Abstract
Purpose
Adiponectin is a promising biomarker linking obesity and disease risk; however, limited data are available regarding adiponectin in black women among whom obesity is highly prevalent.
Methods
A cross-sectional analysis was conducted to assess racial differences and correlates of serum adiponectin measured in 996 black and 996 white women enrolled in the Southern Community Cohort Study through Community Health Centers in twelve southeastern states from 2002–2006.
Results
Blacks had significantly lower adiponectin levels than whites (median 10.9 versus 14.9 ug/ml, Wilcoxon p<0.0001). Among blacks, adiponectin was lower among overweight and obese women compared to healthy weight women but showed no clear decreasing trend with increasing severity of obesity; adjusted geometric means (95% confidence interval) were 15.0 (13.8–16.4), 11.5 (10.6–12.5), 9.7 (9.0–10.6), 11.4 (10.3–12.6), and 10.9 (9.5–12.6) ug/ml for body mass index [BMI] categories of 18.5–24.9, 25–29.9, 30–34.9, 35–39.9, and 40–45 (p for trend<0.0001). In contrast, among whites there was a monotonic reduction in adiponectin over increasing BMI (adjusted geometric means = 19.9 (18.3–21.7), 15.1 (13.9–16.4), 14.3 (13.2–15.5), 12.5 (11.2–13.9), and 11.0 (9.7–12.5) ug/ml, p for trend<0.0001). BMI, age, HDL-cholesterol, and hypertension were important correlates of adiponectin in both groups.
Conclusions
Among women, racial differences exist in both the magnitude and form of the adiponectin-BMI association.
Keywords: Adiponectin, obesity, African Americans
INTRODUCTION
Adiponectin is a protein produced exclusively in adipose tissue that appears to play a critical role in mediating physiological effects such as insulin sensitivity, inflammatory response, and cell proliferation. Adiponectin levels are inversely associated with obesity and are thought to decrease in individuals with increased adiposity through down-regulation of adiponectin receptors (1). Adiponectin may also be affected by diet, physical activity, comorbidities, and other environmental factors as well as variation in genes encoding adiponectin or its receptors (1). Because adiponectin is inversely associated with obesity phenotypes as well as several obesity-related diseases (2), there is speculation that adiponectin activity may be a useful target in the prevention of cardiovascular disease, cancer, and type 2 diabetes (1, 2). With the highest prevalence of obesity found among non-Hispanic black women (39%) and the lowest among non-Hispanic white women (22%) (3), differences in adiponectin levels across race groups may contribute to disparities in obesity-related diseases between black and white women.
Identifying correlates of adiponectin and ascertaining whether they vary by race is likely to enhance the development of effective adiponectin-related chemopreventive strategies. Thus, the goal of this analysis was to examine associations between adiponectin levels, body mass index (BMI), and other potential correlates and to assess whether associations varied by race.
METHODS AND PROCEDURES
Institutional Review Boards at Vanderbilt University, Meharry Medical College, and the University of North Carolina at Chapel Hill approved this study.
Study population
The Southern Community Cohort Study (SCCS) is a prospective epidemiologic cohort study designed to examine racial disparities in cancer incidence and mortality (4). Study enrollment began in 2002 in 12 southeastern states at Community Health Centers (CHC) which are government-funded facilities providing health services primarily to low-income individuals in medically underserved areas (5). Potential participants included anyone entering the CHC such as patients, persons accompanying patients, and individuals utilizing CHC pharmacy or dental services. Individuals were eligible if they had not been under treatment for cancer in the past year. A personal or family history of cancer or elevated risk factors for cancer was not used to determine eligibility. Additionally, as described previously (4), participants were required to be age 40–79 years and English-speaking. From over 47,000 SCCS participants enrolled through early 2006, a sub-sample of 2,000 women who provided a blood sample at study enrollment and self-reported their race as either `Black/African American' or `White' was selected for further biomarker analyses. This included a random sample of 395 women selected in 2005 within three strata (race, BMI, and smoking status) and a second random sample of 1,605 women selected in 2006 in equal numbers across race, BMI, and menopausal status categories.
Data collection
Trained study interviewers led all participants through a structured questionnaire using a computer-assisted interview with extensive skip patterns and range and logic checks. The interview elicited information including demographics, anthropometrics, and several aspects of health and behavior. Physical activity was measured using a questionnaire developed for the SCCS to comprehensively assess active and sedentary behaviors at the time of the interview. Dietary intake in the year prior to the baseline interview was measured using an 89-item food frequency questionnaire (FFQ) designed specifically for the SCCS to elicit information about foods most commonly eaten in the southeastern United States (6, 7). For the 20% of women who were patients in the CHC on the day of the baseline interview, measured height and weight were abstracted from medical records for validation purposes. Participants self-reported diagnoses of medical conditions including diabetes, heart attack or coronary bypass surgery, hypertension, high cholesterol, and depression by responding “Yes” to questions beginning “Has a doctor ever told you that you have…?”
A convenience blood sample was collected at the time of recruitment using one EDTA-containing plasma tube and one serum BD Vacutainer® tube. For this study, the median time (hours) between the last reported meal and blood collection was 6.0 for blacks and 6.3 for whites (p=0.07). Fasting blood, defined as at least 8 hours since last meal, was collected for 44% of the participants. Blood samples were shipped cold to Vanderbilt University in Nashville, TN, where they were processed for storage at −80° C. 84% of the blood samples were received the day after the blood draw and 98% were received within two days. The samples were frozen for an average of 2.6 years (range 3 months to 5 years) prior to analysis.
Laboratory assays
Adiponectin levels were measured in serum by immunoassay using the LINCOplex kit (Luminex® xMAP™ Technology, St. Louis, MO) in the Vanderbilt Hormone Assay and Analytical Services Core Laboratory in duplicate for each woman. The average of the two measurements was used in all analyses. Duplicate sets of samples for five randomly selected women as well as five repeat samples from each of two pooled samples were measured to assess the reliability and validity of the assay. Adiponectin levels were successfully measured in 1,992 of the 2,000 samples (eight samples failed due to a filter plate error or low sample volume). The intra-assay coefficient of variation was 9.4%. High-density lipoprotein (HDL) cholesterol was measured in serum by the Vanderbilt Lipid Laboratory using the ACE Clinical Chemistry System and the ACE HDLC Reagent (#SA1038) following the manufacturer's protocols (Alfa Wassermann, Inc, West Caldwell, NJ). The intra-assay coefficient of variation was 1.6%. Neither adiponectin nor HDL-cholesterol levels differed by fasting status (for adiponectin, p=0.3 for blacks, p=0.6 for whites; for HDL, p=0.5 for blacks, p=0.4 for whites).
Statistical Methods
For this cross-sectional analysis, data from 1,992 women with measured adiponectin were analyzed. BMI was calculated from self-reported values as [weight (kg)] / [height (m)2]. Dietary intakes, total physical activity, and HDL-cholesterol were categorized into quartiles based on the distribution of the entire sample. Other characteristics were categorized as shown in Table 1. The Wilcoxon signed rank test was used to compare adiponectin levels between groups.
Table 1.
Descriptive Statistics for Unadjusted Serum Adiponectin Levels (ug/ml) Among 1,992 Women in the Southern Community Cohort Study, 2002–2006
| Black women | White women | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Median | 25th–75th percentile | N | Median | 25th–75th percentile | |||
| All participants | 996 | 10.9 | 7.0 | 19.1 | 996 | 14.9 | 9.0 | 24.7 |
| Body mass index (kg/m2) | ||||||||
| < 18.5 | 8 | 22.3 | 8.8 | 36.2 | 10 | 28.9 | 13.5 | 48.3 |
| 18.5 – 24.9 | 240 | 15.7 | 9.6 | 25.7 | 239 | 21.8 | 13.6 | 36.6 |
| 25.0 – 29.9 | 248 | 11.0 | 7.4 | 18.7 | 248 | 15.7 | 9.8 | 25.0 |
| 30.0 – 34.9 | 250 | 8.9 | 5.9 | 14.4 | 249 | 13.7 | 8.7 | 20.9 |
| 35.0 – 39.9 | 165 | 10.6 | 6.0 | 17.9 | 146 | 10.7 | 7.7 | 18.5 |
| 40.0 + | 85 | 9.2 | 6.0 | 16.6 | 104 | 9.5 | 5.9 | 15.4 |
| Age at interview | ||||||||
| 40 – 44 | 330 | 9.8 | 6.5 | 18.0 | 341 | 13.9 | 8.5 | 20.8 |
| 45 – 49 | 265 | 10.3 | 6.7 | 18.7 | 243 | 13.9 | 8.2 | 21.6 |
| 50 – 54 | 148 | 12.2 | 7.2 | 19.7 | 136 | 15.4 | 8.6 | 28.0 |
| 55 – 59 | 105 | 11.5 | 7.3 | 21.5 | 120 | 16.2 | 10.1 | 30.1 |
| 60 – 64 | 53 | 13.6 | 8.6 | 19.1 | 93 | 17.3 | 10.6 | 28.2 |
| 65 – 69 | 50 | 12.2 | 7.4 | 25.0 | 29 | 20.9 | 11.8 | 32.9 |
| 70 – 74 | 25 | 12.6 | 8.4 | 25.3 | 24 | 21.1 | 13.7 | 43.3 |
| 75 – 79 | 20 | 18.7 | 10.4 | 21.4 | 10 | 29.5 | 24.8 | 51.4 |
| Household income | ||||||||
| < $15K | 620 | 10.9 | 7.0 | 19.0 | 594 | 14.5 | 9.4 | 24.1 |
| $15 – $25K | 236 | 10.7 | 6.9 | 18.3 | 205 | 14.4 | 8.6 | 25.3 |
| $25 – $50K | 115 | 12.5 | 6.8 | 20.6 | 129 | 16.5 | 8.6 | 29.9 |
| > $50K | 16 | 9.9 | 6.1 | 18.8 | 64 | 16.0 | 11.0 | 22.9 |
| Education (years) | ||||||||
| < 9 | 78 | 11.9 | 7.7 | 22.4 | 85 | 14.7 | 8.3 | 22.0 |
| 9 – 11 | 241 | 11.0 | 7.1 | 18.9 | 224 | 14.2 | 8.9 | 25.2 |
| 12 | 415 | 11.4 | 7.2 | 19.8 | 411 | 14.6 | 9.0 | 25.5 |
| > 12 | 262 | 10.3 | 6.1 | 18.2 | 276 | 15.6 | 9.4 | 22.7 |
| Physical activity (Met-hrs/day) | ||||||||
| Q1 (<10.2) | 248 | 11.6 | 7.5 | 20.3 | 247 | 14.0 | 8.7 | 21.9 |
| Q2 (10.2–18.3) | 256 | 11.6 | 6.9 | 19.5 | 240 | 16.2 | 9.8 | 27.2 |
| Q3 (18.4–29.2) | 255 | 10.6 | 6.9 | 18.5 | 242 | 14.7 | 8.8 | 24.9 |
| Q4 (>29.2) | 233 | 10.8 | 6.8 | 18.5 | 262 | 15.4 | 8.8 | 25.7 |
| HDL cholesterol (mg/dl) | ||||||||
| Q1 (<43) | 170 | 7.1 | 5.0 | 10.8 | 314 | 10.2 | 6.6 | 16.8 |
| Q2 (43–50) | 231 | 8.7 | 5.8 | 14.9 | 272 | 14.2 | 9.6 | 21.9 |
| Q3 (51–60) | 245 | 12.9 | 8.5 | 21.4 | 220 | 16.7 | 10.8 | 29.4 |
| Q4 (>60) | 342 | 14.7 | 9.3 | 24.4 | 182 | 24.2 | 16.2 | 40.9 |
| Total energy intake (kcal/day) | ||||||||
| Q1 (<1352) | 213 | 10.0 | 6.2 | 18.0 | 268 | 15.4 | 9.2 | 27.2 |
| Q2 (1352–1875) | 226 | 11.7 | 7.6 | 18.9 | 255 | 15.1 | 8.8 | 26.5 |
| Q3 (1876–2644) | 217 | 10.9 | 7.2 | 22.7 | 264 | 14.5 | 8.9 | 21.9 |
| Q4 (>2644) | 294 | 11.4 | 7.2 | 18.0 | 187 | 15.0 | 8.7 | 22.8 |
| Total fat intake (g/day) | ||||||||
| Q1 (<49) | 215 | 9.7 | 6.1 | 18.0 | 266 | 15.4 | 9.6 | 29.8 |
| Q2 (49–71) | 234 | 10.9 | 7.5 | 18.7 | 247 | 14.6 | 8.7 | 22.6 |
| Q3 (72–103) | 223 | 11.9 | 7.0 | 22.4 | 258 | 14.3 | 8.9 | 23.2 |
| Q4 (>103) | 278 | 11.7 | 7.3 | 18.4 | 203 | 16.1 | 8.8 | 23.2 |
| Carbohydrate intake (g/day) | ||||||||
| Q1 (<173) | 208 | 10.3 | 6.4 | 18.8 | 273 | 15.4 | 9.4 | 27.5 |
| Q2 (173–240) | 226 | 11.9 | 7.7 | 21.6 | 255 | 15.4 | 9.1 | 27.5 |
| Q3 (241–335) | 227 | 10.4 | 6.5 | 18.7 | 254 | 14.0 | 8.5 | 22.1 |
| Q4 (>335) | 289 | 11.5 | 7.5 | 18.5 | 192 | 14.9 | 8.8 | 22.8 |
| Protein intake (g/day) | ||||||||
| Q1 (<49) | 221 | 9.7 | 6.1 | 18.2 | 260 | 15.2 | 8.7 | 27.6 |
| Q2 (49–70) | 227 | 10.6 | 6.8 | 17.9 | 254 | 14.2 | 8.8 | 23.2 |
| Q3 (71–100) | 234 | 12.4 | 7.3 | 23.1 | 247 | 15.2 | 9.7 | 26.0 |
| Q4 (>100) | 268 | 11.7 | 7.4 | 18.6 | 213 | 14.6 | 8.7 | 22.0 |
| Fiber intake (g/day) | ||||||||
| Q1 (<11) | 220 | 10.0 | 6.8 | 17.6 | 261 | 15.0 | 8.3 | 24.1 |
| Q2 (11–16) | 227 | 10.6 | 6.9 | 19.9 | 254 | 14.4 | 9.2 | 24.3 |
| Q3 (17–24) | 229 | 12.9 | 7.0 | 20.5 | 252 | 15.4 | 9.5 | 28.8 |
| Q4 (>24) | 274 | 11.1 | 7.3 | 18.7 | 207 | 14.5 | 8.9 | 22.2 |
| Alcohol consumption (drinks per day) | ||||||||
| 0 | 496 | 10.9 | 7.1 | 19.7 | 591 | 14.0 | 8.6 | 23.5 |
| 1 – < 2 | 386 | 11.0 | 7.0 | 19.1 | 366 | 15.7 | 9.4 | 25.3 |
| 2+ | 111 | 10.5 | 6.4 | 18.7 | 37 | 18.2 | 13.2 | 36.5 |
| Duration of cigarette smoking (years) | ||||||||
| Never smoker | 414 | 10.6 | 6.8 | 18.0 | 328 | 14.7 | 8.8 | 25.6 |
| <20 | 133 | 10.9 | 6.6 | 19.3 | 121 | 15.4 | 9.8 | 25.1 |
| 20–29 | 236 | 10.9 | 7.1 | 18.3 | 248 | 15.0 | 9.3 | 22.6 |
| 30+ | 209 | 12.6 | 7.2 | 22.5 | 293 | 14.7 | 8.9 | 26.2 |
| Menopausal status | ||||||||
| Pre- | 499 | 10.3 | 6.5 | 17.6 | 497 | 13.8 | 8.4 | 21.6 |
| Post- | 497 | 11.9 | 7.5 | 20.4 | 499 | 16.1 | 10.3 | 28.6 |
| Number of live births | ||||||||
| None | 101 | 12.4 | 7.2 | 22.8 | 96 | 15.3 | 9.1 | 28.6 |
| 1–2 | 332 | 11.7 | 7.2 | 20.6 | 477 | 15.0 | 8.7 | 23.2 |
| 3–4 | 358 | 10.2 | 6.5 | 18.4 | 323 | 14.5 | 9.6 | 25.2 |
| 5+ | 205 | 10.8 | 7.1 | 16.7 | 99 | 15.4 | 8.9 | 25.1 |
| Age at menarche (years) | ||||||||
| <12 | 186 | 10.6 | 6.6 | 18.2 | 217 | 13.6 | 8.3 | 22.1 |
| 12 | 258 | 10.4 | 7.0 | 19.6 | 277 | 13.6 | 8.6 | 22.5 |
| 13 | 219 | 10.9 | 6.6 | 19.2 | 255 | 16.2 | 10.3 | 27.2 |
| 14 | 131 | 12.4 | 8.8 | 20.0 | 93 | 15.8 | 8.9 | 33.0 |
| 15+ | 197 | 10.8 | 7.0 | 19.3 | 147 | 15.8 | 9.6 | 24.3 |
| Diabetesa | ||||||||
| Yes | 220 | 10.0 | 6.2 | 19.8 | 164 | 13.0 | 7.5 | 22.1 |
| No | 776 | 11.0 | 7.1 | 19.0 | 832 | 15.3 | 9.3 | 25.1 |
| Heart attack or coronary bypass surgerya | ||||||||
| Yes | 41 | 12.3 | 8.6 | 23.8 | 51 | 15.0 | 9.8 | 21.5 |
| No | 955 | 10.9 | 6.9 | 18.9 | 945 | 14.9 | 9.0 | 25.0 |
| Hypertensiona | ||||||||
| Yes | 600 | 10.7 | 6.5 | 18.7 | 462 | 13.3 | 7.9 | 22.5 |
| No | 396 | 11.5 | 7.7 | 19.7 | 534 | 16.2 | 9.9 | 25.5 |
| High cholesterola | ||||||||
| Yes | 280 | 10.9 | 6.8 | 18.8 | 371 | 13.7 | 8.6 | 22.1 |
| No | 715 | 10.9 | 7.0 | 19.1 | 624 | 15.6 | 9.3 | 25.6 |
| Depressiona | ||||||||
| Yes | 214 | 10.6 | 6.5 | 18.0 | 479 | 14.4 | 9.0 | 23.1 |
| No | 782 | 11.0 | 7.1 | 19.4 | 515 | 15.4 | 9.1 | 25.4 |
Has a doctor ever told you that you have….?
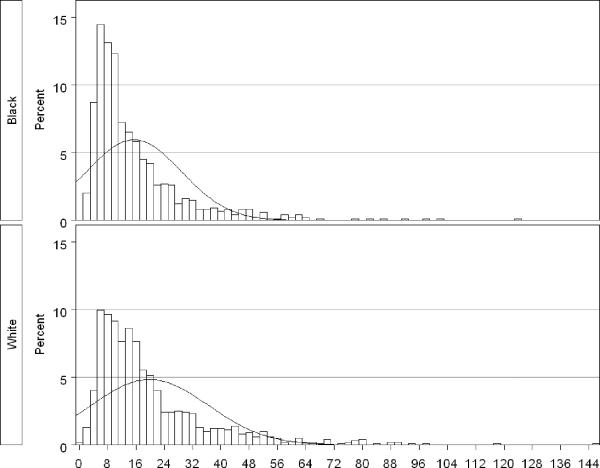
Adiponectin had a skewed distribution (Figure 1), and therefore was log-transformed to better meet modeling assumptions (8). To ease presentation, back-transformed values are shown. Linear regression models were constructed for blacks and whites separately. All models were adjusted for sample selection (395 selected in 2005 versus 1605 selected later) and age at baseline interview. Further adjustment for factors used in the sample selection (cigarette smoking status and menopausal status) did not alter the results and were not included in the models presented here. Categorized covariates with inherent order were modeled using indicator variables rather than as ordinal variables because the assumption of linearity was not generally met.
Figure 1.
Distribution of adiponectin levels (ug/ml) in 996 black and 996 white women in the Southern Community Cohort Study, 2002–2006
The first analytic objective was to characterize the relationship between adiponectin and BMI within each race group. BMI (continuous) was regressed on adiponectin, and potential confounders (determined from the literature and categorized as shown in Table 1) were added to the model using backwards model selection with a change-in-estimate criterion of ≥ 5%. Linear trend tests were conducted via F-tests of the continuous BMI variable. Potential modifiers of the BMI-adiponectin relationship were assessed using the likelihood ratio test (LRT) to compare models with and without interaction terms for BMI and potential modifiers. Additionally, adjusted geometric means for adiponectin were calculated from linear regression models that included standard categories of BMI (<18.5, 18.5–24.9, 25–29.9, 30–34.9, 35–39.9, and 40–45 kg/m2) and the final set of confounders.
The second analytic goal was to determine correlates of adiponectin after adjustment for BMI. BMI was forced into the linear regression model and potential correlates were then added sequentially. Models were compared using Akaike's Information Criterion (AIC) which balances model fit with model complexity (9), and potential correlates were included in the final model as long as their addition resulted in an AIC value at least one unit lower than the AIC for the smaller-order model.
SAS/STAT software, Version 9.1 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
RESULTS
Adiponectin levels were 25% lower among black women compared to white women (medians: 10.9 versus 14.9 ug/ml, respectively, Wilcoxon p<0.0001). After adjustment for BMI and age, adiponectin was still significantly lower in blacks (p<0.0001). Unadjusted adiponectin levels decreased with increasing BMI categories in both groups although not as consistently among blacks (Table 1). There was a strong positive association between adiponectin and both age and HDL-cholesterol while adiponectin was slightly lower among women reporting diabetes or hypertension. Unadjusted adiponectin levels increased with increasing alcohol consumption in whites but not in blacks. Adiponectin was not consistently associated with education, income, physical activity, cigarette smoking, or any of the dietary or reproductive indices.
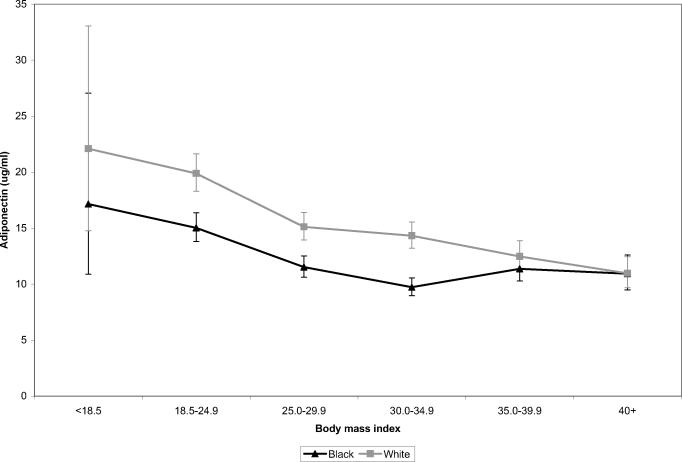
There was a strong linear association between continuous BMI and adiponectin in both race groups (test for linear trend, p<0.0001 for black and white women). Figure 2 illustrates race-specific associations between categories of BMI and adiponectin adjusted for HDL-cholesterol, the only variable found to be a confounder of the BMI-adiponectin relationship. Adjusted adiponectin means for blacks were lower than those for whites within each category of BMI although the differences for women in the highest categories of BMI (35–39.9 and 40+) were small. Among blacks, adiponectin decreased over BMI values up to 30–34.9 before leveling off at the highest levels of BMI. In contrast, among whites, adiponectin levels were lower with each increasing category of BMI. Menopausal status did not modify the BMI-adiponectin relationship (LRT p=0.9 for blacks, p=0.6 for whites) (data not shown). In models restricted to 421 black and 450 white women with fasting blood samples, the association between BMI and adiponectin was essentially unchanged for both groups (data not shown).
Figure 2.
Adjusted geometric means and 95% confidence intervals for adiponectin levels (ug/ml) by body mass index categories in 996 black and 996 white women in the Southern Community Cohort Study, 2002–2006. Geometric means are adjusted for age, sample selection, and HDL-cholesterol.
Table 2 shows race-specific prediction models for adiponectin. Based on the AIC values, BMI was an important correlate of adiponectin in both race groups although the regression coefficient was larger in magnitude among whites. Age, HDL-cholesterol, and hypertension were also significant correlates of adiponectin in both groups based on the AIC comparisons. None of the other factors examined added additional predictive value to the models.
Table 2.
Linear Regression Results from Prediction Modelsa for Log-adiponectin (ug/ml) in 1,992 Black and White Women in the Southern Community Cohort Study, 2002–2006
| Black women | White women | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Beta | Std err | p-value | Partial R2 | Beta | Std err | p-value | Partial R2 |
| Body Mass Index (kg/m2) | −0.017 | 0.0036 | <0.0001 | 0.05 | −0.029 | 0.003 | <0.0001 | 0.11 |
| HDL-cholesterol (mg/dl) | 0.10 | 0.10 | ||||||
| Q1 (<43) | Referent | Referent | ||||||
| Q2 (43–50) | 0.17 | 0.067 | 0.01 | 0.29 | 0.053 | <0.0001 | ||
| Q3 (51–60) | 0.53 | 0.067 | <0.0001 | 0.41 | 0.057 | <0.0001 | ||
| Q4 (>60) | 0.59 | 0.064 | <0.0001 | 0.67 | 0.063 | <0.0001 | ||
| Age at interview (years) | 0.010 | 0.003 | <0.0001 | 0.01 | 0.015 | 0.003 | <0.0001 | 0.02 |
| Hypertension | −0.085 | 0.046 | 0.07 | 0.003 | −0.072 | 0.044 | 0.10 | 0.002 |
| Adjusted model R2 | 0.17 | 0.27 | ||||||
Model estimates for the intercept and sample selection term are not shown
As both diabetes and treatment for diabetes have been shown to affect adiponectin levels (10), we repeated our main analyses in the subset of non-diabetic women in this study (N=776 black and N=832 white). Among the non-diabetics, median adiponectin levels remained significantly lower in blacks compared to whites (median: 11.0 versus 15.3 ug/ml, respectively, Wilcoxon p<0.0001). Adjusted geometric means for adiponectin across categories of BMI were also not appreciably different in either race group among the non-diabetic women compared to the patterns observed in the entire sample (for BMI categories of 18.5–24.9, 25–29.9, 30–34.9, 35–39.9, and 40–45, adjusted adiponectin means were 15.3, 11.4, 9.7, 11.2, and 9.9 in non-diabetic black women and 20.1, 15.4, 14.7, 12.0, and 10.8 in non-diabetic white women).
DISCUSSION
In this largest to-date, cross-sectional study of black and white women from similar geographic and socioeconomic situations, we observed that blacks have lower adiponectin levels than whites even after adjustment for BMI. Our results expand upon previous studies that have also found lower levels of adiponectin in blacks but were limited either by small numbers of black participants (11–14) or limited age ranges (15–17), indicating that racial differences in adiponectin exist across the adult age spectrum.
Adiponectin has consistently been found to be negatively correlated with obesity in white and Asian populations (18–20). Despite racial differences in the prevalence of obesity and risk for obesity-related disease, few large studies have examined adiponectin in relation to obesity in blacks. As did our study, several small studies (12, 14, 21) as well as the Atherosclerosis Risk in Communities Study (15) found that adiponectin decreased over categories of BMI in blacks and that adjusted adiponectin values were lower for black women compared to white women in each BMI category. Our analysis included larger numbers of women with higher values of BMI than in previous studies, and we showed for the first time that the form of the BMI-adiponectin association may differ by race with white women showing a consistent decline in adiponectin over all levels of BMI while among blacks, adiponectin was lower among overweight and obese women compared to healthy weight women but there was little trend with increasing severity of obesity.
Our first-ever extensive examination of potential correlates of adiponectin found few factors that were strongly associated with adiponectin. Adiponectin levels rose with advancing age, consistent with earlier studies (18, 22). The direction of this association has previously been noted to be paradoxical; abdominal fat, which is inversely associated with adiponectin, increases with age indicating that adiponectin levels should decrease with age. One potential explanation for this seeming contradiction is that estrogen, thought to inhibit adiponectin, decreases with age allowing adiponectin to rise (18). We also observed a strong positive association between HDL-cholesterol and adiponectin, as have others (18, 22). Mechanistically, it is hypothesized that decreased adiponectin levels may affect hepatic insulin resistance leading to increased hepatic lipase activity and decreased HDL levels (18, 22). While the cross-sectional nature of our data did not allow us to examine the temporality of the HDL-adiponectin relationship, our data demonstrate that this strong association holds across race and age groups.
Low adiponectin levels have been inversely linked to hypertension (23–25) including in one study of blacks (25), a finding we also observed in both race groups. A few prior studies have found this association only among participants with insulin resistance, but at least some evidence indicates that low adiponectin levels may affect the development of hypertension at an early stage, without involvement of insulin resistance (24).
Based on previous examination in the literature as well as plausible roles in the physiologic pathways through which adiponectin is believed to act, we selected additional medical conditions for inclusion in this study, but they were ultimately not found to be important correlates of adiponectin in our final multivariate model. We found that adiponectin levels were only slightly lower among diabetics, and after adjustment for BMI and age, diabetes and adiponectin were not significant associated. This was somewhat unexpected since adiponectin and diabetes incidence have previously been shown to be inversely linked (10). One explanation may be that many SCCS women with prevalent diabetes were receiving treatment for their diabetes (among diabetics in this study, 75% of white women and 87% of black women reported taking medication for their diabetes), thus reducing effects of hyperinsulinemia on adiponectin levels. We also observed slightly lower adiponectin levels among women reporting depression, a finding consistent with the literature to date that has demonstrated lower adiponectin among patients with major depressive disorder and anxiety scores (26); however, in our study, this difference was non-significant after inclusion in the final multivariate model.
Neither physical activity nor dietary factors were predictive of adiponectin levels despite their known roles as major components of energy balance. In contrast, a prior study found that physical activity was associated with increased adiponectin levels, with moderate/high intensity activity showing stronger effects than low intensity activity (27). It is possible that the activity levels in our population were too low overall to detect effects on adiponectin levels; in fact, less than 20% of the women in either race group in this study met the recommended guidelines for physical activity (28). Little research has been conducted regarding associations between dietary factors and adiponectin. One study found no association between total calories or macronutrient intake and adiponectin (29) while fiber was found to be positively associated with adiponectin among diabetics (30, 31). It seems likely that there are many intermediaries in the pathways linking diet and physical activity to adiponectin, making the detection of associations difficult in our cross-sectional dataset. Additionally, it is possible that once age, BMI, and HDL-cholesterol were included in models for adiponectin, minimal additional predictive value was added by factors such as diabetes, diet, and physical activity which are reasonably expected to be predictive of adiponectin but are also strongly associated with age, BMI, and HDL-cholesterol. While standard in large epidemiologic studies, the physical activity and dietary intakes obtained via questionnaire rather than objective measures may also have limited the detection of associations with adiponectin.
This study is potentially limited by our measurement of adiponectin. The high-molecular weight (HMW) form of adiponectin has been suggested to be the more biologically active (32) and thus, we may have been unable to detect certain associations because we (like most other large, population-based studies) did not specifically measure HMW adiponectin. Additionally, adiponectin was measured only at a single point in time. Studies in white and Chinese individuals found that adiponectin levels measured one year apart were highly correlated, indicating that a single measurement of adiponectin is likely sufficient for large epidemiologic studies (33, 34). Another possible limitation of our adiponectin measurement is that it was not conducted exclusively in fasting samples; however, analyses limited to samples provided more than 8 hours since the last meal did not show any appreciable differences from those using the entire population.
The use of self-reported height and weight measures is also a potential limitation. A recent review indicates that among women, height tends to be over-reported and weight under-reported (35). However, data from the 1999–2004 National Health and Examination Survey show that despite errors in self-report, BMI categories based on self-reported values still generally demonstrate good agreement with BMI categories from measured values (36). These data also showed that under-reporting was more common in whites and among well-educated women (36) which suggests that the BMI values calculated from self-report in the SCCS may be less vulnerable to bias than in other studies of more educated, white participants. Furthermore, in the SCCS, BMI values calculated from self-reported height and weight were very highly correlated with BMI values calculated from medical record data overall (Pearson correlation coefficient > 0.95) as well as across strata of BMI, race, education, and income, indicating that the self-reported values are generally of good quality. An additional study limitation is the absence of a measurement of central obesity as it has been shown that the amount of centrally-deposited adipose tissue differs between black and white women at the same level of BMI (37) and further, that there are differences in the proportion of the various isoforms of adiponectin in relation to measures of central obesity across race groups (38).
A major strength of this study is the utilization of the SCCS resource. While the women included in this study do not reflect the distribution of all women in the US due to the recruitment of SCCS participants via CHCs, the SCCS participants are generally reflective of the 14 million users of CHCs in the US who represent a largely understudied group of individuals at high risk for many diseases. More importantly, within-population comparisons such as those presented here, are valid regardless of generalizability. Indeed, by design, the black and white participants in this study arose from similar geographic and SES backgrounds facilitating the examination of racial differences by minimizing the potential role of SES-driven confounding, a limitation that clouds the interpretation of results from many previous studies. Our study also overcomes limitations from previous studies which were hampered by small numbers of black participants, narrow age ranges, and few participants with BMI greater than 35.
Analysis of this large population of highly comparable black and white women shows that adiponectin levels are lower in blacks than in whites and that adiponectin is inversely associated with obesity but the shape of the BMI-adiponectin association differs by race. Additionally, we demonstrated that age, HDL-cholesterol, and hypertension are strong correlates of adiponectin in both race groups. Future work within the SCCS and other studies with diverse populations will be guided by these findings as the mechanistic role played by adiponectin in the development of disease is examined. Further, efforts to develop treatments that can alter adiponectin levels or even therapeutic applications of adiponectin itself for diseases such as cardiovascular disease, cancer, and diabetes may be guided by the racial differences in adiponectin levels and its correlates observed in this study.
ACKNOWLEDGEMENTS
This project was funded in part by grant OP05-0927-DR1 from Susan G. Komen for the Cure. The Southern Community Cohort Study is funded by grant R01 CA92447 from the National Cancer Institute (NCI). Dr. Cohen also received support from NCI Training Grants T32 CA09330-26 and 5-R25-CA057726. Dr. Gammon is supported in part from P30ES10126 from the National Institute of Environmental Health Sciences. Serum sample preparation was conducted at the Survey and Biospecimen Shared Resource that is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485).
ABBREVIATIONS AND ACRONYMS
- SCCS
Southern Community Cohort Study
- CHC
Community Health Center
- BMI
Body mass index
- FFQ
Food frequency questionnaire
- HDL
High-density lipoprotein
- AIC
Akaike's Information Criterion
- HMW
High molecular weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005 May;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 2.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003 Aug;26(8):2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 3.Differences in Prevalence of Obesity Among Black, White, and Hispanic Adults ---United States, 2006--2008. MMWR: Morbidity and Mortality Weekly Report 17 Jul 2009. 2009 Sep 22;58(27):740–4. [PubMed] [Google Scholar]
- 4.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005 Jul;97(7):972–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Hargreaves MK, Arnold C, Blot WJ. Community health centers: Their role in the treatment of minorities and in health disparities research. In: Satcher D, Pamies R, editors. Multicultural Medicine and Health Disparities. McGraw-Hill; New York: 2006. pp. 485–94. [Google Scholar]
- 6.Buchowski MS, Schlundt DG, Hargreaves MK, Hankin JH, Signorello LB, Blot WJ. Development of a culturally sensitive food frequency questionnaire for use in the Southern Community Cohort Study. Cell Mol Biol (Noisy-le-grand) 2003 Dec;49(8):1295–304. [PubMed] [Google Scholar]
- 7.Signorello LB, Munro HM, Buchowski MS, Schlundt DG, Cohen SS, Hargreaves MK, et al. Estimating nutrient intake from a food frequency questionnaire: incorporating the elements of race and geographic region. Am J Epidemiol. 2009 Jul 1;170(1):104–11. doi: 10.1093/aje/kwp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinbaum DG, Kupper LL. Applied regression analysis and other multivariable methods. Duxbury Press; North Scituate, Mass: 1978. [Google Scholar]
- 9.Akaike H. Fitting Autoregressive Models for Prediction. Annals of the Institute of Statistical Mathematics. 1969;21:243–7. [Google Scholar]
- 10.Li S, Shin JJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009 Jul 8;302(2):179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 11.Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005 Sep;54(9):2772–8. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 12.Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, Saha C, Pratt JH, Considine RV. Lower serum adiponectin levels in African-American boys. Obes Res. 2003 Nov;11(11):1384–90. doi: 10.1038/oby.2003.187. [DOI] [PubMed] [Google Scholar]
- 13.Ferris WF, Naran NH, Crowther NJ, Rheeder P, van der Merwe L, Chetty N. The relationship between insulin sensitivity and serum adiponectin levels in three population groups. Horm Metab Res. 2005 Nov;37(11):695–701. doi: 10.1055/s-2005-870580. [DOI] [PubMed] [Google Scholar]
- 14.Araneta MR, Barrett-Connor E. Adiponectin and ghrelin levels and body size in normoglycemic Filipino, African-American, and white women. Obesity. 2007 Oct;15(10):2454–62. doi: 10.1038/oby.2007.291. Silver Spring, Md. [DOI] [PubMed] [Google Scholar]
- 15.Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004 Sep;53(9):2473–8. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 16.Kanaya AM, Wassel Fyr C, Vittinghoff E, Havel PJ, Cesari M, Nicklas B, et al. Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab. 2006 Dec;91(12):5044–50. doi: 10.1210/jc.2006-0107. [DOI] [PubMed] [Google Scholar]
- 17.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, et al. Serum adiponectin in young adults--interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol. 2004 Aug;14(7):492–8. doi: 10.1016/j.annepidem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003 Apr;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 19.Staiger H, Tschritter O, Machann J, Thamer C, Fritsche A, Maerker E, et al. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res. 2003 Mar;11(3):368–72. doi: 10.1038/oby.2003.48. [DOI] [PubMed] [Google Scholar]
- 20.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 21.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004 Jan;53(1):1–3. doi: 10.1016/j.metabol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Hanley AJ, Bowden D, Wagenknecht LE, Balasubramanyam A, Langfeld C, Saad MF, et al. Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic Hispanics and African-Americans. J Clin Endocrinol Metab. 2007 Jul;92(7):2665–71. doi: 10.1210/jc.2006-2614. [DOI] [PubMed] [Google Scholar]
- 23.Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007 Jun;49(6):1455–61. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- 24.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004 Jun;43(6):1318–23. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 25.Shikany JM, Lewis CE, Freedman BI, Arnett DK, Leiendecker-Foster C, Jones TL, et al. Plasma adiponectin concentrations and correlates in African Americans in the Hypertension Genetic Epidemiology Network (HyperGEN) study. Metab-Clin Exp. 2007 Aug;56(8):1011–6. doi: 10.1016/j.metabol.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Taylor VH, Macqueen GM. The Role of Adipokines in Understanding the Associations between Obesity and Depression. J Obes. 2010;2010 doi: 10.1155/2010/748048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity. 2008 Feb;16(2):241–56. doi: 10.1038/oby.2007.53. Silver Spring, Md. [DOI] [PubMed] [Google Scholar]
- 28.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington, DC: 2008. [DOI] [PubMed] [Google Scholar]
- 29.Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003 Apr;88(4):1730–6. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]
- 30.Qi L, Meigs JB, Liu S, Manson JE, Mantzoros C, Hu FB. Dietary fibers and glycemic load, obesity, and plasma adiponectin levels in women with type 2 diabetes. Diabetes Care. 2006 Jul;29(7):1501–5. doi: 10.2337/dc06-0221. [DOI] [PubMed] [Google Scholar]
- 31.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005 May;28(5):1022–8. doi: 10.2337/diacare.28.5.1022. [DOI] [PubMed] [Google Scholar]
- 32.Imbeault P. Environmental influences on adiponectin levels in humans. Appl Physiol Nutr Metab. 2007 Jun;32(3):505–11. doi: 10.1139/H07-017. [DOI] [PubMed] [Google Scholar]
- 33.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003 Apr;49(4):650–2. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 34.Lee SA, Kallianpur A, Xiang YB, Wen W, Cai Q, Liu D, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer Epidemiol Biomarkers Prev. 2007 Nov;16(11):2464–70. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- 35.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007 Jul;8(4):307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 36.Craig BM, Adams AK. Accuracy of body mass index categories based on self-reported height and weight among women in the United States. Matern Child Health J. 2009 Jul;13(4):489–96. doi: 10.1007/s10995-008-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996 Sep;45(9):1119–24. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 38.Lara-Castro C, Doud EC, Tapia PC, Munoz AJ, Fernandez JR, Hunter GR, et al. Adiponectin multimers and metabolic syndrome traits: relative adiponectin resistance in African Americans. Obesity. 2008 Dec;16(12):2616–23. doi: 10.1038/oby.2008.411. Silver Spring, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]