HUCB cells influence the growth of hippocampal neurons harvested from young adult (7 mo of age) rats.
The effect of HUCB cells on the survival of young adult rat hippocampal cells
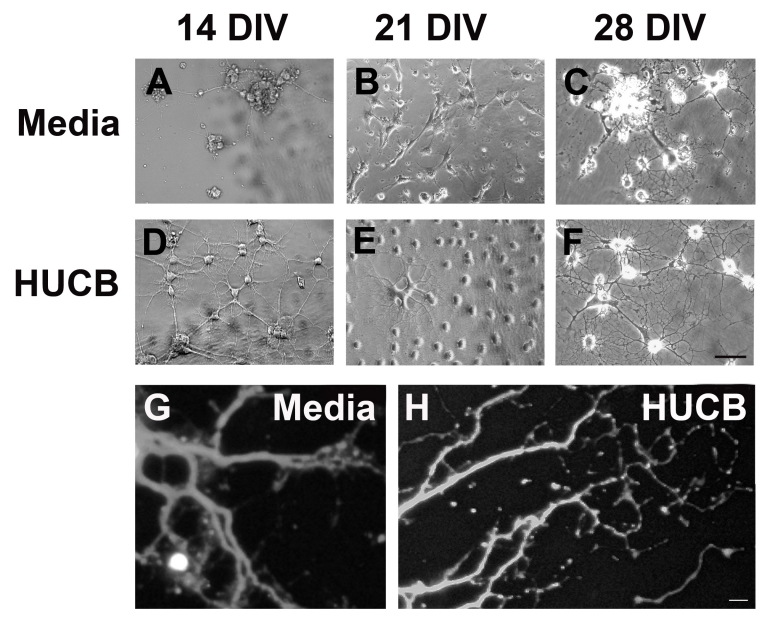
When young adult hippocampal neurons were isolated from healthy host brain and cultured in neural optimized medium without HUCB cells, neuronal survival was low and phenotypic maturation was slow, neurons having few short and poorly elaborated dendrites (Fig. 2, A–C). At 14 DIV, the neurons remained in small clumps (Fig 2A). By 21 DIV, they had begun to spread out across the culture dish and had some neuritic extensions (Fig. 2B) that were also evident at 28 DIV (Fig. 2C, 2G). However, in the hippocampal neuronal HUCB co-cultures, most of the young hippocampal neurons survived and by 14 DIV (Fig 2D) had formed complex networks of connecting neurites that continued to develop and elaborate out to 28DIV (Fig 2E, 2H). Most of the neurons in HUCB treated cultures were morphologically multipolar, reminiscent of sympathetic or parasympathetic neurons.
Figure 2.
Phase contrast photomicrographs illustrating the maturation of young adult hippocampal neurons cultured with or without HUCB cells. A) Cultures of hippocampal neurons in the non-treated, Media group had few surviving cells and sparse, short processes after 14 DIV. B) While neurons in the non-treated culture had spread across the culture dish at 21 DIV, the dendrites were still short and an extensive network of intercellular connections had not yet formed. C) Even after 28 DIV, hippocampal neurons grown in culture without HUCB, remained predominantly clustered together and had simple, short neuritic processes. D) In contrast, hippocampal neurons co-cultured with HUCB cells even for as little as 14 DIV exhibited normal, healthy neuronal morphology with multiple, long processes (dendritic arbors) which interconnected with each other and formed networks. E) After 21 DIV, the HUCB treated adult hippocampal neurons exhibited even more extensive dendritic arborization, similar in morphology to sympathetic neurons. F) After 28 DIV, the HUCB treated hippocampal neurons formed extremely complex tree-like dendritic structures. G) Many of the hippocampal cells expressed the mature neuronal marker, MAP2 regardless of whether they were treated with HUCB. Consistent with the phase contrast images in A–F, MAP2 immuno-labeling further demonstrated the development of an extensive dendritic tree over time. Fluorescent photomicrographs taken after 21 DIV of G) hippocampal neurons grown in Media alone and H) hippocampal neurons co-cultured with HUCB cells. Scale bar = 50 μm.
The role of HUCB cells on the phenotypic maturation of hippocampal neurons
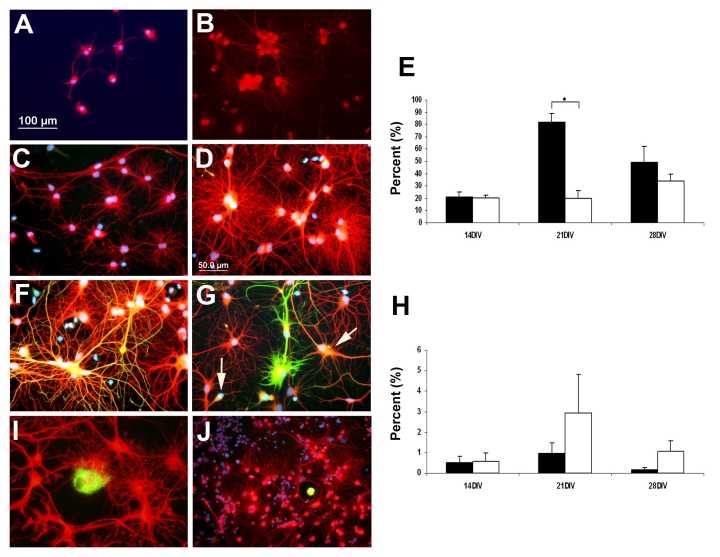
Many of the surviving hippocampal cells in both HUCB and Media treated conditions were positive for the mature neuronal marker MAP2 (Fig. 3, A–D), although the cells in the non-treated cultures (Fig 3A, 3B) were fewer and smaller with less neuritic outgrowth than in the HUCB-treated cultures (Fig 3C, 3D). When the percentage of MAP2 positive cells was determined, there was no significant difference between the HUCB and Media treated cultures after 14 DIV (Fig 3E); however, there were significantly more MAP2 positive (+) cells in the HUCB -treated cultures after 21 DIV (p < 0.05).
Fig. 3.
Neuronal and glial antigens were expressed in co-cultures of HUCB and neural cells from young adult hippocampal neurons. The majority of hippocampal neurons in both Media and the HUCB treated cultures were MAP2+ (red). A) At 14 DIV, MAP2+ cells in Media group survived in small numbers and grew slowly. The dendritic processes were mostly short and ill developed. B) At the same time point, the HUCB treated cultures had many MAP2+ cells growing in clusters with a network of neurites forming. C) At 28 DIV, the Media cultures had several small clusters of MAP2+ cells with small neuritic networks, D) In the HUCB treated cultures the bottom of the culture plates were covered with a massive network of neurites. E) Percentage of total cells that were MAP2+ over time in culture. F, G) Cultures grown for 28 DIV, had GFAP+ cells scattered throughout (green). Some of cells expressed both the neuronal MAP2 (red) and astrocytic GFAP (green) antigens and appeared orange (arrows, G). H) Percentage of total cells that were GFAP+ over time in culture. I, J) Although HUCB cells were plated at a relatively high density (105/cm2), only a small number of cells were observed expressing human mitochondria antigen (hMito, green), while most of cells were positive for neuronal TuJ1 (red). DAPI was used to visualize cell nuclei (blue). Scale bar = 100 μm in A, B, J; Scale bar = 50 μm in C, D, F, G, I. * p < 0.05
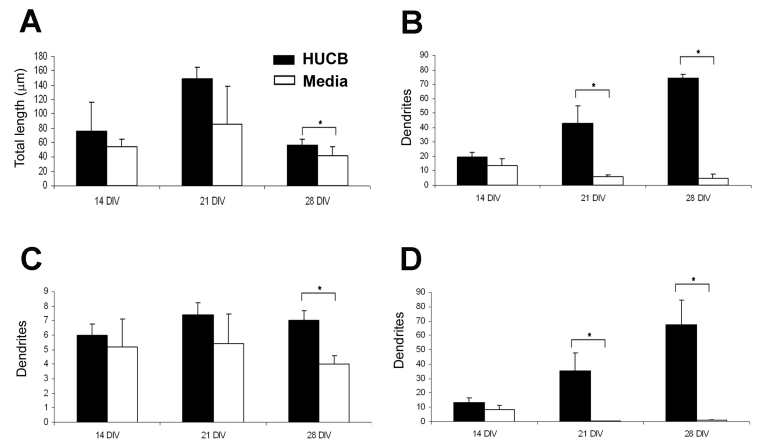
Not only did HUCB cells effectively increase survival of the adult hippocampal neurons in vitro, we found that the non-adherent HUCB cells also promoted extensive dendritic outgrowth (Fig. 3C, 3D) compared to the Media treated groups (Fig. 3A, 3B). Hippocampal neurons in the treated group were well-differentiated with long processes that interconnected to form networks, particularly as time in culture increased. The average dendrite length per neuron (including primary, secondary and tertiary dendritic processes) in the HUCB treated group tended to be longer than in the Media group (Fig. 4A) but only reached significance at 28 DIV (p < 0.05). There were significantly more dendrites per neuron at 21 DIV and 28 DIV (p < 0.05). Upon further examination, it appeared that this increased arborization was primarily a result of more secondary and tertiary arbors in the treated group (13.4 ± 2.97 on day 14, 35.4 ± 12.55 on day 21 and 67.4 ± 17.1 per cell (Fig. 4D). The difference between treated and non-treated groups reached significance on days 21 and 28 (p < 0.05, paired t-test). In contrast to treated group, there were fewer dendritic branches exhibited by the MAP2 positive neurons in the Media group and dendrites were shorter (Fig. 3A, 3B; Fig. 4).
Fig. 4.
HUCB cells enhance dendritic arborization of young adult hippocampal neurons. A) Average length of total dendritic length. B) Average total numbers of dendrites per neuron. C) Average number of primary dendrites per neuron. D) Average number of secondary and tertiary dendrites per neuron. * p < 0.05
The expression of glial antigen in HUCB treated and non-treated cultures
Some of cells in culture expressed the glial marker, GFAP (Fig. 3F, 3G; green) and expression increased with time in culture (Fig. 3H). Most of the GFAP+ cells appeared in the Media group, but there was no significant difference between HUCB treated and non-treated groups (Fig. 3H; green).
HUCB cells in co-cultures
We also identified the HUCB cells in our co-cultures using immunolabeling with a human-specific marker for human mitochondria (hMito) (Fig. 3I, 3J; hMito, green and TuJ1, red). The HUCB cells were added to the co-culture at a high density of 106 cells/cm2; even so, far fewer human mitochondrial positive cells were observed. Most of these were scattered individually throughout the co-cultures; only a few cells were observed in close association with the adult hippocampal neurons.
2. The effect of HUCB cells on aging hippocampal neurons
In the aging group, we pre-cultured aging hippocampal cells obtained from 21 month old Fisher 344 rats for 1 day after isolation and then co-cultured them with non-adherent HUCB cells for up to 42 days. These HUCB cells were pre-cultured prior to combining in co-culture for 7 days in regular serum-containing medium.
The effect of HUCB cells on the viability and proliferative capacity of aging hippocampal neurons
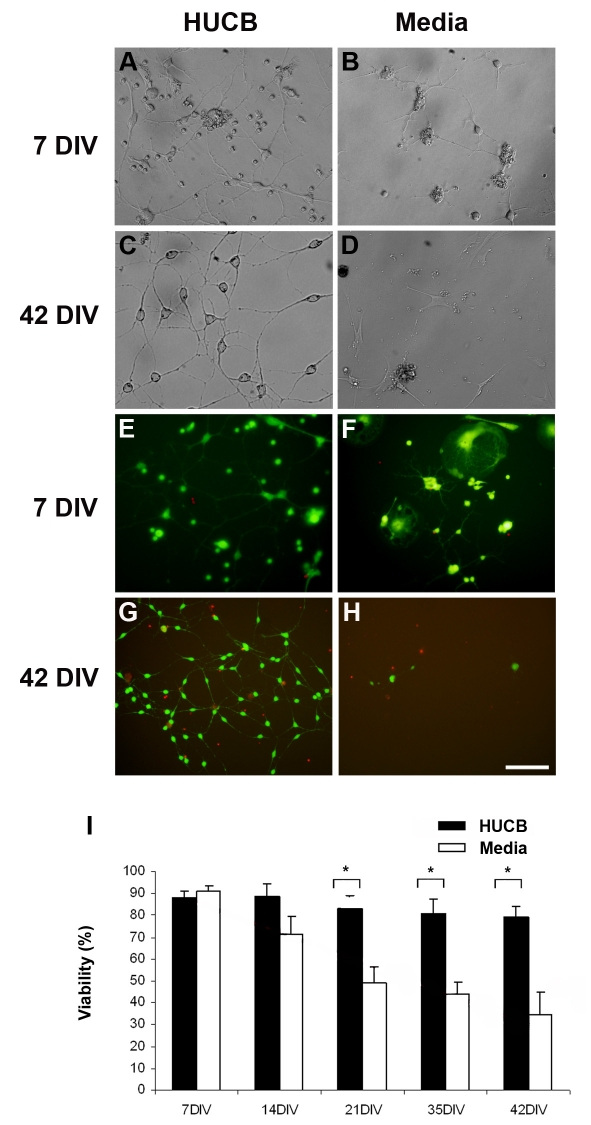
We examined the cellular morphologies of the aging hippocampal neurons at weekly intervals out to 42 DIV. At 7 DIV, the cells in the Media treated group remained in small clusters with little neurite outgrowth (Fig 5B). In contrast, the neurons in the HUCB treated cultures spread out more across the culture dish and formed a network of neuritic connections at 7 DIV (Fig 5A). By 42 DIV there were few surviving cells and neurites remained small in the Media group (Fig. 5D) while HUCB-treated cultures formed a well-connected network of cells (Fig. 5C).
Fig. 5.
Aging hippocampal cells survive better when co-cultured with HUCB cells. A) After 7 DIV, most of the aging hippocampal cells in the HUCB treated group survived and exhibited long neural processes. B) In the Media treated group, clusters of cells appeared smaller and fewer neurons had well-differentiated processes. C) After 42 DIV, the HUCB treated cultures had many healthy neurons with long interconnecting neuritis. D) In comparison, there were few surviving cells after 42 DIV in the Media treated group and those that did survive were small and round with few processes. When we examined cell viability using the FDA/PI assay, we observed many viable cells (green) and few dead cells (red) in both E) HUCB and F) Media treated cultures after 7DIV. G) At 42 DIV, there were many viable cells in the HUCB treated group, and few in the Media treated group (H). This impression was bourne out when the percentage of surviving cells was calculated over time (I). Scale bar = 50 μm in A–C, E and 100 μm in D, F–H. * p < 0.05
FDA/PI staining was also used to investigate the viability of aging hippocampal cells in HUCB and Media treated groups at the 7, 14, 21, 35 and 42 DIV. We found many FDA positive hippocampal cells in the HUCB treated cultures at 7 DIV (Fig. 5E, green)out to 42 DIV (Fig.5G). In comparison, by the end of 42 DIV, the Media group had few surviving cells and those present were not healthy-looking (Fig. 5H, green). The number of FDA+ and total number of cells per field were counted and percentage of surviving cells (viability) determined (Fig 5I). At 7 DIV, viability in the media and HUCB treated cultures was similar. Over the ensuing time in culture, survival in the Media treated group continually decreased. Cell survival in the HUCB treated cultures remained stable out to 42 DIV. It was not until 21 DIV that there were significant differences between HUCB and Media treated and differences remained significant at 35 and 42 DIV (p < 0.05).
The effect of HUCB cells on proliferation of aging hippocampal neurons
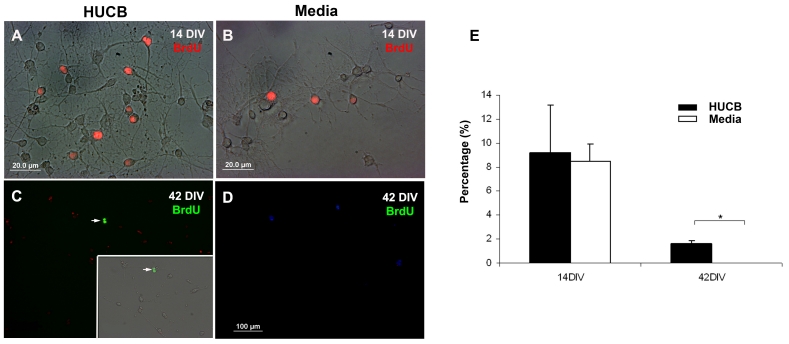
We also examined the proliferative capacity of aging hippocampal neurons exposed to HUCB cells by labeling cells with BrdU which is incorporated into S-phase of the cell cycle. There were BrdU+ cells in both the HUCB treated group (Fig. 6A, red; Fig. 6C, green, arrow) and the Media group (Fig. 6B, red; 6D, green). The difference between both groups was not significant in the early cultures but was significant by 42 DIV (Fig. 6E).
Fig. 6.
BrdU incorporation in aging hippocampal cells.Panel A. Immunofluorescent photomicrographs showing BrdU+ cells in both HUCB and Media treated cells. A) A combined phase-contrast, fluorescent image demonstrating that BrdU (red) was incorporated into the nucleus of cells in the HUCB treated group at 14 DIV and (B) also in the Media treated group. (C) Although BrdU was incorporated into fewer cells over time, BrdU labeled cells antibody (green) were still observed at 42 DIV in the HUCB treated group. (D) There were no BrdU+ cells in the Media group on 42 DIV. Scale bar = 20μm in (A) and (B), and 100 μm in (C) and (D).E) The percentage of BrdU+ cells in both HUCB and Media treated groups at 14 and 42 DIV. * p < 0.05
Expression of neural markers in both HUCB treated and non-treated groups
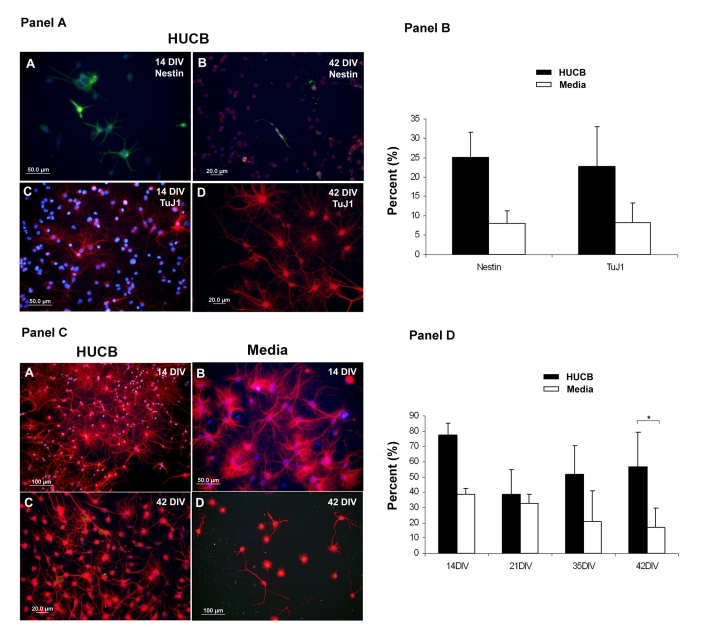
The observed increase in proliferation suggested that some of the surviving cells may be stem cells with the ability to generate new neurons. To explore whether HUCB cells promote neurogenesis of hippocampal cells, we labeled cultures with both nestin and the immature neuronal marker TuJ1. There was a tendency for more Nestin+ (Fig. 7A, 7B in Panel A, green) and TuJ1+ (Fig. 7C, 7D in Panel A, red) cells to be observed in the HUCB treated group but the difference between treated and non-treated groups did not gain statistical significance even on day 42 of culture (Fig. 7, Panel B).
Fig. 7.
The expression of neural antigens on aging hippocampal neurons. Panel A. Expression of the stem cell (Nestin) and immature neuronal (TuJ1) antigens. (A, B) Several Nestin+ cells (green) were detected in the HUCB treated hippocampal cells at A) 14 DIV and B) 42 DIV. Similarly, a number of TuJ1+ cells (red) were observed in the HUCB treated hippocampal cultures at 14 DIV (C) and 42 DIV (D). DAPI staining was used as a counterstain to visualize nuclei (blue). Scale bar = 50 μm in (A) and (C), and 20 μm in (B) and (D). Panel B. Percentage of total cells that were Nestin and TuJ1 positive after 42 DIV. Panel C. The mature neuronal marker MAP2 is expressed on aging hippocampal neurons in culture. (A, C) show that the majority of HUCB treated hippocampal neurons expressed MAP2 (red) and had extremely rich neurite outgrowth on days 14 (A) and 42 (C). (B, D) MAP2+ hippocampal neurons (red) in the non-treated group at 14 DIV (B) and 42 DIV (D). MAP2+ cells in this group were similar to those in the treated group in the early stage of culture (14 DIV). However, the number of MAP2+ cells dramatically declined and most of them lost their processes by 42 DIV. Scale bar = 100 μm in (A) and (D), 50 μm in (B) and 20 μm in (C). Panel D. Percentage of MAP2+ hippocampal cells in culture over time. * p < 0.05.
Similar to the cultures of young hippocampal cells, the majority of the aging hippocampal cells in both HUCB (Fig 7, Panel C; A, C) and Media (Fig 7, Panel C; B, D) treated cultures were positive for the mature neuronal marker MAP2 at early time points (day 14) but there were no significant differences between the groups (Fig. 7, Panel D). At later time points there were still many cells in the HUCB treated cultures, but the percentage of MAP2+ positive cells in Media group decreased over time. By 42 DIV, there were significantly fewer MAP2+ cells in the Media group compared to the treated group (p < 0.05). At this time, the HUCB treated cells still had extensive arborization while the non-treated cultures did not.
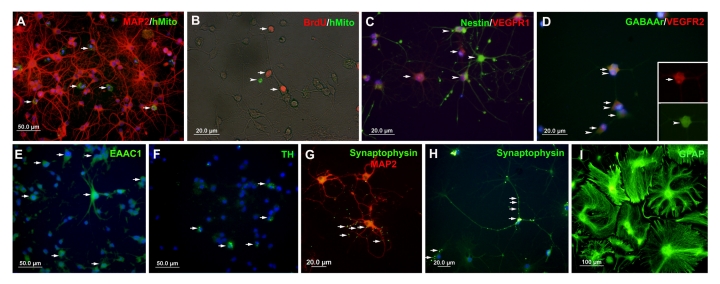
We also examined the expression of other neuronal proteins expressed by the hippocampal cells. We detected synaptophysin (Fig. 8, G and H; green, arrows), GABAAr (Fig. 8, D; green), EAAC1 (Fig. 8, E; green, arrows) and TH (Fig. 8, F; green, arrows), in the HUCB treated groups after both 14 and 21 DIV. While we found each of these proteins in the Media group at 14 DIV, we found only a few synaptophysin positive cells at 21 DIV and did not observe GABAAr, EAAC1, or TH at this time (Table 1). We also investigated the expression of VEGF receptor 1 (VEGFR1) and 2 (VEGFR2) in both HUCB treated and non-treated groups. We found that the majority of hippocampal cells (around 90%), especially in early culture, were positive for VEGFR1 (Fig. 8, C; red, arrowhead) and 2 (Fig. 8, D; red, arrows). Interestingly, these positive cells did not co-express Nestin (Fig. 8, C; green, arrowheads), but we found that some VEGFR2 cells (Fig. 8, D, red, arrowheads) co-expressed GABAAr (Fig. 8, D, green, arrows).
Fig. 8.
Expression of neuron related antigens and human antigen in the co-culture of aging hippocampal neurons and HUCB cells after 14 DIV.A) Numerous human mitochondria positive cells (green, arrows) were scattered and around MAP2+ aging hippocampal neurons. B) The human mitochondria positive cells (green, arrowhead) did not co-express the proliferative marker BrdU (red, arrows). C) Most of the aging hippocampal cells were positive for VEGFR1 antigen (red, arrow) but there was no co-expression of VEGFR1 with the immature marker, Nestin (green). D) Similar to VEGFR1, a large number of aging hippocampal cells expressed antigen for VEGFR2 (red, arrows) and some of these VEGFR2+ cells also expressed GABAAr (green, arrowheads). E) Numerous cells were detected that expressed antigen to EAAC1 (green, arrows) and a few cells were observed expressing TH (F; green, arrows) and Synaptophysin (G and H; green, arrows). I) Many GFAP+ cells (green) were found in aging hippocampal cultures. Scale bar = 50 μm in (A, E, F), 20 μm in (B–D, G, H), and 100 μm in (I).
Table 1.
Relative Expression of Neural Antigens by Cultured Hippocampal Neurons
| 14 DIV | 21 DIV | |||
|---|---|---|---|---|
| Media | HUCB | Media | HUCB | |
| Synaptophysin | + | + | +/− | + |
| TH | + | + | − | + |
| GABAAr | + | + | − | +/− |
| EAAC1 | + | + | − | + |
| VEGFR1 | + | + | +/− | + |
| VEGFR2 | + | + | +/− | + |
HUCB cells in aging hippocampal cultures
We double labeled aging hippocampal cultures with antibody to human mitochondria and MAP2, to examine the relationship of the HUCB and hippocampal cells in the co-culture system. Human mitochondria labeled HUCB cells (Fig. 8A; green, arrows) were observed distributed throughout the cultures and were surrounded by MAP2+ hippocampal neurons (Fig. 8A; red). These HUCB cells rarely co-expressed MAP2 (Fig. 8A) or the proliferative marker, BrdU (Fig. 8B; red, arrows).
3. Factor production by HUCB cells cultured for 45 DIV
To examine whether the HUCB cells produced any factors that may act to support survival and growth of neuronal cultures, we used a multiplexed ELISA to examine factor production when these cells were grown in mono-culture. The long-term cultured non-adherent fraction from the HUCB cells produced relatively more V-CAM-1, I-CAM-1, L-selectin, E-selectin, IL-6, G-CSF, GM-CSF, SDF-1b and VEGF in contrast to a small amount of expression of the neurotrophins CTNF and BDNF (Table 2).
Table 2.
Factors Secreted by HUCB cells Grown for 45 DIV
| Factor | Concentration in Culture Media (pg/ml) | |
|---|---|---|
| Media | HUCB | |
| β-NGF | 0 | 17.8 |
| BDNF | 0 | 23.4 |
| NT3 | 1.4* | 37.1 |
| CNTF | 0 | 31.9 |
| PDGF-BB | 3.6* | 67 |
| VEGF | 0.6* | 136.1 |
| SDF-1b | 0 | 193.8 |
| G-CSF | 2.1* | 36.5 |
| GM-CSF | 0.7* | 31.2 |
| IL-1a | 4.4 | 9.7 |
| IL-3 | 0.7 | 25.7 |
| IL-6 | 1.6 | 127.5 |
| IL-7 | 0.5* | 4.4* |
| IL-8 | 303.5 | 808.9 |
| IL-11 | 6.9 | 29.9 |
| IFNγ | 1.2 | 8.3 |
| TNFα | 0.8 | 25.2 |
| E-Selectin | 142.9 | 373.9 |
| L-Selectin | 0 | 779.7 |
| ICAM | 0 | 2908.8 |
| VCAM | 20.5 | 190.4 |
Below the level of sensitivity for the assay
DISCUSSION
In the present study we examined the ability of non-adherent HUCB cells to extend life-span and maintain the morphology (outgrowth and enriched arborization of the dendritic tree) of adult hippocampal neurons harvested from both young and aged rat brain. We showed that HUCB cells were able to protect hippocampal neurons harvested from young adult and aging rat brain grown in vitro and promote the growth of dendrites. Further, HUCB cells induced proliferation in a population of hippocampal neurons. These protective effects may be a function of the production of growth factors and cytokines produced by the HUCB cells.
HUCB cells promote survival and proliferation of adult hippocampal neurons
In this study, HUCB cells enhanced survival and neurite outgrowth of adult hippocampal neurons grown in vitro. It was possible to maintain neurons obtained from 21 month old rats in culture for more than 42 days when the neurons were co-cultured with HUCB cells. These cells maintained an extensive dendritic tree and formed a network of dendrites interconnecting neurons. In the absence of HUCB cells, few of these aging cells survived and these had fewer dendrites even though bFGF was added to all cultures.
The difference between HUCB treated and non-treated cultures was more dramatic in the aging cultures than in those from the younger adult brain. There were other differences between cultures of hippocampal cells from the young and aged adult brain. For example, there was a tendency for the percentage of MAP2+ young cells to increase with DIV with exposure to HUCB cells (Fig 3). In contrast, the percentage of aging cells initially decreased and then was fairly stable (Fig 7). Survival of untreated cells was similar in the young and old neurons. In addition, there appeared to be more GFAP+ cells in the aging culture (Fig. 8) than in the young culture (Fig 3). Glial cells produce numerous nutrient factors that support the survival and development of neurons [19]. The mechanism by which the HUCB cells extend the life of the hippocampal cells may be either by 1) enhancing the proliferative capacity of hippocampal cells or 2) protecting existing and newly generated hippocampal neurons from damage and death.
New neurons are generated in the subgranular zone of the dentate gyrus throughout life. Most of these newly generated cells will not survive [44], but those neurons that do survive, do so for extended lengths of time (8 months in rat [45] and 2 years in human [13]). When we examined the proliferative capacity of the aging hippocampal neurons using BrdU incorporation, approximately 10% of the cells incorporated BrdU regardless whether they were cultured with HUCB cells or not. By 42 DIV, however, BrdU+ cells were only observed in the HUCB treated cultures. The presence of BrdU+ cells in these cultures, however, does not necessarily mean that a stem cell population survived in these cultures. BrdU may be incorporated into cells undergoing DNA repair [46, 47] or mature neurons or astrocytes may have dedifferentiated into a progenitor-like cells [48]. Since we did not characterize the freshly isolated cells for BrdU incorporation or expression of stem cell markers such as nestin or SOX-2, we cannot fully address this issue. However, after 42 DIV, both nestin and an early neuronal marker, TuJ1 were present, suggesting the presence of a stem cell. Both antigens were expressed by the aging hippocampal cells and while there was no significant difference in expression in the HUCB-treated and untreated cultures, there tended to be more nestin and TuJ labeling in the HUCB treated cultures.
The HUCB cells promote the maturation and dendritic arborization of adult hippocampal neurons
In the course of maturation, neurons develop a distinct, extensive dendritic tree that is shaped by the cells location and function. Our data revealed that HUCB treated groups contained more mature neurons which expressed the mature neuronal marker MAP2, especially in longer term (21 DIV) cultures from both young adult and aging rat brain. In addition to expressing mature microtubule proteins these cells also expressed neurotransmitter (TH), GABA and VEGF receptors and glutamate transporter so the cells were likely receptive to local chemical signals. Further, they also expressed synaptophysin, suggesting that synapses may have been forming between these cultured cells. The expression of these neural markers is consistent with the observed development of the rich dendritic arbors in HUCB treated cultures. There was extensive arborization principally at the level of secondary and tertiary dendritic branches, where numerous spines are likely to be found. However, we did not characterize these MAP2+ cells further to determine if they were CA1 or CA3 pyramidal cells or dentate granule cells. This remains to be performed in future studies.
The influence of the factors produced by HUCB cells on adult hippocampal neurons
Thus far we have shown that HUCB cells benefit adult/aging hippocampal neurons by increasing their survival, growth, differentiation, maturation, and arborization. It is likely that HUCB cells induce these effects through the release of growth or trophic factors. We have previously shown that HUCB mononuclear cells secrete the cytokines (IL-6 and IL-10], chemokines (IL-8 and MCP-1), and VEGF [49]. Other research groups have determined that HUCB cells secrete angiopoietin, hepatocyte growth factor, VEGF, epidermal growth factor and PDGF [50]. Neurotrophin mRNA (NGF, glial derived neurotrophic factor (GDNF), BDNF, NT 3 and NT 5) has also been found in HUCB cells [51]. We therefore investigated whether the HUCB cells produce and secrete factors that could play a critical role in the development of hippocampal cells. In agreement with our previous study, HUCB cells released VEGF, SDF-1b, IL-6 and IL-8 as determined with a multiplex ELISA. VEGF has been found to decrease in the hippocampus with age [19]. Similarly, there is a decrease in VEGFR1 in the hippocampus in the adult compared to the neonate, although expression of VEGFR2 is highest in the adult brain [52]. The concentration of SDF-1b in the media of the HUCB culture was higher than any of the other growth factors. Expression of SDF-1 and its receptor is depleted in Tg2576 mouse model of AD [53]. Further, treating young nontransgenic mice with an antagonist resulted in impaired learning and memory. Among the cytokine/chemokines, IL-8 expression was significantly elevated. The IL-8 CXCR-2 receptor is most highly expressed on hippocampal neurons [54]. Indeed, IL-8 has been shown to significantly increase survival of hippocampal neurons, especially in long term cultures [55]. In addition, multiple neurotrophins were secreted, but at much lower concentrations.
The factors secreted in the greatest amount from our HUCB cells were ICAM-1, E and L – selectins, and IL-8. The high concentration of adhesion molecules may have been a contributing factor to the extensive neurite outgrowth observed in the co-cultures. ICAM-5 is a major contributor to dendritic outgrowth and synpatogenesis [56]. A consistent observation in the aging brain and hippocampus is the background upregulation of pro-inflammatory cytokines such as TNFα and IL-1β which induce ICAM-1 expression [57]. Recently it was reported that when ICAM-5 is shed from hippocampal neurons there is a decrease in T cell infiltration into the hippocampus, a decrease in TNFα and a stimulation of TGF-β and IFNγ producing an anti-inflammatory environment [58]. While our cell culture preparation cannot address this issue directly, the observed increase in adhesion molecule expression could explain the decrease in microglial activation observed by Bachstetter et al in the dentate gyrus [59].
HUCB cells in adult hippocampal co-cultures
The HUCB cell mononuclear fraction contains a population of stem / progenitor cells that can be expanded in the culture and express multiple phenotypes including neural phenotypes. In our study, however, we found only a small number of HUCB cells in the co-culture system that developed into neural-like cells and integrated into the primary hippocampal cell cultures. Actually, the number of HUCB cells decreased with time. The HUCB cells in our co-culture system were less able to proliferate as evidenced by lack of BrdU double labeling and poor expression of neural markers. One reason for that may be that our culture system was optimized to support neural cells and was not suitable to induce proliferation and expansion HUCB stem / progenitor cells.
Effect of HUCB cells on aging rat brain and their functions
In our studies with aging brain cells, most of the hippocampal neurons in the HUCB treated group grew very well over time with normal morphologies and rich dendritic arbors. Compared to the non-treated Media group, the viability (percentage of FDA+ cells) and proliferative capacity (percentage of BrdU+ cells) of these treated hippocampal cells was higher. In the early culture period (14 days in culture), both groups of hippocampal neurons (HUCB and Media treated) were found to be positive for GABAAr, a marker for GABAergic synapses; Synapophysin, a marker for synapses; TH, a marker for dopaminergic neurons and EAAC1, a marker for glutamate neurons. However, in the late cultures (day 21], these antigens were mostly found in the HUCB treated group, and rarely detected in the non-treated group (Table 2). The HUCB cells appeared to protect neuronal functions while they effectively stabilized the cytoarchitecture of neurons in hippocampus [39]. Noticeably, in the late aging culture, we found a large number of cells in the HUCB treated group while most of the cells died in the non-treated group. We observed both GFAP+ and MAP2+ cells, although the longer we cultured the cells, the more GFAP+ cells there were. There were also many cells in these cultures that were neither GFAP+ nor MAP2+; they were also negative for CD11b (microglia) and human mitochondria (HUCB). These cells may have been oligodendrocytes or endothelial cells.
Acknowledgements and Disclosures
We would like to thank Ms. Amy Simmens and Mr. Siddharth Kamath for their technical assistance in the completion of this study. This study was supported in part by a grant from the National Institutes of Health (R01AG020927) to AEW. Human umbilical cord blood cells were provided by Saneron CCEL Therapeutics, Inc. SGD, CDS, PRS, and AEW are consultants to Saneron CCEL Therapeutics. SGD, AEW, CDS and PRS are inventors on cord-blood related patents. PRS is co-founder of Saneron CCEL Therapeutics, Inc. CDS is Vice President for Research at Saneron CCEL Therapeutics.
Reference
- [1].Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- [2].Kalaria RN. Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther. 1996;72(3):193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- [3].Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann NY Acad Sci. 1999;893:154–75. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- [4].Backman C, Rose GM, Hoffer BJ, Henry MA, Bartus RT, Friden P, Granholm AC. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. J Neurosci. 1996;16(17):5437–42. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baxter DA, Canavier CC, Clark JW, Jr, Byrne JH. Computational model of the serotonergic modulation of sensory neurons in aplysia. J Neurophysiol. 1999;82(6):2914–35. doi: 10.1152/jn.1999.82.6.2914. [DOI] [PubMed] [Google Scholar]
- [6].Gallagher M, Colombo PJ. Ageing: The cholinergic hypothesis of cognitive decline. Curr Opin Neurobiol. 1995;5(2):161–8. doi: 10.1016/0959-4388(95)80022-0. [DOI] [PubMed] [Google Scholar]
- [7].Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci. 1994;14(8):4815–24. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ridley RM, Barefoot HC, Maclean CJ, Pugh P, Baker HF. Different effects on learning ability after injection of the cholinergic immunotoxin me20.4igg-saporin into the diagonal band of broca, basal nucleus of meynert, or both in monkeys. Behav Neurosci. 1999;113(2):303–15. doi: 10.1037//0735-7044.113.2.303. [DOI] [PubMed] [Google Scholar]
- [9].Wrenn CC, Lappi DA, Wiley RG. Threshold relationship between lesion extent of the cholinergic basal forebrain in the rat and working memory impairment in the radial maze. Brain Res. 1999;847(2):284–98. doi: 10.1016/s0006-8993(99)02099-5. [DOI] [PubMed] [Google Scholar]
- [10].Chappell J, McMahan R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacology. 1998;37(4–5):481–7. doi: 10.1016/s0028-3908(98)00032-x. [DOI] [PubMed] [Google Scholar]
- [11].Shetty AK, Turner DA. Hippocampal interneurons expressing glutamic acid decarboxylase and calcium-binding proteins decrease with aging in Fischer 344 rats. J Comp Neurol. 1998;394:252–69. [PubMed] [Google Scholar]
- [12].Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- [13].Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [14].Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16ink4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–52. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [17].van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia. 2005;51(3):173–86. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- [20].Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, McKnight SL. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. PNAS. 2005;102(39):14052–7. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lai M, Hibberd CJ, Gluckman PD, Seckl JR. Reduced expression of insulin-like growth factor 1 messenger RNA in the hippocampus of aged rats. Neurosci Lett. 2000;288(1):66–70. doi: 10.1016/s0304-3940(00)01170-8. [DOI] [PubMed] [Google Scholar]
- [22].Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neurosci. 1999;88(1):269–79. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- [23].Anderson MF, Åberg MAI, Nilsson M, Eriksson PS. Insulin-like growth factor-1 and neurogenesis in the adult mammalian brain. Dev Brain Res. 2002;134(1–2):115–22. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- [24].Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- [25].Shaw KN, Commins S, O’Mara SM. Lipopolysaccharide causes deficits in spatial learning in the water maze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- [26].Nunzi MG, Milan F, Guidolin D, Toffano G. Dendritic spine loss in hippocampus of aged rats. Effect of brain phosphatidylserine administration Neurobiol Aging. 1987;8(6):501–10. doi: 10.1016/0197-4580(87)90124-2. [DOI] [PubMed] [Google Scholar]
- [27].Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987;8(6):521–45. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- [28].Mervis RF, Pope D, Lewis R, Dvorak RM, Williams LR. Exogenous nerve growth factor reverses age-related structural changes in neocortical neurons in the aging rat. A quantitative Golgi study. Ann NY Acad Sci. 1991;640:95–101. doi: 10.1111/j.1749-6632.1991.tb00198.x. [DOI] [PubMed] [Google Scholar]
- [29].Tashiro T, Komiya Y. Maturation and aging of the axonal cytoskeleton: Biochemical analysis of transported tubulin. J Neurosci Res. 1991;30:192–200. doi: 10.1002/jnr.490300120. [DOI] [PubMed] [Google Scholar]
- [30].Parhad IM, Scott JN, Cellars LA, Bains JS, Krekoski CA, Clark AW. Axonal atrophy in aging is associated with a decline in neurofilament gene expression. J Neurosci Res. 1995;41(3):355–66. doi: 10.1002/jnr.490410308. [DOI] [PubMed] [Google Scholar]
- [31].Van der Zee EA, Naber PA, Disterhoft JF. Age-dependent changes in the immunoreactivity for neurofilaments in rabbit hippocampus. Neurosci. 1997;79(1):103–16. doi: 10.1016/s0306-4522(96)00634-3. [DOI] [PubMed] [Google Scholar]
- [32].Timiras PS, Hudson DB, Segall PE. Lifetime brain serotonin: Regional effects of age and precursor availability. Neurobiol Aging. 1984;5(3):235–42. doi: 10.1016/0197-4580(84)90068-x. [DOI] [PubMed] [Google Scholar]
- [33].Gluckman E, Rocha V. History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy. 2005;7(3):219–27. doi: 10.1080/14653240510027136. [DOI] [PubMed] [Google Scholar]
- [34].Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C. Outcome of cord-blood transplantation from related and unrelated donors. N Eng J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- [35].Yerebakan C, Sandica E, Prietz S, Klopsch C, Ugurlucan M, Kaminski A, Abdija S, Lorenzen B, Boltze J, Nitzsche B, Egger D, Barten M, Furlani D, Ma N, Vollmar B, Liebold A, Steinhoff G. Autologous umbilical cord blood mononuclear cell transplantation preserves right ventricular function in a novel model of chronic right ventricular volume overload. Cell Transplantation. 2009;18(8):855–68. doi: 10.3727/096368909X471170. [DOI] [PubMed] [Google Scholar]
- [36].Das H, Abdulhameed N, Joseph M, Sakthivel R, Mao H-Q, Pompili VJ. Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transplant. 2009;18(3):305–18. doi: 10.3727/096368909788534870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen N, Hudsion JE, Walczak P, Misiuta I, Garbuzova-Davis S, Jiang L, Sanchez-Ramos J, Sanberg PR, Zigova T, Willing AE. Human umbilical cord blood progenitors: The potential of these hematopoietic cells to become neural. Stem Cells. 2005;23:1560–70. doi: 10.1634/stemcells.2004-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: Distribution, migration, and differentiation. Journal of Hematotherapy & Stem Cell Research. 2003;12(3):255–70. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- [39].Garbuzova-Davis S, Willing AE, Desjarlais T, Davis Sanberg C, Sanberg PR. Transplantation of human umbilical cord blood cells benefits an animal model of sanfilippo Syndrome type B. Stem Cells Dev. 2005;14(4):384–94. doi: 10.1089/scd.2005.14.384. [DOI] [PubMed] [Google Scholar]
- [40].Newcomb JD, Ajmo CT, Davis Sanberg C, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006;15(3):213–23. doi: 10.3727/000000006783982043. [DOI] [PubMed] [Google Scholar]
- [41].Vendrame M, Cassady CJ, Newcomb J, Butler T, Pennypacker KR, Zigova T, Davis Sanberg C, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–5. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- [42].Saporta S, Kim JJ, Willing AE, Fu ES, Davis CD, Sanberg PR. Human umbilical cord blood stem cells infusion in spinal cord injury: Engraftment and beneficial influence on behavior. J Hemato Stem Cell Res. 2003;12(3):271–8. doi: 10.1089/152581603322023007. [DOI] [PubMed] [Google Scholar]
- [43].Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile krabbe's disease. N Eng J Med. 2005;352(20):2069–81. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- [44].Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- [45].Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- [46].Bauer S, Patterson PH. The cell cycle–apoptosis connection revisited in the adult brain. J Cell Biol. 2005;17(4):641–50. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53(1):198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [48].Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and ink4a/arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–77. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- [49].Newman MB, Willing AE, Manresa JJ, Davis Sanberg C, Sanberg PR. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: Implications for brain repair. Exp Neurol. 2006;199:201–8. doi: 10.1016/j.expneurol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [50].Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, Meier C. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood–derived mononuclear cells in vitro. Exp Hematol. 2007;35:1119–31. doi: 10.1016/j.exphem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- [51].Fan C-G, Zhang Q-J, Tang F-W, Han Z-B, Wang G-S, Han Z-C. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett. 2005;380:322–5. doi: 10.1016/j.neulet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- [52].Yang S-Z, Zhang L-M, Huang Y-L, Sun F-Y. Distribution of flk-1 and flt-1receptors in neonatal and adult rat brains. Anat Rec. 2003;274A:851–6. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- [53].Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28(2):143–53. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Horuk R, Marfin AW, Wang Z-X, Schweitzer L, Gerassimides A, Guo H, Lu Z-H, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–90. [PubMed] [Google Scholar]
- [55].Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: Potential involvement of glial-derived factors. Brain Res. 1993;600(1):49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- [56].Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K, Yoshihara Y. Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J Neurosci. 2007;27(33):8866–76. doi: 10.1523/JNEUROSCI.1047-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1b expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood– brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27(35):9301–9. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tian L, Lappalainen J, Autero M, Hanninen S, Rauvala H, Gahmberg CG. Shedded neuronal ICAM-5 suppresses T-cell activation. Blood. 2008;111:3615–25. doi: 10.1182/blood-2007-09-111179. [DOI] [PubMed] [Google Scholar]
- [59].Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Meth. 1997;71(2):143–55. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- [61].Chen N, Kamath S, Newcomb J, Hudson J, Garbuzova-Davis S, Bickford P, Davis-Sanberg C, Sanberg P, Zigova T, Willing A. Trophic factor induction of human umbilical cord blood cells in vitro and in vivo. J Neur Eng. 2007;4:130–45. doi: 10.1088/1741-2560/4/2/013. [DOI] [PMC free article] [PubMed] [Google Scholar]