Abstract
In polygynous insect species, male reproductive success is directly related to lifetime mating success. However, the costs for males of sexual activities such as courting, signaling, and mating are largely unknown. We studied the cost of sexual activities in male Mediterranean fruit flies, Ceratitis capitata (Tephritidae), a polygynous lekking species, by keeping cohorts of individual male flies under relaxed crowding conditions in the laboratory. We used 5 cohorts among which individuals differed in their opportunities to interact with con-specifics and recorded life span, and in one treatment, mating rate. We found that males kept singly lived more than twice as long as males that interacted intensively with mature virgin females, while male-male interactions caused a smaller reduction in longevity. Because longevity of males that could court but not mate was not significantly different from those that could court and mate, we conclude that courting (not mating) was responsible for the observed longevity reduction. Moreover, we detected high variability in male mating success, when 5 virgin females were offered daily. In contrast to the cohort level, individual males that mated at a high rate lived relatively long, thus indicating heterogeneity in quality or sexual strategy among males.
Keywords: Cost of mating, Cost of sexual courting, sexual signaling, Ceratitis capitata, Tephritidae
1. Introduction
Cost of reproduction, defined as reduction in survival or future fecundity as a result of reproduction (Prowse and Partridge, 1997), significantly affects life history evolution including key traits such as survival and age-specific fecundity schedules in animals that reproduce sexually (Maynard Smith, 1958, Partridge and Farquhar, 1983; Partridge et al. 1985). The cost of sexual activities can be partitioned into extrinsic - behavioral and ecological (predation risk, allocation of time and energy to sexual activities; Martin and Hosken, 2004), and intrinsic (physiological; Sakaluk et al., 2004).
There are numerous studies demonstrating cost of reproduction in females (e. e. Bell and Koufopanou, 1986; Chapman et al., 1998; Harshman and Zera, 2006; Langley and Clutton-Brock, 1998; Mangan, 1997; Martin and Hosken, 2004; Molleman et al., 2008; Müller et al., 2002; Sivinski 1993), but fewer in males, and even fewer attempt to quantify the cost of different male sexual activities (Burton-Chellew et al., 2007; Cordts and Partridge, 1996; Gems and Riddle, 1996; Kotiaho and Simmons, 2003; Martin and Hosken, 2004; Perez-Staples and Aluja, 2006). Theory and empirical evidences suggest that sexual signaling, intensive courting, and ejaculate production all confer costs in polygynous species (Byrne and Rice, 2006, and references there in). Recent life time behavioral studies suggest that sexual signaling has no obvious effects on male Mediterranean fruit fly (medfly) lifespan (Papadopoulos et al., 2004; Zhang et al., 2006). However, whether mating or courting are negatively correlated with male longevity is not known. A cost of copulation has been demonstrated recently for males of several insect species (Burton-Chellew et al., 2007; Kotiaho and Simmons, 2003; Martin and Hosken, 2004; McNamara et al., 2008; Simmons and Kotiaho, 2007). For male medflies the relationship between lifetime mating success and life span has not been investigated. However, in another, male lekking, polygynous tephritid the number of copulations was not correlated with life span within individuals (Perez-Staples and Aluja, 2006). The latter study did not include a control cohort that did not interact with females as in our study.
To separate cost of courtship from costs of more general interaction with other individuals we also need to know the costs of non-sexual interactions. It is often assumed that male–male antagonistic interactions confer major longevity reductions compared with male–female interactions. Male medflies are believed to defend territories and exhibit intensive aggressive behavior against intruders (Arita and Kaneshiro, 1989; Prokopy and Hendrichs, 1979). An experiment with high densities of medflies in cages suggested that male – male interactions are costly to male medflies, while male – female interactions do not confer additional costs (Gaskin et al., 2002).
Over the last two decades the Mediterranean fruit fly (medfly; Ceratitis capitata) has become an important model system in demographic, aging, and genetic research (i.e. Carey and Vaupel, 2003; Loukeris et al., 1995; Malacrida et al., 2007). The sexual behavior of medfly has been studied in great detail (see Aluja and Norrbom, 1999). Reproductive maturity is attained within 5 days for most laboratory strains and considerably longer (7 – 13 days) in wild flies (Papadopoulos et al., 1998; Liedo et al., 2002). Males are polygynous, and form leks (Yuval and Hendrichs, 1999). It is well documented (in field cages and laboratory studies) that a small proportion of males account for most of the matings (Shelly, 2000). Females are monoandrous or oligoandrous (Bonizzoni et al., 2006; Bonizzoni et al., 2002; Kraaijeveld et al., 2005). Within leks, males perform sexual signaling while releasing a sex pheromone to attract virgin, mature females (Prokopy and Hendrichs, 1979). Upon female arrival males initiate a complex courtship behavior, and females select their mates based on male courtship performance. Successful copulation that leads to sperm transfer lasts on average 2 – 3 hours depending on environmental conditions and the medfly biotype (Papadopoulos unpublished data). Nonetheless, most of the data regarding medfly sexual behavior were obtained from studies focusing on relatively young individuals, and the association between sexual signaling and life span has only recently been determined (Papadopoulos et al., 2004; Zhang et al., 2006).
The aims of this study were better understanding of (a) the cost of reproduction in male medflies, and (b) the division of cost of reproduction into sexual courting and male sexual signaling, male–male interaction (contest and others), and mating per se. We specifically tested the following hypotheses: (1) Intensive sexual activities reduce the life span of male medflies; (2) Sexual courting and mating are both costly for male medflies; and (3) male-male interactions are more costly than male-female interactions.
2. Methods
2.1. Experimental conditions and insects
The flies were obtained from the Moscamed mass-rearing facility in Metapa, Mexico. Those flies used in our experiments had been reared in the mass-rearing facility for approximately 25 generations. Experiments and observations were conducted under laboratory conditions (25 ± 2 °C, 65% r.h. and 12:12 L:D, lights turned on at 06:00) in autumn and winter 2002 – 2003. Light in the experimental room was provided by day light tubes, and the light intensity in the experimental arena ranged from 1,000 to 1,500 lux. Adults were sexed upon emergence, maintained in groups of 100 individuals, and offered a diet consisting of sugar and yeast hydrolysate in a ratio of 3:1 respectively.
2.2. Cost of reproduction
Under laboratory conditions cost of reproduction in male medflies may arise because of intrasexual male-male agonistic interaction, sexual signaling, courting, and mating per se. To test whether (and if so which) male reproductive activities incur a life span cost, one day old males were randomly assigned to one of the following five treatments: (a) no mating—males engaged in sexual signaling only; individually kept males (one male per cage) having no access to females; (b) intensive mating—one male offered five mature virgin females each day until death (one male plus five females per cage). Thus number of matings/day/male could range from 0 to 5; (c) no mating, courting—one male was kept with five females that could not mate (one male plus five females per cage); females were mature, mated, and had their ovipositor sealed to preclude any chance of mating; Female ovipositor was cauterized with hot forceps; (d) no mating, male–male interactions—males kept in groups of six per cage; (e) moderate mating—three males offered three virgin females daily (3 males plus 3 females per cage). There were 60, 60, 60, 15, and 25 cages (replicates) for treatments a, b, c, d, and e respectively, i.e. 60, 60, 60, 90, and 75 males per treatment respectively. Thus number of matings/day/male could range from 0 to 3. We used clear plastic, cubic cages of 2 liters capacity for all treatments. The density of flies within each cage was rather relaxed (1–6 individuals in 2 liters [10 by 20 cm base by 10 cm height] volume) and kept constant by replacing dead flies with similarly treated ones. Replacement flies were marked with a color dot on the thorax to be distinguished from the males of the experimental cohort. A perforated 100 cm2, mesh-covered window, served for ventilation and access to the cage. Food (yeast hydrolysate and sugar, 1: 4 respectively) and water were provided ad libitum throughout the experiment. The number of matings was recorded in treatment b (intensive mating). Observers monitored the cages every 15 minutes, from 08:00 to 17:00, to record mating pairs. To be able to distinguish different matings of the same male within a day (assuming that each female would not mate more than once on a day), virgin females were marked with a within-cage unique color dot on the thorax. Earlier studies have demonstrated that such a marking has no effect on both male and female sexual behavior (McInnis et al. 2002; Shelly and McInnis 2003). At the end of each day females were removed from cages. New females were place into experimental cages at 07:00 next day. Within the experimental room there were several males and females held in cages located in close distance to the experimental arena. Neither sexual signaling nor sexual courting was systematically recorded.
2.3. Statistical Methods
Statistical analyses included Cox model and Log – rank test to detect differences in survival among the above treatments, time – dependent Cox proportional hazard model to detect possible associations between number of matings and life span in treatment (b) intensive mating, and Kaplan Meier estimates of age-specific survival rates (Collett, 2003; Sokal and Rohlf, 1995). Hazard functions for each treatment were obtained by smoothing following the procedure described in Müller et al. (1997) and Wang et al. (1998).
3. Results
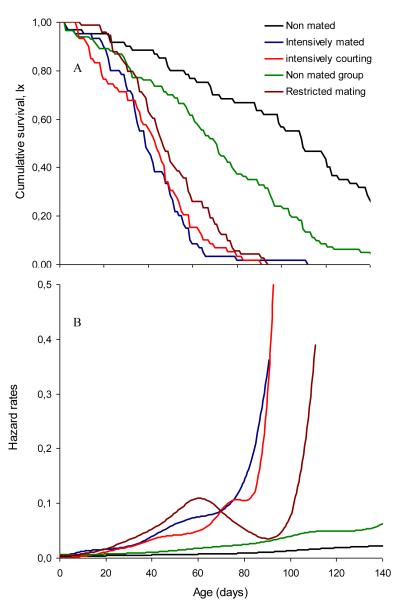
Average and record life spans for each of the cohorts tested are given in Table 1. Males maintained individually lived on average of 2.2 to 2.7 times longer than males exposed to females, and 1.6 times longer than males kept in groups of six. Longevity of males kept in groups of six was 1.7 times longer than that of males exposed daily to five virgin females. The 25% longest lived males that were kept individually (no mating) lived an average of nearly 5 months (i.e. 140 days), while the 25% longest lived males that had the opportunity to mate intensively lived an average of less than 2 months (i.e. 51 days). Average and maximum longevities were positively correlated (r = 0.95, P < 0.05). Our results clearly demonstrate that sexual activities incur a significant cost in terms of reduced life span for male medflies (Log rank test, Chi-square = 145.0; df = 4; P < 0.0001) (Fig. 1, Table 1). Age-specific mortality rates were low in non-mated males up to day 100 and then gradually increased. A similar pattern was observed in the mortality of males kept in groups (Fig. 1B). Mortality of males that interacted with females was higher and increased dramatically much earlier than males that did not interact with females (Fig. 1B). Intensively mated and courting males suffered approximately 9 times higher hazard rate compared with non-mated individually kept males (Table 2). Moderately mated males exhibit increased hazard rates by 6.5 times compared with non-mated males, and intensive male – male interactions increased hazard rates by only 2.6 times. There was no evidence for significant differences in longevity between the two treatments where individual males interacted with females (either only courting or both courting and mating; see Table 2).
Table 1.
Longevity of the males related to their reproductive activity
| Treatment of males | N | Average life span (days ± SE) |
Percentile (day ± SE) |
Max (days) |
||
|---|---|---|---|---|---|---|
| 25% | 50% | 75% | ||||
| Non mated | 60 | 106.9 ± 7.1a | 140 ± 11.5 | 109 ± 10.9 | 61 ± 10.6 | 201 |
| Intensive mating | 60 | 39.8 ± 2.4d | 51 ± 2.4 | 38 ± 3.2 | 29 ± 5.7 | 111 |
| Intensive courting | 59 | 40.4 ± 2.7d | 54 ± 3.2 | 43 ± 2.9 | 21 ± 5.6 | 90 |
| Non mated - group | 82 | 69.9 ± 4.2b | 96 ± 4.7 | 70 ± 4.6 | 41 ± 7.5 | 120 |
| Restricted mating | 74 | 48.6 ± 2.3c | 64 ± 4.5 | 46 ± 2.6 | 35 ± 3.4 | 9 |
Averages followed by different letters are significantly differed (pair-wise comparison, log-rank test; P < 0.05)
Figure 1.
Survivorship (A) and hazard rate (B) curves for different categories of male medflies.
Table 2.
Variables of the Cox proportional hazards model for the five male categories. Non mated males formed the baseline.
| Source of variation | B | Exp(B) (CL) | Z |
|---|---|---|---|
| Male category | |||
| Intensive mating | 2.246 ± 0.224 | 9.45 (6.10 – 14.65) | 10.04*** |
| Intensive courting | 2.175 ± 0.226 | 8.80 (5.65 – 13.69) | 9.64*** |
| Non mated - group | 0.952 ± 0.188 | 2.59 (1.79 – 3.74) | 5.07*** |
| Restricted mating | 1.872 ± 0.216 | 6.50 (4.26 – 9.93) | 8.68*** |
p < 0.001.
To identify association between the number of matings per day and life span for individual males we fitted a Cox-proportional hazard model to treatment b (intensive mating) with both successful mating and not successful mating attempts (lasting 15 minutes or less) as time dependent predictors. Successful matings were found to be a significant predictor for the hazard rates (coefficient = − 1.23; Z = −4.75; P = 0.000002), while unsuccessful mating attempts were not a significant predictor (coefficient = −0.28, Z = −0.8; P = 0.39). Fitting a Cox-proportional hazard model with only successful matings as a predictor yielded similar results (coefficient = −1.25, Z = −4.69; P = 0.000002). The negative coefficient indicates a positive association between mating and life span. i.e. flies that mate at a high rate live longer.
4. Discussion
Our results clearly demonstrate that sexual activities dramatically reduce life span in male medflies. Below we will discuss candidate components of sexual activities that could be responsible for this finding.
4.1 Male-female interactions versus mating
In all three treatments where males were confined with females, longevity was reduced by 55 to 70% in comparison to males kept individually. A significant longevity cost of reproduction of exposure to females has been recently reported for Onthophagus binodis (Coleoptera) males (Kotiaho and Simmons, 2003; Simmons and Kotiaho, 2007). Because sexual courting and mating are inextricably linked in medflies we were unable to separate mating from courtship. However, we were able to let males court without mating (by caging one male with five females that were not able to mate), and we assume that the difference between the cost of courting and mating versus cost of courting alone, is the cost of mating. Because there was no such difference, our results demonstrate that courtship, not mating, confers a significant cost to males. Nevertheless, in the wild, survival may be reduced during copulation when pairs are less mobile because of predation (Hendrichs et al., 1991; Hendrichs et al., 2007). On the other hand, comparison of survival rates between males kept in groups of 6 and males kept with either virgin or not able to mate females revealed that male interaction with females, and not crowding was responsible for reducing survival.
That cost of male sexual activity is concentrated in courtship rather than mating per se is in agreement with studies in Drosophilidae and Tephritidae (Cordts and Partridge, 1996; Perez-Staples and Aluja, 2006). In contrast, Martin and Hosken (2004) demonstrated a cost of copulation for males of the seaweed fly Saltella sphondylli (Sepsidae): the mating behavior of this species does not include pre-copulation courtship, and therefore, the cost of copulation could be disentangled from that of pre-copulatory courtship in this system.
Our results showed a negative relationship between lifetime male mating success (details will be treated elsewhere) and hazard rate. Thus, long lived males can carry the cost of intensive mating. Intensive sexual activities and a long life span can be found in males bearing “good genes” or those experiencing “good conditions” (Hunt et al., 2005; Jennions et al., 2001; Kokko, 1998; Kotiaho et al., 1999). Although flies in our experiment enjoyed similar larval conditions our data cannot distinguish between “good genes” or the “conditions dependent” hypotheses. Apart from heterogeneity in ‘quality’, males may also differ in sexual strategy, where some males invest highly in obtaining mates, but have small ejaculates, while others expend less on obtaining mates but obtain more offspring with those they mate with by transferring more sperm or accessory gland products (Del Castillo and Gwynne, 2007).
Production of sperm and accessory fluids might be costly for males (Prowse and Partridge, 1997; Tregenza and Wedell, 2002), and ejaculate management, in polygynous species, might comprise a major determinant of male fitness. In other insect species, such as butterflies (Ferkau and Fischer, 2006), males suffer a significant reduction of ejaculate size in successive matings (Rogers et al., 2005). Males may be constrained in the amount of sperm that they produce and therefore, fewer sperm might be transferred to females in consecutive matings. Therefore, males may suffer a fertility cost of reproduction (Prowse and Partridge, 1997; Simmons and Kotiaho, 2007). However, males of Anastrepha obliqua balance sperm transfer in successive matings within the same day (Perez-Staples and Aluja, 2006). Whether medfly males suffer sperm and/or accessory fluid depletion and whether they can regulate sperm transfer in successive matings, under intensive mating throughout life time, remains to be addressed.
4.2 Interactions between individuals (male-male interactions)
Intra-sexual contest, aggressive interactions, and male-male interactions are common among medfly males in crowded laboratory conditions and are believed to incur a major cost, while male-female interactions, under intense crowding conditions, add no further cost to males (Gaskin et al., 2002). However, our results suggest that in relaxed laboratory holding conditions (1 – 6 individuals per 2 litter cage capacity) male – female interactions cause a dramatic reduction in male longevity compared with that of male – male interactions. Male – female interactions (comparing treatments with same density of individuals within cages; b, c, and d above) reduced male life span by approximately 45% compared with that of males kept in groups of the same size. Differences in the experimental set up, in particular the size of the cages, provisioning of a paper ‘leaf’ for lekking and intensity of crowding, may account for the discrepancy between our findings and that of Gaskin et al. (2002).
Besides direct sexual or agonistic encounters among individuals in flies held in groups that may affect longevity, social interactions may increase food consumption and elicit effects upon the endocrine system of the fly, and the metabolic regulation of food consumed (Zur et al., 2009). Increased protein intake, because of social interaction, has been suggested to account for reducing adult longevity (Lee at al., 2008). Management of food resources and longevity seems to be inextricably linked. Individually caged males can regulate food intake and slowly consume part of their reserves entering a “waiting mode” of reproduction that may increase longevity (Zur et al., 2009). This physiological mechanism may also account for differences in longevity in solitarily kept males compared with males kept in groups with other individuals (either males or females) that we found in the current study.
The development and mass-rearing of genetic sexing strains resulted in the new concept of “filter” or “mother colony management”. Following this method of mass rearing, the mother colony is maintained in small numbers, of low adult density per cage, and relaxed semi-natural conditions (Fisher and Caceres, 2001; Franz, 2005). Therefore, recombinants can be easily detected and discarded, and selection for unfavorable behavioral traits can be avoided. Our results suggest that male biased sex ratio under relaxed rearing conditions would not affect male longevity; however, it might increase male sexual competitiveness directly affecting the quality of the produced flies.
In conclusion male medflies experience an extreme cost of reproduction arising mainly from courtship. While on the cohort level, intensive lifetime interaction with mature virgin females is negatively correlated with life span, on the individual level long-lived flies mate at high frequencies, thus indicating extensive heterogeneity among males. Male-male interactions only bear a small cost under particular non-crowded conditions.
Acknowledgments
We thank Ying Zhang for assistance with data analysis, Boaz Yuval (The Hebrew University, Israel) for comments on an earlier draft of this paper, and Leslie Sandberg for editorial assistance. Suggestions and comments of two anonymous reviewers substantially improved this paper. This study was supported by the National Institute on Aging through grants P01-AG022500-01, and P01-AG08761-10.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluja M, Norrbom AL, editors. Fruit flies (Tephritidae): phylogeny and evolution of behavior. Taylor and Francis CRC Press; London: 1999. [Google Scholar]
- Arita LH, Kaneshiro KY. Sexual selection and lek behavior in Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae) Pacific Science. 1989;43:135–143. [Google Scholar]
- Bell G, Koufopanou V. The cost of reproduction. In: Dawkins R, Ridley M, editors. Oxford Surveys in Evolutionary Biology. vol 3. Oxford University Press; Oxford: 1986. pp. 83–131. [Google Scholar]
- Bonizzoni M, Gomulski LM, Mossinson S, Guglielmino CR, Malacrida AR, Yuval B, Gasperi G. Is polyandry a common event among wild populations of the pest Ceratitis capitata? Journal of Economic Entomology. 2006;99:1420–1429. doi: 10.1603/0022-0493-99.4.1420. [DOI] [PubMed] [Google Scholar]
- Bonizzoni M, Katsoyannos BI, Marguerie R, Guglielmino CR, Gasperi G, Malacrida A, Chapman T. Microsatellite analysis reveals remating by wild Mediterranean fruit fly females, Ceratitis capitata. Molecular Ecology. 2002;11:1915–1921. doi: 10.1046/j.1365-294x.2002.01602.x. [DOI] [PubMed] [Google Scholar]
- Burton-Chellew MN, Sykes EM, Patterson S, Shuker DM, West SA. The cost of mating and the relationship between body size and fitness in males of the parasitoid wasp Nasonia vitripennis. Evolutionary Ecology Research. 2007;9:921–934. [Google Scholar]
- Byrne PG, Rice WR. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proceedings of the Royal Society B-Biological Sciences. 2006;273:917–922. doi: 10.1098/rspb.2005.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Vaupel JW. Biodemopgraphy. In: Delamater J, editor. Handbook of the Social Phychology. Kluwer Academic/Plenum Publisher; New York: 2003. pp. 625–658. [Google Scholar]
- Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D. Modelling survival data in medical research. 2nd edn Taylor & Francis CRC Press; London: 2003. [Google Scholar]
- Cordts R, Partridge L. Courtship reduces longevity of male Drosophila melanogaster. Animal Behaviour. 1996;52:269–278. [Google Scholar]
- Del Castillo RC, Gwynne DT. Increase in song frequency decreases spermatophore size: correlative evidence of a macroevolutionary trade-off in katydids (Orthoptera: Tettigoniidae) Journal of Evolutionary Biology. 2007;20:1028–1036. doi: 10.1111/j.1420-9101.2006.01298.x. [DOI] [PubMed] [Google Scholar]
- Ferkau C, Fischer K. Costs of reproduction in male Bicyclus anynana and Pieris napi butterflies: Effects of mating history and food limitation. Ethology. 2006;112:1117–1127. [Google Scholar]
- Fisher K, Caceres C. A filter rearing system for mass reared genetic sexing strains of Mediterranean fruit fly (Diptera: Tephritidae). In: Tan K-H, editor. Area-wide Control of Fruit Flies and Other Insect Pests; Joint Proceedings of the International Conference on Area-wide Control of Insect Pests and of the Fifth International Symposium on Fruit Flies of Economic Importance; Penang, Malaysia. 1-5 June 1998; Penang: Penerbit Universiti Sains Malaysia; 2000. pp. 543–550. [Google Scholar]
- Franz G. Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing for the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; Amsterdam, the Netherlands: 2005. pp. 427–451. [Google Scholar]
- Gaskin T, Futerman P, Chapman T. Increased density and male-male interactions reduce male longevity in the medfly, Ceratitis capitata. Animal Behaviour. 2002;63:121–129. [Google Scholar]
- Gems D, Riddle DL. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature. 1996;379:723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Zera AJ. The cost of reproduction: The devil in the details. Trends in Ecology and Evolution. 2006;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hendrichs J, Katsoyannos BI, Papaj DR, Prokopy RJ. Sex differences in movement between natural feeding and mating sites and tradeoffs between food consumption, mating success and predator evasion in Mediterranean fruit flies (Diptera: Tephritidae) Oecologia. 1991;86:223–231. doi: 10.1007/BF00317534. [DOI] [PubMed] [Google Scholar]
- Hendrichs M, Wornoayporn W, Katsoyannos B, Hendrichs J. Quality control method to measure predation evasion in wild and mass-rared medflies. Florida Entomologist. 2007;90:64–70. [Google Scholar]
- Hunt J, Brooks R, Jennions MD. Female mate choice as a condition-dependent life-history trait. American Naturalist. 2005;166:79–92. doi: 10.1086/430672. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Moller AP, Petrie M. Sexually selected traits and adult survival: A meta-analysis. Quarterly Review of Biology. 2001;76:3–36. doi: 10.1086/393743. [DOI] [PubMed] [Google Scholar]
- Kokko H. Good genes, old age and life-history trade-offs. Evolutionary Ecology. 1998;12:739–750. [Google Scholar]
- Kotiaho JS, Alatalo RV, Mappes J, Parri S. Sexual signalling and viability in a wolf spider (Hygrolycosa rubrofasciata): measurements under laboratory and field conditions. Behavioral Ecology and Sociobiology. 1999;46:123–128. [Google Scholar]
- Kotiaho JS, Simmons LW. Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. Journal of Insect Physiology. 2003;49:817–822. doi: 10.1016/S0022-1910(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld K, Katsoyannos BI, Stavrinides M, Kouloussis NA, Chapman T. Remating in wild females of the Mediterranean fruit fly, Ceratitis capitata. Animal Behaviour. 2005;69:771–776. [Google Scholar]
- Langley PA, Clutton-Brock TH. Does reproductive investment change with age in tsetse flies, Glossina morsitans morsitans (Diptera: Glossinidae)? Functional Ecology. 1998;12:866–870. [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proceedings of the National Academy of Sciences. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedo P, De Leon E, Barrios MI, Valle-Mora JF, Ibarra G. Effect of age on the mating propensity of the Mediterranean fruit fly (Diptera: Tephritidae) Florida Entomologist. 2002;85:94–101. [Google Scholar]
- Loukeris TG, Livadaras I, Arca B, Zabalou S, Savakis C. Gene-Transfer Into the Medfly, Ceratitis capitata, With a Drosophila-Hydei Transposable Element. Science. 1995;270:2002–2005. doi: 10.1126/science.270.5244.2002. [DOI] [PubMed] [Google Scholar]
- Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, Gugliclmino CR. Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica. 2007;131:1–9. doi: 10.1007/s10709-006-9117-2. [DOI] [PubMed] [Google Scholar]
- Mangan RL. Effects of strain and access to males on female longevity, lifetime oviposition rate, and egg fertility of the Mexican fruit fly (Diptera: Tephritidae) Journal of Economic Entomology. 1997;90:945–954. [Google Scholar]
- Martin OY, Hosken DJ. Copulation reduces male but not female longevity in Saltella sphondylli (Diptera : Sepsidae) Journal of Evolutionary Biology. 2004;17:357–362. doi: 10.1046/j.1420-9101.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. The Theory of Evolution. Penguin Books; London: 1958. [Google Scholar]
- McInnis DO, Rendon P, Komatsu J. Mating and remating of medflies (Diptera : Tephritidae) in Guatemala: Individual fly marking in field cages. Florida Entomologist. 2002;85:126–137. [Google Scholar]
- McNamara KB, Elgar MA, Jones TM. A longevity cost of re-mating but no benefits of polyandry in the almond moth, Cadra cautella. Behavioral Ecology and Sociobiology. 2008;62:1433–1440. [Google Scholar]
- Molleman F, Ding J, Wang J-L, Brakefield PM, Carey JR, Zwaan BJ. Amino acid sources in the adult diet do not affect life span and fecundity in the fruit-feeding butterfly Bicyclus anynana. Ecological Entomology. 2008;33:429–438. doi: 10.1111/j.1365-2311.2008.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Chiou JM, Carey JR, Wang JL. Fertility and life span: late children enhance female longevity. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2002;57:B202–B206. doi: 10.1093/gerona/57.5.b202. [DOI] [PubMed] [Google Scholar]
- Müller HG, Wang JL, Capra WB. From life tables to hazard rates: the transformation approach. Biometrika. 1997;84:881–892. [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Carey JR, Muller HG, Zhang Y. High sexual signalling rates of young individuals predict extended life span in male Mediterranean fruit flies. Oecologia. 2004;138:127–134. doi: 10.1007/s00442-003-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Economopoulos AP, Carrey JR. Effect of adult age, food, and time of day on sexual calling incidence of wild and mass-reared Ceratitis capitata males. Entomologia Experimentalis et Applicata. 1998;89:175–182. [Google Scholar]
- Partridge L, Farquhar M. Lifetime mating success of male fruitflies (Drosophila melanogaster) is related to their size. Animal Behaviour. 1983;31:871–877. [Google Scholar]
- Partridge L, Mackay TFC, Aitken S. Male Mating Success and Fertility in Drosophila-Melanogaster. Genetical Research. 1985;46:279–285. [Google Scholar]
- Perez-Staples D, Aluja M. Sperm allocation and cost of mating in a tropical tephritid fruit fly. Journal of Insect Physiology. 2006;52:839–845. doi: 10.1016/j.jinsphys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Prokopy RJ, Hendrichs J. Mating behavior of Ceratitis capitata (Diptera, Tephritidae) on a field caged host tree. Annals of the Entomological Society of America. 1979;72:642–648. [Google Scholar]
- Prowse N, Partridge L. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. Journal of Insect Physiology. 1997;43:501–512. doi: 10.1016/s0022-1910(97)00014-0. [DOI] [PubMed] [Google Scholar]
- Rogers DW, Baker RH, Chapman T, Denniff M, Pomiankowski A, Fowler K. Direct and correlated responses to artificial selection on male mating frequency in the stalk-eyed fly Cyrtodiopsis dalmanni. Journal of Evolutionary Biology. 2005;18:642–650. doi: 10.1111/j.1420-9101.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- Sakaluk SK, Campbell MTH, Clark AP, Chadwick-Johnson J, Keorpes PA. Hemolymph loss during nuptial feeding constrains male mating success in sagebrush crickets. Behavioral Ecology. 2004;15:845–849. [Google Scholar]
- Shelly TE. Male signalling and lek attractiveness in the Mediterranean fruit fly. Animal Behaviour. 2000;60:245–251. doi: 10.1006/anbe.2000.1470. [DOI] [PubMed] [Google Scholar]
- Shelly TE, McInnis DO. Influence of adult diet on the mating success and survival of male Mediterranean fruit flies (Diptera: Tephritidae) from two mass - rearing strains on field-caged host trees. Florida Entomologist. 2003;86:340–344. [Google Scholar]
- Simmons LW, Kotiaho JS. The effects of reproduction on courtship, fertility and longevity within and between alternative male mating tactics of the horned beetle, Onthophagus binodis. Journal of Evolutionary Biology. 2007;20:488–495. doi: 10.1111/j.1420-9101.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- Sivinski JM. Longevity and fecundity in the Caribbean fruit fly (Diptera: Tephritidae) Effects of mating, strain and body size. Florida Entomologist. 1993;76:635–644. [Google Scholar]
- Sokal RR, Rohlf EJ. Biometry. 3rd edn Freedman; New York: 1995. [Google Scholar]
- Tregenza T, Wedell N. Polyandrous females avoid cost of inbreeding. Nature. 2002;415:71–73. doi: 10.1038/415071a. [DOI] [PubMed] [Google Scholar]
- Yuval B, Hendrichs J. Behavior of flies in the genus Ceratitis (Dacinae: Ceratitidini) In: Aluja M, Norrbom A, editors. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior. CRC Press; Boca Raton, Florida, USA: 1999. pp. 429–457. [Google Scholar]
- Wang J-L, Müller HG, Capra WB. Analysis of oldestold mortality: lifetables revisited. Annals of Statistics. 1998;26:126–163. [Google Scholar]
- Zhang Y, Muller HG, Carey JR, Papadopoulos NT. Behavioral trajectories as predictors in event history analysis: Male calling behavior forecasts medfly longevity. Mechanisms of Ageing and Development. 2006;127:680–686. doi: 10.1016/j.mad.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur T, Nemny-Lave E, Papadopoulos NT, Nestel D. Social interactions regulate resource utilization in a Tephritid fruit fly. Journal of Insect Physiology. 2009;55:890–897. doi: 10.1016/j.jinsphys.2009.05.013. [DOI] [PubMed] [Google Scholar]