SUMMARY
Caloric restriction (CR) extends the lifespan and healthspan of a variety of species, and slows the progression of age-related hearing loss (AHL), a common age-related disorder associated with oxidative stress. Here we report that CR reduces oxidative DNA damage in multiple tissues and prevents AHL in wild-type mice, but fails to modify these phenotypes in mice lacking the mitochondrial deacetylase Sirt3, a member of the sirtuin family. In response to CR, Sirt3 directly deacetylates and activates mitochondrial isocitrate dehydrogenase 2 (Idh2), leading to increased NADPH levels and an increased ratio of reduced to oxidized glutathione in mitochondria. In cultured cells, overexpression of Sirt3 and/or Idh2 increases NADPH levels and protects from oxidative stress-induced cell death. Therefore, our findings identify Sirt3 as an essential player in enhancing the mitochondrial glutathione antioxidant defense system during CR, and suggest that Sirt3-dependent mitochondrial adaptations may be a central mechanism of aging retardation in mammals.
Keywords: caloric restriction, Sirt3, age-related hearing loss, oxidative stress, ROS, glutathione, isocitrate dehydrogenase 2, NADPH, mitochondria
INTRODUCTION
It is well established that reducing food consumption by 25–60% without malnutrition consistently extends both the mean and maximum lifespan of rodents (Weindruch et al., 1988; Kouboa et al., 2003). Caloric restriction (CR) is also known to extend lifespan in yeast, worms, fruit flies, spiders, birds, and monkeys and delays the progression of a variety of age-associated diseases such as cancer, diabetes, cataract, and age-related hearing loss (AHL) in mammals (Weindruch et al., 1988; Sohal et al., 1996; Someya et al., 2007; Colman et al., 2009). Furthermore, CR reduces neurodegeneration in animal models of Parkinson's disease (Mattson, 2000) as well as Alzheimer's disease (Zhu et al., 1999). The mitochondrial free radical theory of aging postulates that aging results from accumulated oxidative damage caused by reactive oxygen species (ROS), originating from the mitochondrial respiratory chain (Balaban et al., 2005). Consistent with this hypothesis, mitochondria are a major source of ROS and of ROS-induced oxidative damage, and mitochondrial function declines during aging (Wallace, 2005). A large body of evidence suggests that CR reduces the age-associated accumulation of oxidatively damaged proteins, lipids, and DNA through reduction of oxidative damage to these macromolecules and/or enhanced antioxidant defenses to oxidative stress (Weindruch et al., 1988; Sohal et al., 1996; Masoro, 2000). Yet, whether the anti-aging action of CR in mammals is a regulated process and requires specific regulatory proteins such as sirtuins still remains unclear.
Sirtuins are NAD+-dependent protein deacetylases that regulate lifespan in lower organisms, and have emerged as broad regulators of cellular fate and mammalian physiology (Donmez et al., 2010; Finkel et al., 2009). A previous report has shown that lifespan extension by CR in yeast requires Sir2, a member of the sirtuin family (Lin et al., 2000), linking sirtuins and CR-mediated retardation of aging. In mammals, there are seven sirtuins that display diverse cellular localization (Donmez et al., 2010; Finkel et al., 2009). Previous studies have focused on the role of Sirt1 as the major sirtuin mediating the metabolic effects of CR in mammals (Chen et al., 2005; Bordone et al., 2007; Chen et al., 2008). However, recent studies indicate that upregulation of Sirt1 in response to CR is not observed in all tissues examined (Cohen et al., 2004; Barger et al., 2008) and currently, no study has provided conclusive evidence that sirtuins play an essential role in CR-mediated aging retardation in mammals. Sirt3 is a member of the mammalian sirtuin family that is localized to mitochondria and regulates levels of ATP and the activity of Complex I of the electron transport chain (Ahn et al., 2008), and as such may play a role in the metabolic reprogramming mediated by CR. A recent study has shown that CR increases Sirt3 levels in liver mitochondria (Schwer et al., 2009). Fasting also increases Sirt3 protein expression in liver mitochondria, and mice lacking Sirt3 display the hallmarks of fatty-acid oxidation disorders, indicating that Sirt3 modulates mitochondrial fatty-acid oxidation in mammals (Hirschey et al., 2010). Furthermore, CR increases expression of Sirt3 in primary mouse cardiomyocytes, while overexpression of Sirt3 protects these cells from oxidative stress-induced cell death (Sundaresan et al., 2008), suggesting a potential role of Sirt3 in the aging retardation associated with CR in mammals.
AHL is a universal feature of mammalian aging and is the most common sensory disorder in the elderly (Someya et al., 2010; Liu et al., 2007). AHL is characterized by an age-associated decline of hearing function associated with loss of spiral ganglion neurons and sensory hair cells in the cochlea of the inner ear (Someya et al., 2010; Liu et al., 2007). The progressive loss of neurons and hair cells in the inner ear leads to the onset of AHL because these postmitotic cells do not regenerate in mammals. The onset of AHL begins in the high frequency region and spreads toward the low frequency region during aging (Keithley et al., 2004; Hunter et al., 1987). This is accompanied by the loss of neurons and hair cells beginning in the basal region and spreading toward the apex of the cochlea of the inner ear with age. A previous study has shown that CR slows the progression of AHL in CBA/J mice (Sweet et al., 1988), while we have shown previously that CR prevents AHL in C57BL/6J mice, reduces cochlear degeneration, and induces Sirt3 in the cochlea (Someya et al., 2007). Both strains of mice have been extensively used as a model of AHL, although the age of onset of AHL varies from 12 to 15 months of age in C57BL/6J mice to 18–22 months of age in CBA/J mice (Zheng et al., 1999). Experimental evidence suggest that oxidative stress plays a major role in AHL (Jiang et al., 2007; Someya et al., 2009) and that CR protects cochlear cells through reduction of oxidative damage and/or by enhancing cellular antioxidant defenses to oxidative stress (Someya et al., 2007). Yet, the molecular mechanisms by which CR reduces oxidative cochlear cell damage remain unknown.
In this report, we show that the mitochondrial deacetylase Sirt3 is required for the CR-mediated prevention of AHL in mice. We also show that Sirt3 is required for the reduction of oxidative damage in multiple tissues under CR conditions, as evidenced by DNA damage levels. At the mechanistic level, Sirt3 directly deacetylates isocitrate dehydrogenase 2 (Idh2), an enzyme that converts NADP+ to NADPH in mitochondria. In response to CR, Sirt3 stimulates Idh2 activity in mitochondria, leading to increased levels of NADPH and an increased ratio of reduced glutathione/oxidized glutathione, the major redox couple in the cell. In cultured cells, overexpression of Sirt3 and/or Idh2 increases NADPH levels and protects these cells from oxidative stress. The data presented here provides the first conclusive evidence that CR-mediated reduction of oxidative damage, and prevention of a common age-related phenotype (AHL) requires a member of the sirtuin family in mammals.
RESULTS
Sirt3 is Required for the CR-mediated Prevention of Age-related Cochlear Cell Death and Hearing Loss
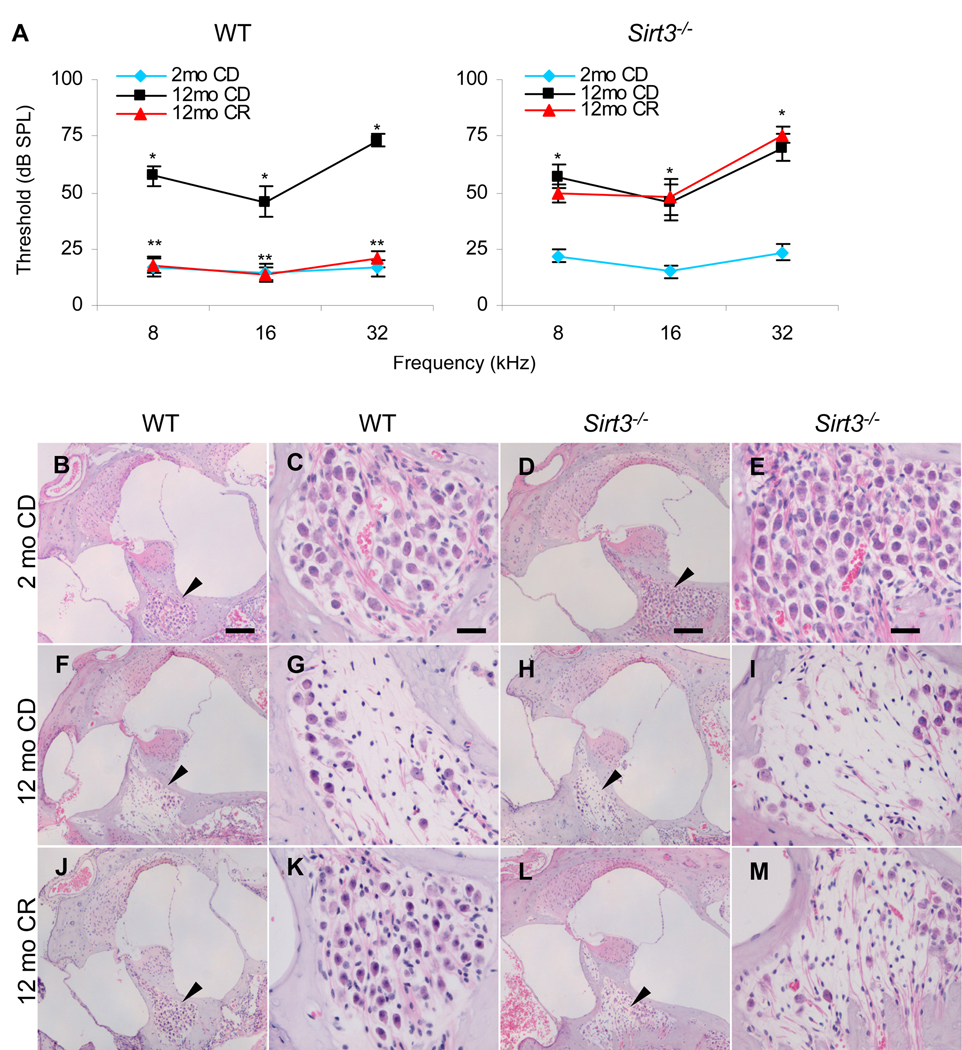
First, to investigate whether Sirt3 plays a role in the CR prevention of AHL, we conducted a 10-month CR dietary study using WT and Sirt3−/− mice that have been backcrossed onto the C57BL/6J background. The C57BL/6J strain is considered an excellent model to study the anti-aging action of CR since this mouse strain is the most widely used mouse model for the study of aging and responds to CR with a robust extension of lifespan (Weindruch et al., 1988) and prevention of AHL (Someya et al., 2007). We reduced the calorie intake of WT and Sirt3−/− mice to 75% (a 25% CR) of that fed to control diet (CD) mice in early adulthood (2 months of age), and this dietary regimen was maintained until 12 months of age. The auditory brainstem response (ABR), a common electrophysiological test of hearing function, was used to monitor the progression of AHL in these mice (Someya et al., 2009). We first confirmed that aging resulted in increased ABR hearing thresholds at the high (32 kHz), middle (16 kHz), and low (8 kHz) frequencies in 12-month-old WT mice (Figure 1A), indicating that these mice displayed hearing loss. As predicted, CR delayed the progression of AHL at all tested frequencies in WT mice (Figure 1A). Strikingly, CR did not delay the progression of AHL in Sirt3−/− mice (Figure 1A), although CR had the same effect on body weight reduction in both WT and Sirt3−/− mice (Figure S2A and B). Neural and hair cell degeneration are hallmarks of AHL (Keithley et al., 2004). In agreement with the hearing test results, basal regions of the cochleae from calorie restricted WT mice displayed only minor loss of spiral ganglion neurons (Figure 1J and K, See also Figure 1B, C, F, and G) and hair cells (Figure S1E, See also Figure S1A and C), while CR failed to protect these cells in Sirt3−/− mice (Figure 1L and M, See also Figure 1D, E, H, and I, Figure S1F, See also Figure S1B and D). Collectively, these results demonstrate that Sirt3 plays an essential role in the CR-mediated prevention of age-related cochlear cell death and hearing loss in mice.
Figure 1. CR Prevents AHL and Protect Cochlear Neurons in WT Mice, But not in Sirt3−/− Mice.
(A) ABR hearing thresholds were measured at 32, 16, and 8 kHz from control diet and/or calorie restricted WT (left) and Sirt3−/− (right) mice at 2 and 12 months of age (n = 9–12). *Significantly different from 2-month-old WT or Sirt3−/− mice (P < 0.05), **significantly different from 12-month-old WT mice (P < 0.05). CD = control diet, CR = calorie restricted diet.
(B–M) Neurons in the basal cochlear regions from WT mice in control diet at 2 (B and C) and 12 (F and G) months of age, and calorie restricted diet at 12 months of age (J and K). Neurons from control diet Sirt3−/− mice at 2 (D and E) and 12 (H and I) months of age, and calorie restricted Sirt3−/− mice at 12 months of age (L and M)(n = 5). Arrows in the lower magnification photos indicate neuron regions. Scale bar = 100 µm (B, F, J, D, H, and L), 20 µm (C, G, J, E, I, and M). Data are means ± SEM. See also Figure S1, Figure S2, and Figure S3.
Next, to investigate whether Sirt3 plays a role in the metabolic effects induced by CR, we conducted a 3-month CR dietary study using WT and Sirt3−/− mice starting at 2 month of age. Mice lacking the Sirt3 gene appeared phenotypically normal under basal and CR conditions: Sirt3−/− mice were viable and fertile, and no significant changes were observed in body weight (Figure S2A and B), bone mineral density (Figure S2C), body fat (Figure S2D), tissue weight (Figure S2E), serum glucose levels (Figure S3A), glucose tolerance (Figure S3B), serum Igf-1 (Figure S3C) and cholesterol (Figure S3D) levels between control diet WT and Sirt3−/− mice or calorie restricted WT and Sirt3−/− mice at 5 months of age. However, while we found that WT mice displayed lower levels of serum insulin (Figure S3E) and triglycerides (Figure S3F) in response to CR, no significant changes were observed in these serum markers between control diet fed and calorie restricted Sirt3−/− mice, suggesting a possible role of Sirt3 in metabolic adaptations to CR.
Sirt3 is Required for the CR-mediated Reduction of Oxidative Damage in Multiple Tissues
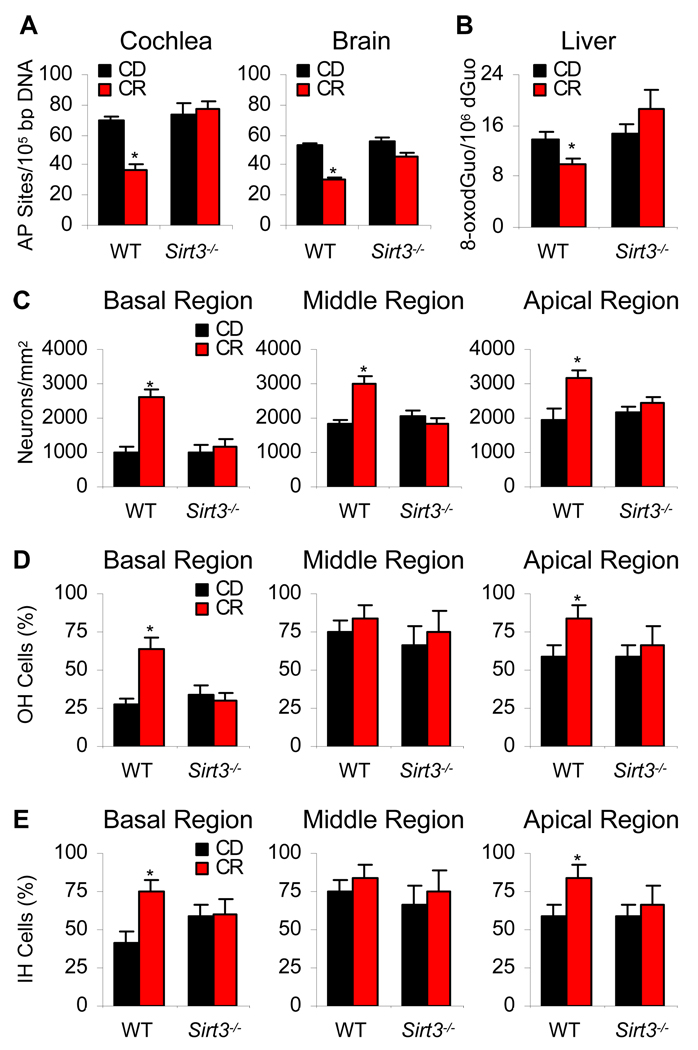
How does Sirt3 reduce cochlear cell degeneration and slow the progression of AHL in response to CR? It is well established that CR reduces oxidative damage to DNA, proteins, and lipids in multiple tissues in mammals (Sohal et al., 1996; Masoro, 2000; Hamilton et al., 2001). Hence, we hypothesized that Sirt3 may play a role in the CR-mediated reduction of oxidative damage in the cochlea and other tissues. To test this hypothesis, we measured oxidative damage to DNA in the cochleae, brain (neocortex), and liver of control diet and calorie restricted WT and Sirt3−/− mice at 12 months of age. We found that CR reduced oxidative DNA damage in WT mice as determined by measurements of 8-hydroxyguanosine and apurinic/aprimidinic (AP) sites, but failed to reduce oxidative DNA damage in tissues from Sirt3−/− mice (Figure 2A and B). In agreement with the oxidative damage results, CR increased spiral ganglion neuron survival (Figure 2C), outer hair cell survival (Figure 2D), and inner hair cell survival (Figure 2E) in the basal regions of the cochleae of WT mice, while CR failed to protect these cells in Sirt3−/− mice (Figure 2C–E). Together, these results provide evidence that Sirt3 plays an essential role in the CR-mediated reduction of oxidative DNA damage in multiple tissues.
Figure 2. CR Reduces Oxidative DNA Damage and Increases Cell Survival in the Cochleae from WT Mice, But not from Sirt3−/− Mice.
(A) Oxidative damage to DNA (apurinic/apyrimidinic sites) was measured in the cochlea and neocortex from control diet and calorie restricted WT and Sirt3−/− mice at 12 months of age (n = 4–5). AP sites= apurinic/apyrimidinic sites. *Significantly different from 12-month-old WT mice (P < 0.05).
(B) Oxidative damage to DNA (8-oxodGuo) was measured in the liver from control diet and calorie restricted WT and Sirt3−/− mice at 12 months of age (n = 4–5).
(C) Neuron survival (neuron density) of basal, middle, and apical cochlear regions was measured from control diet and calorie restricted WT and Sirt3−/− mice at 12 months of age (n = 4–5).
(D) OH (outer hair) cell survival (%) of basal, middle, and apical cochlear regions was measured from control diet and calorie restricted WT and Sirt3−/− mice at 12 months of age (n = 4–5).
(E) IH (inner hair) cell survival (%) of basal, middle, and apical cochlear regions was measured from control diet and calorie restricted WT and Sirt3−/− mice at 12 months of age (n = 4–5). Data are means ± SEM. See also Figure 1B–M.
Sirt3 Enhances the Mitochondrial Glutathione Antioxidant Defense System in Response to CR
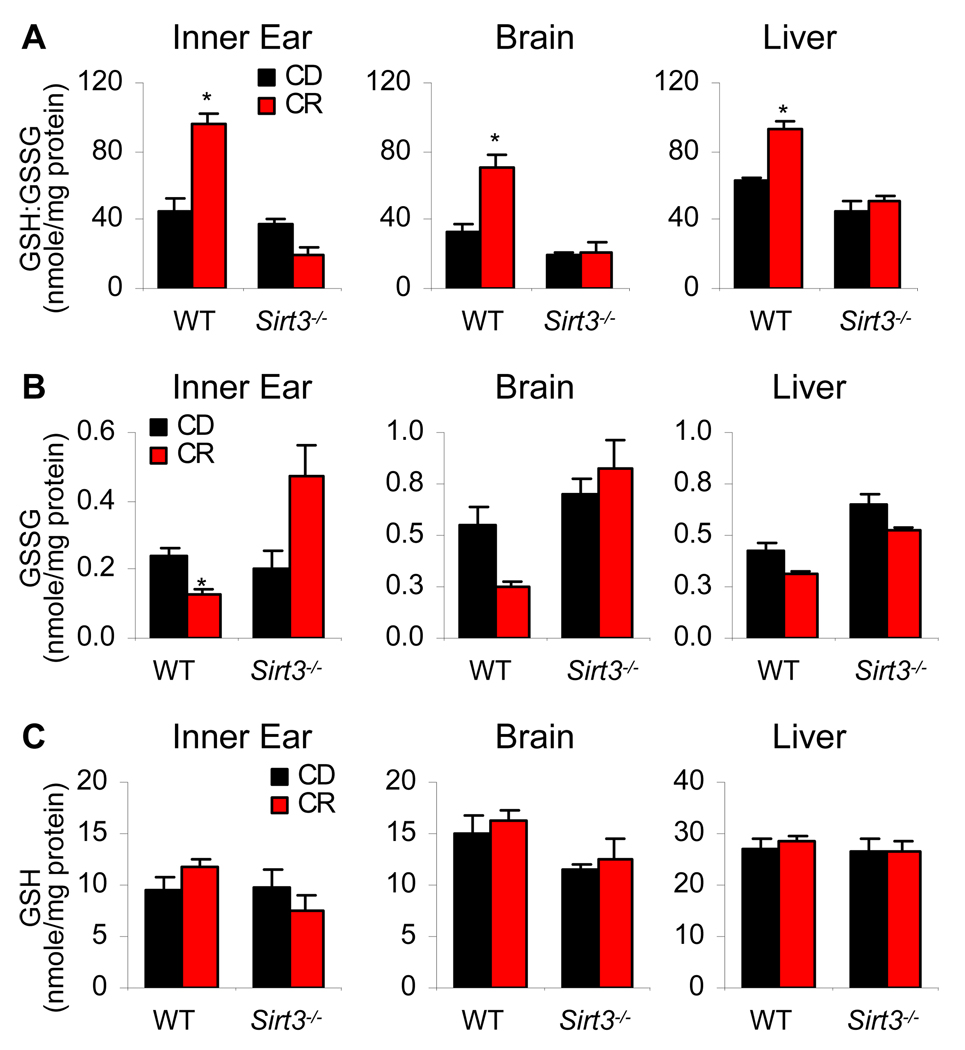
A previous study has shown that overexpression of Sirt3 increased mRNA expression of the antioxidant genes manganese superoxide dismutase (MnSOD) and catalase (Cat) in primary cardiomyocytes and that Sirt3−/− primary cardiomyocytes displayed higher levels of ROS compared to those of WT cells (Sundaresan et al., 2009), suggesting that Sirt3 may regulate the antioxidant systems. Glutathione acts as the major small molecule antioxidant in cells (Anderson, 1998; Halliwell et al., 2007; Mari et al., 2009; Rebrin et al., 2003) and NADPH-dependent glutathione reductase regenerates reduced glutathione (GSH) from oxidized glutathione (GSSG) (Anderson, 1998; Mari et al., 2009). In healthy mitochondria from young mice, glutathione is found mostly in the reduced form, GSH (Mari et al., 2009). During aging, oxidized glutathione accumulates, and hence an altered ratio of mitochondrial GSH to GSSG is thought to be a marker of both oxidative stress and aging (Rebrin et al., 2003; Schafer et al., 2001; Mari et al., 2009). Thus, we hypothesized that Sirt3 may regulate the mitochondrial glutathione antioxidant system under CR conditions. To test this hypothesis, we measured the ratio of GSH:GSSG in the mitochondria of the inner ear, brain, and liver of control diet and calorie restricted WT and Sirt3−/− mice at 5 months of age. Mitochondrial GSSG levels decreased during CR in the inner ear from WT mice, but not from Sirt3−/− mice (Figure 3B, See also Figure 3C). We also found that the ratios of GSH:GSSG in mitochondria increased during CR in all the tested WT tissues (Figure 3A); however, CR failed to increase the ratios of GSH:GSSG in Sirt3−/− tissues (Figure 3A). These results are consistent with the histological, cochlear cell counting, and oxidative DNA damage results which demonstrated that CR reduces oxidative damage in WT tissues, but not in the Sirt3−/− tissues. Thus, during CR, Sirt3 promotes a more reductive environment in mitochondria of multiple tissues, thereby enhancing the glutathione antioxidant defense system.
Figure 3. Sirt3 Increases the Ratios Of GSH:GSSG in Mitochondria During CR.
(A–C) Ratios of GSH:GSSG (A), GSSG (B), and GSH (C) were measured in the inner ear, brain (neocortex), and liver from control diet and calorie restricted WT and Sirt3−/− mice at 5 months of age (n = 4–5). *Significantly different from 12- or 5-month-old WT mice (P < 0.05). Data are means ± SEM.
Sirt3 Stimulates Idh2 Activity and Increases NADPH levels in Mitochondria in Response to CR
Enzymes of mitochondrial antioxidant pathways require NADPH to perform their reductive functions. NADP+-dependent Idh2 from mitochondria converts NADP+ to NADPH, thereby promoting regeneration of GSH by supplying NADPH to glutathione reductase (Jo et al., 2001). A previous in vitro study suggested that Idh2 might be a target of Sirt3, as incubation of Sirt3 with isocitrate dehydrogenase led to an apparent increase in dehydrogenase activity (Schlicker et al., 2008). Thus, we hypothesized that in response to CR, the mitochondrial deacetylase Sirt3 might directly deacetylate and activate Idh2, thereby regulating the levels of NADPH and consequently, the glutathione antioxidant defense system.
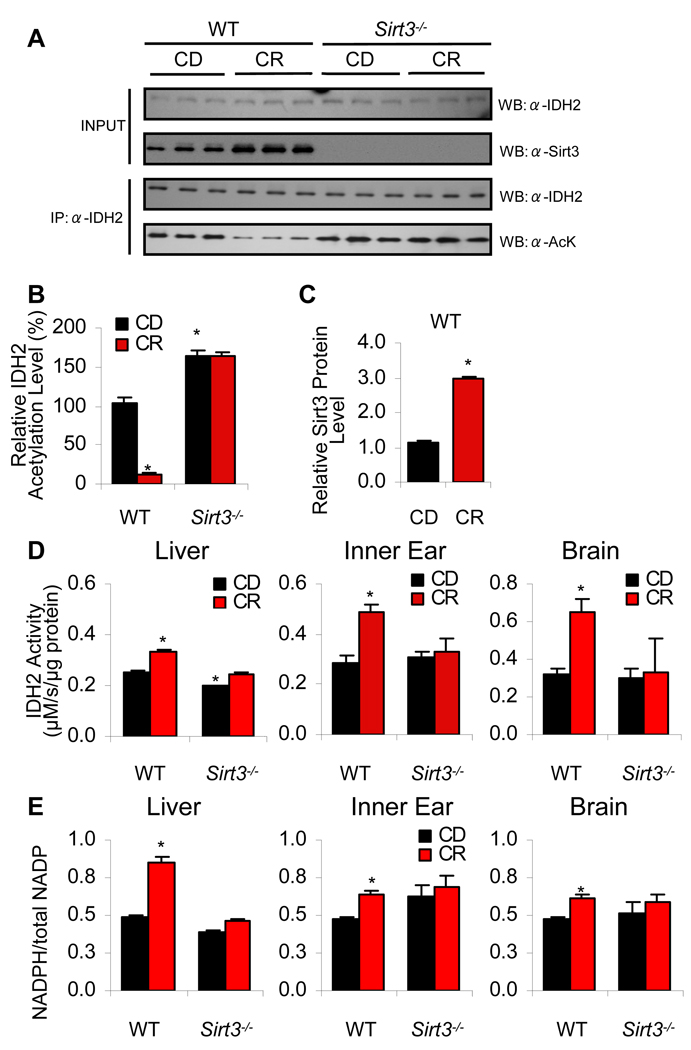
To provide initial support for the hypothesis that Sirt3 regulates Idh2 activity through deacetylation, we measured the acetylation levels of Idh2 in the liver mitochondria of WT and Sirt3−/− mice fed control and CR diets. In WT tissues, acetylation of Idh2 was substantial in the control diet fed tissues, but CR induced an eight-fold decrease in acetylation (Figure 4A and B). Robust acetylation of Idh2 was observed in Sirt3−/− mice from both control and CR diet fed conditions, indicating that Sirt3 is required for the CR-induced deacetylation of Idh2 (Figure 4A and B). As predicted, CR induced Sirt3 protein levels that were ~3-times higher than those observed with control diet tissues in WT mice (Figure 4C).
Figure 4. Sirt3 Increases Idh2 Activity and NADPH Levels in Mitochondria by Decreasing the Acetylation State of Idh2 During CR.
(A) Top panels: Western blot analysis of Sirt3 and Idh2 levels in the liver from 5-month-old WT or Sirt3−/− fed either control or calorie restricted diet. Lower panels: Endogenous acetylated Idh2 was isolated by immunoprecipitation with anti-Idh2 antibody followed by western blotting with anti-acetyl-lysine antibody (n = 3).
(B–C) Quantification of the amounts of total Idh2 acetylation (B) and Sirt3 protein (C) from (A). Western blot was normalized with Idh2 levels or Sirt3 levels quantified and analyzed by Image software (n = 3).
(D) Idh2 activities were measured in the liver, inner ear (cochlea), and brain (neocortex) from control diet and calorie restricted WT and Sirt3−/− mice at 5 months of age (n = 3–5).
(E) Ratios of NADPH:total NADP (NADP+ + NADPH) were measured in the liver, inner ear, and brain (neocortex) from control diet and caloric restricted WT and Sirt3−/− mice at 5 months of age (n = 3–5). *Significantly different from control diet fed WT mice (P < 0.05). Data are means ± SEM.
To establish whether Idh2 activity is stimulated by Sirt3 under CR conditions, we measured Idh2 activity in the mitochondria from the liver, inner ear, and brain of control diet and calorie restricted WT and Sirt3−/− mice. We found that Idh2 activity significantly increased during CR in all the WT tissues (Figure 4D); however, CR failed to increase Idh2 activity in the Sirt3−/− tissues (Figure 4D). If CR can induce a Sirt3-dependent increase in Idh2 activity, we anticipated increased levels of NADPH, providing the primary source of reducing equivalents for the glutathione antioxidant system (Jo et al., 2001; Schafer et al., 2001). To test this hypothesis, we measured NADPH levels in mitochondria of WT and Sirt3−/− mice. We found that levels of NADPH increased during CR in all tissues tested from WT mice (Figure 4E); however, no significant changes in NADPH levels were observed between control diet and CR Sirt3−/− tissues. Collectively, these results provide evidence that during CR, Sirt3 induces the deacetylation and activation of Idh2, leading to increased levels of NADPH in mitochondria of multiple tissues. We note that we observed a reduction in Idh2 activity in liver from Sirt3−/− mice fed the control diet, and that this correlates with a slightly increased level of acetylated Idh2 as compared to WT mice (Figure 4B). However, we did not observe reduced Idh2 activity or reduced NADPH levels in the inner ear or brain of Sirt3−/− mice. We postulate that under basal conditions (control diet fed), additional factors regulate mitochondrial Idh2 activity and NADPH levels.
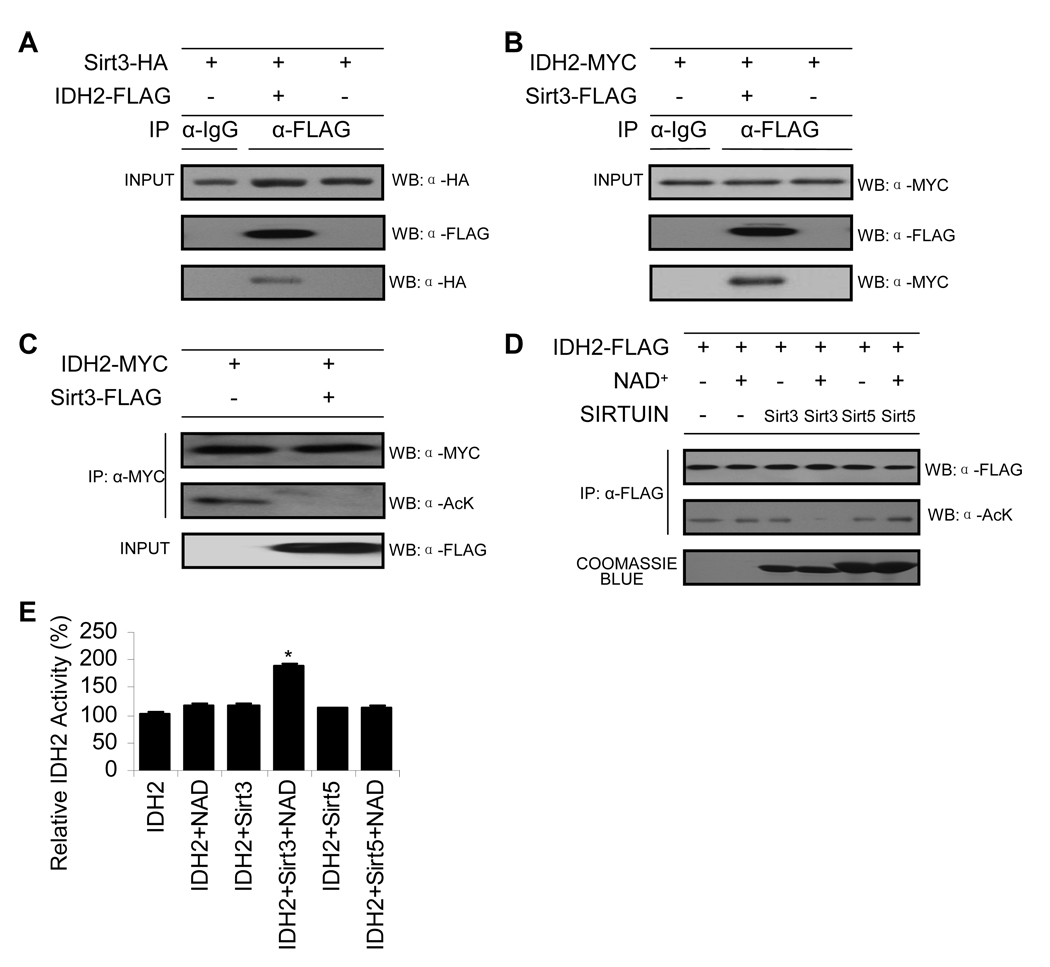
To provide direct evidence that Sirt3 deacetylates Idh2, a number of biochemical experiments were performed. Although most enzyme:substrate reactions are necessarily transient interactions to promote rapid turnover, co-immunoprecipitation (co-IP) experiments can sometimes trap these interactions. Co-IP experiments were performed in human kidney cells (HEK293) co-transfected with Sirt3 and Idh2. We found that precipitated Idh2-FLAG was able to co-IP Sirt3-HA (Figure 5A), while precipitated Sirt3-FLAG was able to co-IP Idh2-MYC (Figure 5B), suggesting that a physical interaction can occur between Sirt3 and Idh2 in human cells. However, co-IP experiments do not prove a direct functional interaction. To provide support for a functional interaction between Sirt3 and acetylated Idh2, deacetylation assays were carried out in HEK293 cells (Figure 5C) and in vitro using purified components (Figure 5D). Utilizing HEK293 cells, Idh2 was cotransfected with or without Sirt3, isolated by immunoprecipitation with anti-MYC antibody followed by western blotting with anti-acetyl-lysine antibody. Coexpression with Sirt3 induced the deacetylation of Idh2 to background levels (Figure 5C). For the in vitro analysis, acetylated Idh2 was prepared (see Figure S4 and Experimental Procedures) and utilized as a substrate for purified recombinant Sirt3 or Sirt5. Acetylation status was assessed by western blotting with anti-acetyl-lysine antibody (Figure 5D) and the resulting change in Idh2 activity was measured separately (Figure 5E). We found that Sirt3, but not Sirt5, deacetylated IDH2 in an NAD+-dependent fashion (Figure 5E). The corresponding Idh2 activity measurements indicated that deacetylation by Sirt3, but not Sirt5, stimulated Idh2 activity by ~100% (Figure 5E). Together, these data provide strong biochemical evidence that Sirt3 deacetylates and stimulates Idh2 activity and increases NADPH levels in mitochondria in response to CR.
Figure 5. Sirt3 Directly Deacetylates Idh2 and Stimulates Activity.
(A–B) Sirt3 interacts with Idh2. Idh2 or Sirt3 were immunoprecipitated from HEK293 cell lysates with IgG antibody or FLAG beads. Precipitated Idh2-FLAG was detected by anti-FLAG antibody and co-IP Sirt3-HA was detected by anti-HA as indicated (A). Precipitated Sirt3-FLAG was detected by anti-FLAG antibody and co-IP Idh2-MYC was detected by anti-MYC as indicated (B) (n = 3).
(C) Sirt3 deacetylates Idh2 in HEK293 cells. Idh2 was cotransfected with or without Sirt3, isolated by immunoprecipitation with anti-MYC antibody followed by western blotting with anti-acetyl-lysine antibody (n = 3).
(D) Sirt3, but not Sirt5, deacetylates Idh2 in vitro. Acetylated Idh2 was prepared as outlined in Experimental Procedure, and was incubated with purified recombinant Sirt3 or Sirt5 with or without NAD+ at 37 °C for 1 hour. Acetylation status was assessed by western blotting with anti-acetyl-lysine antibody (n = 3). An anti-FLAG western shows equivalent Idh2 protein levels were used and coomassie staining shows purified Sirt3 and Sirt5.
(E) In vitro deacetylation of Idh2 by Sirt3, but not Sirt5, stimulates Idh2 activity. Acetylated Idh2 in buffer (Tris (pH 7.5), with or without 1 mM NAD, and 1 mM DTT) was incubated with purified 50 nM Sirt3 or Sirt5 (Hallows et al., 2006) at 37°C for 1 hour, followed by Idh2 activity assay (n = 3). *Significantly different from Idh2 alone (P < 0.05). Data are means ± SEM. See also Figure S4.
Overexpression of Sirt3 and/or Idh2 Increases NADPH Levels and Protects Cells from Oxidative Stress-induced Cell Death
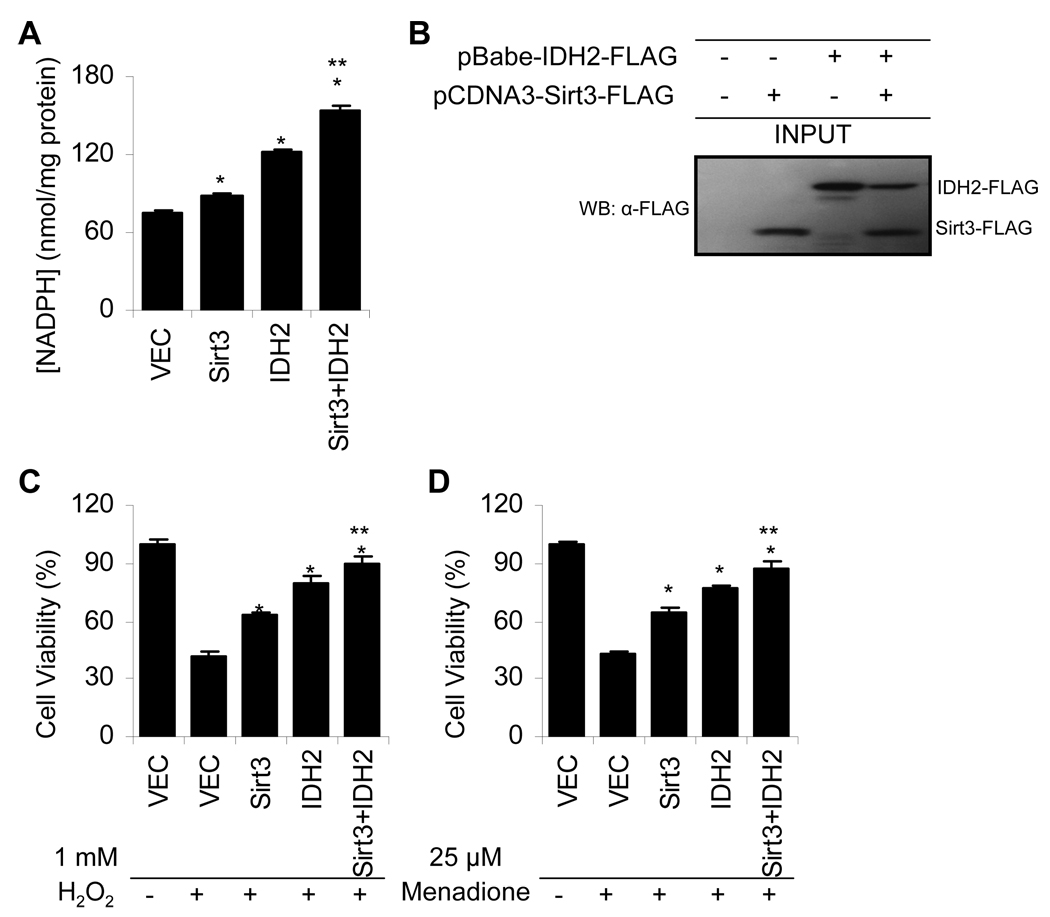
Our physiological, histological, and biochemical results indicate that Sirt3 mediates reduction of oxidative damage by deacetylation and stimulating the activity of Idh2, which increases NADPH levels for antioxidant systems in mitochondria during CR. To provide support for this mechanism, we investigated whether Sirt3 and Idh2 are sufficient to alter the NADPH levels in cultured cells. HEK-293 cells stably transfected with either vector, Sirt3, Idh2, or Sirt3 with Idh2 were generated and their NADPH levels were measured. NADPH levels were significantly increased when either Idh2 or Sirt3 or both proteins were stably overexpressed in HEK293 cells (Figure 6A–B). Importantly, overexpression of both Sirt3 and Idh2 yielded a greater increase in NADPH levels than either Sirt3 or Idh2 overexpressed alone (Figure 6A). Finally, to investigate whether overexpression of Sirt3, Idh2, or Sirt3 with Idh2 can protect cells from oxidative stress, the four HEK293 cell lines were treated with oxidants H2O2 (hydrogen peroxide)(Figure 6C) or menadione (Figure 6D), and cell viability was measured. Overexpression of Sirt3 or IDH2 was sufficient to protect cells from oxidative stress induced by both oxidants (Figure 6C–D). Again, overexpression of both Sirt3 and Idh2 led to higher cell viability than either Sirt3 or Idh2 overexpressed alone (Figure 6C–D). These results provide strong biochemical evidence that Sirt3 mediates reduction of oxidative stress by stimulating Idh2 activity and increasing NADPH levels under stress conditions.
Figure 6. Overexpression of Sirt3 and/or Idh2 Is Sufficient to Increase NADPH Levels and Protects HEK293 Cells from Oxidative Stress.
(A–B) NADPH concentrations were significantly increased when either Idh2 or Sirt3 or both were stably overexpressed in HEK293 cells. Measurements with errors are shown for the four different stable cell populations from each type of transfection (vector alone, Sirt3, Idh2 and Sirt3 with Idh2) (n = 3). *Significantly different from vector alone (P < 0.05), **Significantly different from Idh2 or Sirt3 (P < 0.05), (B) Western blotting confirms Idh2 and Sirt3 stable expression.
(C–D) Sirt3 and/or Idh2 overexpression is sufficient to protect HEK293 cells from the exogenous oxidants hydrogen peroxide (H2O2)(C) and menadione (D). The four different stable cells were transiently exposed to either 1 mM H2O2 or 25 µM menadione (n = 16). Data are means ± SEM.
DISCUSSION
Sirt3 Reduces Oxidative Damage and Enhances the Glutathione Antioxidant Defense System under CR Conditions
A widely accepted hypothesis of how aging leads to age-related hearing loss is through the accumulation of oxidative damage in the inner ear (Someya et al., 2010; Liu et al., 2007). In support of this hypothesis, oxidative protein damage increases in the cochlea of CBA/J mice (Jiang et al., 2007) and oxidative DNA damage increases in the cochlea of C57BL/6J mice during aging (Someya et al., 2009). Age-related hair cell loss is also enhanced in mice lacking the antioxidant enzyme superoxide dismutase 1 (McFadden et al., 1990), while the same mutant animals show enhanced susceptibility to noise-induced hearing loss (Ohlemiller et al., 1999). We have shown recently that overexpression of mitochondrially-targeted catalase delays the onset of AHL in C57BL/6J mice, reduces hair cell loss, and reduces oxidative DNA damage in the inner ear (Someya et al., 2009). Interestingly, overexpression of catalase in the mitochondria leads to extension of lifespan in C57BL/6J mice, but overexpression of catalase in the peroxisome or nucleus does not (Schriner et al., 2005). Under normal conditions, catalase decomposes hydrogen peroxide in the peroxisome, while in mitochondria, hydrogen peroxide is decomposed into water by glutathione peroxidase or peroxiredoxin (Finkel et al., 2000; Mari et al., 2009). Hence, these results suggest that mitochondrial ROS play a critical role in cochlear aging, AHL, and aging in general.
We have demonstrated that Sirt3 mediates the CR reduction of oxidative DNA damage in multiple tissues, and that these effects are likely to arise through an enhanced mitochondrial glutathione antioxidant defense system. As discussed earlier, the GSH:GSSG ratio is thought to be a marker of oxidative stress (Rebrin et al., 2008). Experimental evidence indicates that aging results in a decrease in the ratio of GSH:GSSG in the mitochondria of brain, liver, kidney, eye, heart, and testis from aged C57BL/6J mice due to elevated levels of GSSG, while CR decreases the ratio of GSH:GSSG in the mitochondria of these tissues by lowering GSSG levels (Rebrin et al., 2003; Rebrin et al., 2007). Our findings demonstrate that CR increases these ratios of GSH:GSSG in the mitochondria of brain, liver, and inner ear from WT mice, but fails to increase the ratios in the same tissues from Sirt3−/− mice. Consistent with these results, CR reduced oxidative DNA damage in tissues from WT mice, but failed to reduce such damage in tissues from Sirt3−/− mice. CR also increased spiral ganglion neuron and hair cell survival in the WT cochlea, but not in Sirt3−/− mice. Tissues that are composed of postmitotic cells such as the brain and the inner ear are particularly vulnerable to oxidative damage because of their high energy requirements, and inability to undergo regeneration. Therefore, we speculate that the Sirt3-mediated modulation of the glutathione antioxidant defense system may play a central role in reduction of oxidative stress in multiple tissues under CR conditions, leading to aging retardation. We also note that other mitochondrial effects of Sirt3 such as regulation of fatty acid oxidation (Hirschey et al., 2010) and modulation of Complex I activity (Ahn, et al., 2008) are likely to contribute to the metabolic adaptations in response to CR.
Idh2 Regulates the Redox State of Mitochondria under CR Conditions
A large body of evidence indicates that the antioxidant defense systems do not keep pace with the age-related increase in ROS production, and thus the balance between antioxidant defenses and ROS production shifts progressively toward a more pro-oxidant state during aging (Sohal et al., 1996; Rebrin et al., 2008). This balance is determined in part by the ratios of interconvertible forms of redox couples, such as GSH/GSSG, NADPH/NADP+, NADH/NAD+, thioredoxinred/thioredoxinoxid, and glutaredoxinred/glutaredoxinoxid. The GSH/GSSH couple is thought to be the primary cellular determinant of the cellular redox state, since its abundance is 3 to 4 orders of magnitude higher than the other redox couples (Rebrin et al., 2008). NADPH is the reducing equivalent required for the regeneration of GSH and the GSH-mediated antioxidant defense system, which includes glutathione peroxidases, glutathione transferases, and glutathione reductase, playing a critical role in oxidative stress resistance (Halliwell et al., 2007). GSH is synthesized in the cytosol and transported into the mitochondria through protein channels in the outer mitochondrial membrane (Halliwell et al., 2007; Anderson, 1998). Although GSH can cross the outer mitochondrial membrane through these channels, GSSG cannot be exported into the cytosol (Olafsdottir et al., 1988). Thus, GSSG is reduced to GSH by mitochondrial NADPH-dependent glutathione reductase, preventing accumulation of GSSG in the mitochondrial matrix (Schafer et al., 2001; Mari et al., 2009). We have demonstrated that Sirt3 directly deacetylates and activates Idh2 under CR conditions. In response to CR, deacetylated Idh2 displays increased catalytic activity, which is correlated with increased NADPH levels in the mitochondria of multiple tissues from WT mice, but not from Sirt3−/− mice. Hence, we speculate that Idh2 may be a major player in regulating the redox state of mitochondria under CR conditions given its role in mitochondrial NADPH production. A previous study has shown that Idh2 is induced in response to ROS in mouse fibroblasts, while decreased levels of Idh2 leads to higher ROS and accumulation of oxidative damage to DNA and lipids (Jo et al., 2001). Our in vitro findings demonstrate that overexpression of Sirt3 and/or Idh2 increases NADPH levels and protects cells from oxidative stress-induced cell death. Thus, these observations underlie a critical role for Idh2 in the generation of NADPH in mitochondria under conditions of CR, providing reducing capacity for the glutathione antioxidant system, and increasing oxidative stress resistance.
A Role for Sirt3 in CR-mediated Prevention of AHL
The mouse is considered a good model for the study of human AHL since the mouse cochlea is anatomically similar to that of humans (Steel et al., 1996; Steel et al., 1983). Most inbred mouse strains display some degree of AHL, and the age of onset of AHL is known to vary from 3 months in DBA/2J mice to over 20 months in CBA/CaJ mice (Zheng et al., 1999). The C57BL/6J mouse strain, which is the most widely used mouse model for the study of aging, displays the classic pattern of AHL by 12 to 15 months of age (Hunter and Willott, 1987; Keithley et al., 2004). We have previously shown that AHL in C57BL/6J mice occurs through Bak-mediated apoptosis, and that it can be prevented by the intake of small molecule antioxidants (Someya et al., 2009). We note that C57BL/6J and many other mouse strains carry a specific mutation (Cdh23753A) in the Cdh23 gene, which encodes a component of the hair-cell tip link, and this mutation is known to promote early onset of AHL in these animals (Noben-Trauth et al., 2003). Interestingly, the Cdh23753A allele may increase the susceptibility to oxidative stress in hair cells, since a Sod1 mutation greatly enhaces AHL in mice carrying Cdh23753A, but not in mice wild-type for Cdh23 (Johnson, et al., 2010). However, oxidative damage increases with age in the cochlea of both C57BL/6J mice and the CBA/J mouse strain that does not carry the Cdh23753A allele, indicating that oxidative stress plays a role in AHL independent of Cdh23 (Someya et al., 2009; Jiang et al., 2007; Zheng et al., 1999). In both strains, the loss of hair cells and spiral ganglion neurons begins in the base of the cochlea and spreads towards the apex with age (Keithley et al., 2004; Hunter et al., 1987). Importantly, CR slows the progression of AHL in both C57BL/6J and CBA/J strains (Someya et al., 2007; Sweet et al., 1988). Therefore, the protective effects of Sirt3 in AHL are likely to be of general relevance to AHL.
It is thought that some of the effects of CR in aging retardation require significant reduction of body weight through reducing food consumption. In agreement with this hypothesis, obesity promotes a variety of age-related diseases, such as cardiovascular disease, diabetes, high blood pressure, hypertension, and certain cancers (Paeratakul et al., 2002; Poirier et al., 2006). Obesity is also associated with an increased risk of mortality (Poirier et al., 2006; Lee et al., 1993). Interestingly, CR failed to reduce oxidative damage in multiple tissues and to slow the progression of AHL in CR Sirt3−/− mice, despite the fact that these mice were lean (Figure S2A and B). Thus, these results suggest that weight loss may not be sufficient for the anti-aging action of CR. Instead, we postulate that critical metabolic effectors such as Sirt3 mediate the positive effects of CR.
Concluding Remarks
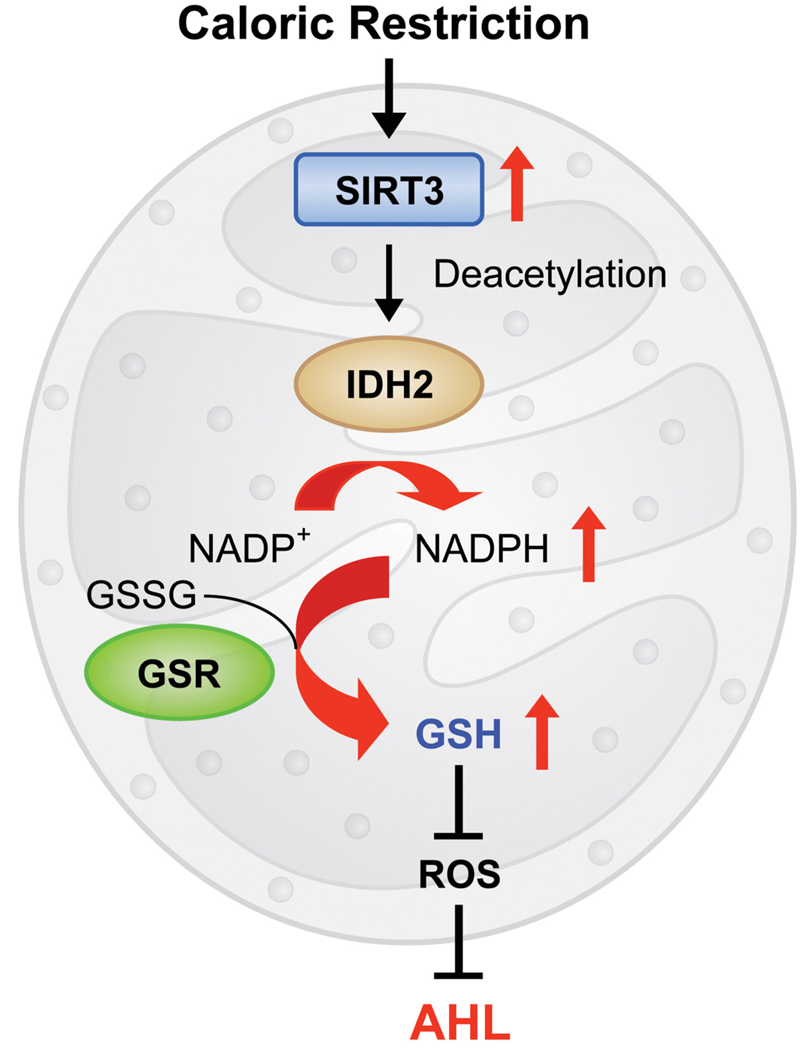
In summary, we propose that in response to CR, Sirt3 activates Idh2, thereby increasing NADPH levels in mitochondria. This in turn leads to increased ratios of GSH:GSSG in mitochondria and decreased levels of ROS, resulting in protection of inner ear cells and prevention of AHL in mammals (Figure 7). Because we observed similar effects of CR in the mitochondrial GSH/GSSG ratios in multiple tissues, we postulate that this may be a major mechanism of aging retardation by CR. We also postulate that pharmaceutical interventions that induce Sirt3 activity in multiple tissues will mimic CR by increasing oxidative stress resistance and preventing the mitochondrial decay associated with aging.
Figure 7. A Model for the CR-mediated Prevention of AHL in Mammals.
In response to CR, SIRT3 activates Idh2, thereby increasing NADPH levels in mitochondria. This in turn leads to an increased ratio of GSH:GSSG and decreased levels of ROS, thereby resulting in protection from oxidative stress and prevention of AHL in mammals.
EXPERIMENTAL PROCEDURES
Animals
Male and female Sirt3+/− mice were purchased from the Mutant Mouse Resource Centers (MMRRC) at the University of North Carolina-Chapel Hill (Chapel Hill, NC). Briefly, these mice were created by generating embryonic stem (ES) cells (Omni bank no. OST341297) bearing a retroviral promoter trap that functionally inactivates one allele of the Sirt3 gene (MGI, 2010). Male and female C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Sirt3+/− mice have been backcrossed for 4 generations onto the C57BL/6J background. All animal studies were conducted at the AAALAC-approved Animal Facility in the Genetics and Biotechnology Center of the University of Wisconsin-Madison. Experiments were performed in accordance with protocols approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (Madison, WI).
Dietary Study
Details on the methods used to house and feed mice have been described previously (Pugh et al., 1999). Mice are housed individually. Control diet (CD) groups were fed 86.4 kcal/week of the precision pellet diet AIN-93M (BioServ, Frenchtown, NJ), while caloric restricted (CR) groups were fed 64.8 kcal/week (a 25% CR) of the precision pellet diet AIN-93M 40%DR (BioServ, Frenchtown, NJ). The schedule of feeding for control diet was 7 grams on Mondays and Wednesdays, and 10 grams on Fridays, while the schedule of feeding for calorie restricted diets was 5 grams on Mondays and Wednesdays, and 8 grams on Fridays. This dietary regimen was maintained from 2 month of age until 5 months of age for a 3-month CR study and from 2 month of age until 12 months of age for a 10-month CR study.
ABR Hearing Test
At 12 months of age, ABRs were measured with a tone burst stimulus at 8, 16, and 32 kHz using an ABR recording system (Intelligent Hearing System, Miami, FL) as previously described (Someya et al., 2009). Mice were anesthetized with a mixture of xylazine hydrochloride (10 mg/kg, i.m.) (Phoenix Urology of St. Joseph, St. Joseph, MO) and ketamine hydrochloride (40 mg/kg, i.m.) (Phoenix Urology of St. Joseph).
Measurement of DNA Oxidation Levels
At 12 months of age, cochlea and neocortex were collected, and DNA was extracted with ethanol precipitation. DNA concentrations for each sample were adjusted to 0.1 µg/ml, and numbers of apurinic/apyrimidinic (AP) sites were determined using the DNA Damage Quantification Kit (Dojindo, Rockville, MD) and performed according to the manufacturer’s instructions and as previously described (Kubo et al., 1992; Meira, et al., 2009; McNeill et al., 2007). Liver was also collected from the same mice, and 8-hydroxyguanosine levels (8-oxo-7,8-2’-deoxyguanosine/106 deoxyguanosine) in the DNA were determined using a HPLC-ECD method as previously described (Hofer et al., 2006).
Measurement of Total GSH and GSSG
Just after mitochondrial lysate preparation, 100 µl of the lysate was mixed with 100 µl of 10% metaphosphoric acid, incubated for 30 min at 4°C, and centrifuged at 14,000 g for 10 min at 4°C. The supernatant was used for the measurements of mitochondrial glutathione contents. Total glutathione (GSH + GSSG) and GSSG levels were determined by the method of Rahman et al. (Rahman et al., 2006). All samples were run in duplicate. The rates of 2-nitro-5-thiobenzoic acid formation were calculated and the total glutathione (tGSH) and the GSSG concentrations in the samples were determined by using linear regression to calculate the values obtained from the standard curve. The GSH concentration was determined by subtracting the GSSG concentration from the tGSH concentration.
Idh2 Acetylation Analysis
Antibodies used for western blotting included anti-Idh2 antibody (Santa Cruz, Santa Cruz, CA), anti-Sirt3 antibody (gift of Dr. Eric Verdin, UCSF), protein A/G plus agrose (Santa Cruz, Santa Cruz, CA), pan-acetylated lysine (generated following the procedure of Zhao, et al., 2010, GeneTel Laboratories LLC, Madison, WI). For immunoprecipitation, liver mitochondria lysates were incubated with anti-Idh2 antibody overnight at 4°C. Then protein A/G plus agarose were added and incubated for 3 hours. After resins were washed, samples were boiled with SDS loading buffer and subjected to western blotting (Smith et al., 2009).
Idh2 Activity
Activities of Idh2 were measured by the Kornberg method (Kornberg, 1955). Briefly, 20 µl of the mitochondrial lysate sample was added in each well of a 96-well plate and then 180 µl of a reaction mixture (33 mM KH2PO4•K2HPO4, 3.3 mM MgCl2, 167 µM NADP+, 167 µM (+)-potassium Ds-threo-isocitrate monobasic) was added in each well. The absorbance was immediately read at 340 nm every 10 sec for 1 min in a microplate reader (Bio-Rad, Hercules, CA). All samples were run in duplicate. The reaction rates were calculated and the Idh2 activity in the sample was defined as the production of one µmole of NADPH per sec.
In vitro deacetylation assay
Idh2-FLAG was transfected into HEK293 cells, which were then treated with 5 mM nicotinamide for 16 hours. Nicotinamide is a widely used sirtuin inhibitor. Nicotinamide treatment leads to increased acetylation of Idh2, with a corresponding decrease in enzymatic activity (Fig. S4). Idh2 from cell lysates was immunoprecipitated with anti-FLAG beads at 4 °C for 2 hours, then Idh2-FLAG on beads was utilized in 200 ul deacetylation buffer (Tris (pH 7.5), with or without 1 mM NAD, and 1 mM DTT) and incubated with purified 50 nM Sirt3 or Sirt5 (Hallows et al., 2006) at 37°C for 1 hour. Aliquots were removed for Idh2 activity assay and western blotting with anti-FLAG antibody or anti-acetyl-lysine antibody.
Statistical analysis
All Statistical analyses were carried out by one-way ANOVA with post-Tukey multiple comparison tests using the Prism 4.0 statistical analysis program (GraphPad, San Diego, CA). All tests were two-sided with statistical significance set at P < 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S. Kinoshita for histological processing. This research was supported by NIH grants AG021905 (T.A.P.) and GM065386 (J.M.D), the National Projects on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science, and Technologies of Japan, and Marine Bio Foundation.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes Supplemental Experimental Procedures, four figures, and one table can be found at http://www.cell.com/xxxxxxxxx.
REFERENCES
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barger JL, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes. Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol. Chem. 2006;387:103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear Res. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol. Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SH, et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Yu H, Ding D, Jiang H, Gagnon LH, Salvi RJ. Separate and combined effects of Sod1 and Cdh23 mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. Hear Res. 2010;268:85–92. doi: 10.1016/j.heares.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear. Res. 2004;188:21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A. Isocitric dehydrogenase of yeast (TPN) Method in Enzymology. 1955;1:705–707. [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Kubo K, Ide H, Wallace SS, Kow YW. A novel, sensitive, and specific assay for abasic sites, the most commonly produced DNA lesion. Biochemistry. 1992;31:3703–3708. doi: 10.1021/bi00129a020. [DOI] [PubMed] [Google Scholar]
- Lee IM, Manson JE, Hennekens CH, Paffenbarger RS., Jr Body weight and mortality. A 27-year follow-up of middle-aged men. JAMA. 1993;270:2823–2828. doi: 10.1001/jama.270.23.2823. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox. Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Exp. Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell. Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McNeill DR, Wilson DM., III A dominant-negative form of the major human abasic endonuclease enhances cellular sensitivity to laboratory and clinical DNA-damaging agents. Mol Cancer Res. 2007;5:61–70. doi: 10.1158/1541-7786.MCR-06-0329. [DOI] [PubMed] [Google Scholar]
- Meira LB, Moroski-Erkul CA, Green SL, Calvo JA, Bronson RT, Shah D, Samson LD. Aag-initiated base excision repair drives alkylation-induced retinal degeneration in mice. Proc Natl Acad Sci U S A. 2009;106:888–893. doi: 10.1073/pnas.0807030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MGI. Sirt3Gt(OST341297)Lex. 2010 Available at http://www.informatics.jax.org/searches/accession_report.cgi?id=MGI:3529767. [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Flood DG, Reaume AG, Hoffman EK, Scott RW, Wright JS, Putcha GV, Salvi RJ. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol. 1999;4:237–246. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- Olafsdottir K, Reed DJ. Retention of oxidized glutathione by isolated rat liver mitochondria during hydroperoxide treatment. Biochim Biophys Acta. 1988;964:377–382. doi: 10.1016/0304-4165(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord. 2002;26:1205–1210. doi: 10.1038/sj.ijo.0802026. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiolo. Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–18. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free. Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Schriner SE, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, Giallourakis C, Comb MJ, Alt FW, Lombard DB. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604–606. doi: 10.1111/j.1474-9726.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BC, Hallows WC, Denu JM. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal. Biochem. 2009;394:101–109. doi: 10.1016/j.ab.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol. Aging. 2007;28:1613–1622. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Someya S, et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. U S A. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev. 2010 doi: 10.1016/j.mad.2010.04.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel KP, Bock GR. Hereditary inner-ear abnormalities in animals. Relationships with human abnormalities. Arch Otolaryngol. 1983;109:22–29. doi: 10.1001/archotol.1983.00800150026005. [DOI] [PubMed] [Google Scholar]
- Steel KP, Moorjani P, Bock GR. Mixed conductive and sensorineural hearing loss in LP/J mice. Hear Res. 1996;28:227–236. doi: 10.1016/0378-5955(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol. Cell. Biol. 2008;28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RJ, Price JM, Henry KR. Dietary restriction and presbyacusis: periods of restriction and auditory threshold losses in the CBA/J mouse. Audiology. 1988;27:305–312. doi: 10.3109/00206098809081601. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C Thomas Publishing, LTD; 1988. [Google Scholar]
- Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Guo Q, Mattson MP. Dietary restriction protects hippocampal neurons against the death-promoting action of a presenilin-1 mutation. Brain Res. 1999;842:224–229. doi: 10.1016/s0006-8993(99)01827-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.