Abstract
Background
The timing of onset of the rise in incidence of esophageal adenocarcinoma (EAC) has not been clearly defined, and doing so may provide clues with regard to exposures associated with the changed epidemiology of this malignancy. We therefore aimed to investigate historical trends in the incidence of EAC and other upper gastrointestinal malignancies.
Methods
We performed a population-based study using Connecticut Tumor Registry (1940–2007) and Surveillance, Epidemiology, and End Results (SEER) (1973–2007) data. Age-adjusted incidence rates (per 100,000 person-years) were calculated for esophageal adenocarcinoma and other upper gastrointestinal malignancies.
Results
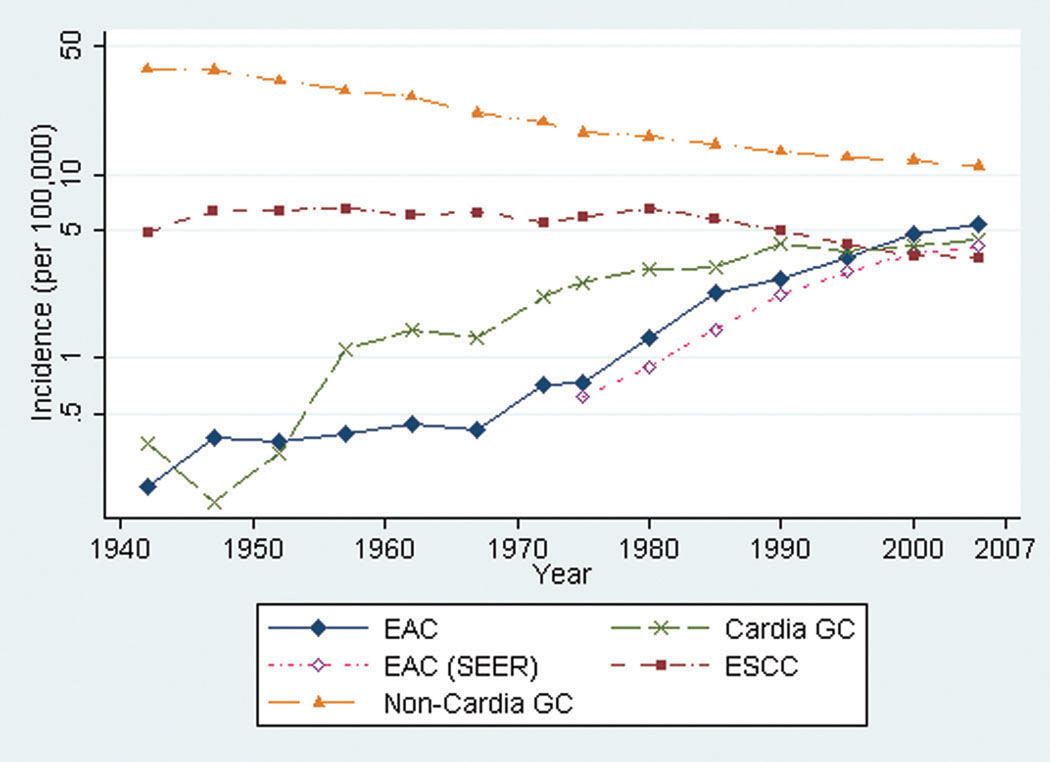
The incidence of EAC remained relatively constant until 1965–69, and then rose from 0.41 (95%CI, 0.26–0.56) to 1.31 (95%CI 1.07–1.54) in 1978–82 and 5.31 (95%CI 4.89–5.73) in 2003–07. The incidence of gastric cardia cancer began to rise in the 1950s and plateaued in the 1990s. The incidence of esophageal squamous cell carcinoma began to decrease around 1980. The trends from Connecticut Tumor Registry data closely mirrored those from SEER data.
Conclusions
The incidence of EAC began to rise in the late 1960s, predating the rise in obesity by a decade. Reduced infection rates of Helicobacter pylori, changes in microbiome, or other exposures may have contributed to the changed epidemiology of this malignancy.
Impact
Analysis of historical data of trends in EAC incidence implicate a change in environmental factors from the mid-20th century as primarily responsible for the initial rise in EAC incidence, predating the rise in obesity prevalence in the United States by over a decade.
Keywords: esophageal adenocarcinoma, epidemiology, incidence, historical trends, population-based research
INTRODUCTION
There has been a widely recognized dramatic rise in the incidence of esophageal adenocarcinoma over the past three decades. In particular, this increase has been demonstrated using data from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute, which found a roughly five-fold increase in incidence of esophageal adenocarcinoma (EAC) in the United States from 1975–2005 (1). This increase was paralleled by a similar rise in the incidence of gastric cardia adenocarcinoma, but the rise in gastric cardia cancer plateaued around 1990 (2). However, there is scant published data on EAC incidence in the U.S. from the pre-SEER era (3).
Factors precipitating this dramatic rise are incompletely understood, although a corresponding rise in the prevalence of obesity is frequently cited. Obesity increases the risk of EAC via increased gastro-esophageal reflux disease (GERD) and potentially through other mechanisms as well (4). Other risk factors for EAC have been identified, including a protective effect of Helicobacter pylori (5–6). The prevalence of H. pylori infection in the United States has decreased substantially over the past several decades and is presumably responsible in part for the marked decline in the incidence of non-cardia gastric cancer. It is unknown whether other historical exposures have played a role in the changed epidemiology of EAC. We therefore decided to investigate trends in the incidence of EAC before and after SEER reporting began in order to determine the timing of the initial rise in incidence of EAC.
METHODS
The Connecticut Tumor Registry is the oldest population-based tumor registry in the United States, with data collection dating back to 1935. Description of the Tumor Registry data have been published previously (7). The case registry was >75% complete in 1940–44 and considered nearly complete (>97%) by 1968–72 (8). Data were obtained with regard to number of cases of EAC, esophageal squamous cell cancer (ESCC), gastric cardia, and gastric non-cardia cancers. Starting in the 1970s, the Connecticut Tumor Registry submitted case data to SEER. Five-year summary data were available from 1935–39 through 1975–79 and from 1973–77 through 2003–07. In order to minimize overlap, we excluded the 1975–79 data from analyses. Presumably, the first five years of the registry (1935–39) had the least complete reporting, and this period was also excluded from analyses.
Age-adjusted incidence rates were calculated using corresponding five-year summary statistics for the population 25 and older, broken down by sex and age group. Population data were provided by the Connecticut Tumor Registry and downloaded from the SEER website (9). The proportion of esophageal cancer cases with histologic confirmation was available for every year of the study period, and ranged from 45% (1940–44) to 97% (2003–07). In order to account for variable rates of histologic confirmation, “corrected” age-adjusted incidence rates for EAC and ESCC were calculated. The “corrected” rate was obtained by dividing the calculated age-adjusted incidence by the proportion of cases with histologic confirmation for the associated five-year time period.
Age-adjusted incidence rates for EAC for the population 25 and older were also calculated using SEER data from 1973–2007 (10). These data encompassed nine state and regional registries, representing approximately 10% of the U.S. population. EAC cases were identified using International Classification of Diseases for Oncology (ICD-O-3) topography codes C15.0–C15.9 for esophageal cancer, and histology codes 8140–8573 for adenocarcinoma. Age-adjusted rates were calculated based on the 2000 U.S. standard. Analyses were performed using SEER*Stat 6.6.2.
RESULTS
The incidence of EAC remained relatively constant from 1940–44 through 1965–69 (Figure 1). The corrected age-adjusted incidence then steadily increased from 0.41 cases per 100,000 person-years (95%CI, 0.26–0.56) in 1965–69 to 1.31 per 100,000 person-years (95%CI 1.07–1.54) in 1978–82. The incidence continued to rise, with 5.31 cases per 100,000 person-years (95%CI 4.89–5.73) in 2003–07. The patterns of incidence for males and females were similar, with both rates beginning to rise in the late 1960s. The male:female ratio for age-adjusted incidence ranged from 3–5:1 and did not change appreciably over time. The uncorrected incidence rates for EAC, ESCC, and esophageal cancer without histologic confirmation are shown in Table 1.
Figure 1.
Age-adjusted incidence of esophageal adenocarcinoma (EAC), esophageal squamous cell cancer (ESCC), gastric cardia cancer (Cardia GC), and gastric non-cardia cancer (Non-Cardia GC), Connecticut 1940–2007. Age-adjusted incidence of EAC from SEER data also shown (EAC (SEER)). (note: y-axis scale is logarithmic)
Table 1.
Percentage of esophageal cancer cases with histologic confirmation as well as uncorrected age-adjusted incidence (per 100,000) of EAC, ESCC, and esophageal cancer without histologic confirmation, Connecticut Tumor Registry, 1940–2007.
| Time period | % histologic confirmation for EC |
Incidence of EC without histologic confirmation |
Uncorrected EAC incidence |
Uncorrected ESCC incidence |
|---|---|---|---|---|
| 1940–1944 | 45% | 3.34 | 0.09 | 2.19 |
| 1945–1949 | 54% | 3.33 | 0.20 | 3.44 |
| 1950–1954 | 66% | 2.64 | 0.23 | 4.22 |
| 1955–1959 | 76% | 1.88 | 0.29 | 5.00 |
| 1960–1964 | 82% | 1.31 | 0.35 | 4.90 |
| 1965–1969 | 88% | 0.95 | 0.36 | 5.49 |
| 1970–1974 | 90% | 0.70 | 0.64 | 4.99 |
| 1973–1977 | 92% | 0.59 | 0.67 | 5.55 |
| 1978–1982 | 94% | 0.46 | 1.23 | 6.07 |
| 1983–1987 | 95% | 0.40 | 2.15 | 5.45 |
| 1988–1992 | 97% | 0.31 | 2.59 | 4.78 |
| 1993–1997 | 97% | 0.27 | 3.39 | 4.04 |
| 1998–2002 | 97% | 0.17 | 4.64 | 3.51 |
| 2003–2007 | 97% | 0.20 | 5.15 | 3.42 |
EC, esophageal cancer; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell cancer
The pattern of incidence of EAC based on SEER data closely mirrored the results from the Connecticut Tumor Registry (Figure 1). The incidence of gastric cardia cancer began to rise approximately a decade earlier than EAC, and has plateaued since the mid-1990s. The incidence of ESCC was relatively constant until the mid-1980s, and has been declining since this time. As expected, the incidence of non-cardia gastric cancer has steadily decreased since the early 1950s. The age-adjusted incidence curves using SEER data from 1973–2007 for ESCC, cardia cancer, and non-cardia gastric cancer all closely paralleled the corresponding curves generated from Connecticut Tumor Registry data from the same time period.
DISCUSSION
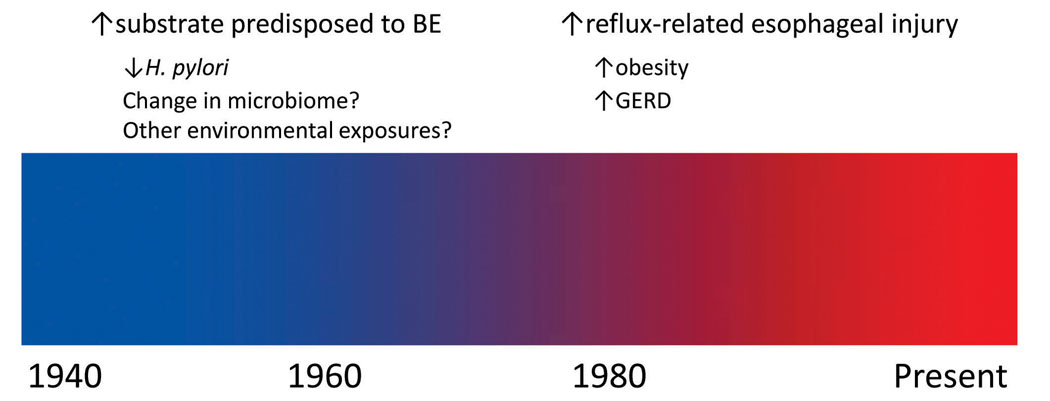
These data from the Connecticut Tumor Registry suggest that the rise in incidence in EAC began in the late 1960s. This trend is consistent with two prior reports from Sweden and the United Kingdom (11–12). The data from the present study suggest that environmental exposures prior to the late 1960s may have contributed to a subsequent rise in EAC incidence (Figure 2). According to results from the National Health and Nutrition Examination Survey (NHANES), a marked increase in the prevalence of obesity (defined as body mass index (BMI) ≥30) in the United States was not observed until the period between NHANES II (1976–80) and NHANES III (1988–94) (13). Interestingly, there was no marked change in the prevalence of overweight individuals (25.0≤BMI≤29.9) from 1960 through 1994 (14). Therefore, obesity does not appear to play a major role in the initial 3- to 4-fold rise in incidence of EAC.
Figure 2.
Schematic of potential historical factors responsible for the observed rise in incidence of esophageal adenocarcinoma. (BE, Barrett’s esophagus; GERD, gastro-esophageal reflux disease)
Barrett’s esophagus (BE) is the primary precursor lesion for the development of EAC, and a rise in BE incidence would likely have preceded a rise in EAC incidence. While difficult to estimate precisely, the average time for progression to EAC (among those BE patients who ultimately progress) is approximately 5 years (15). One would expect an additional lag time between a new or altered exposure and a rise in the incidence of BE, which may have begun in the 1950s.
Infection with H. pylori is associated with a moderately decreased risk of EAC (5–6), and infection rates with H. pylori have been decreasing since the middle part of the 20th century (16–17). H. pylori infection appears to be associated with reduced odds of Barrett’s esophagus (18). Data are inconsistent with regard to a potential inverse association between H. pylori and other complications of GERD such as erosive esophagitis (18–20). A recent meta-analysis demonstrated that there is no association between eradication of H. pylori and subsequent risk of GERD symptoms or erosive esophagitis (21), suggestive that H. pylori may impact risk of EAC at the level of development of BE. It is unclear whether H. pylori itself protects against the development of EAC, or whether the absence of H. pylori reflects an overall change in the microbiome of the upper gastrointestinal tract (22), which in turn increases the risk of EAC.
Cigarette smoking is an established risk factor for EAC (23), and the prevalence of smoking rose markedly during the first half of the 20th century (24). However, it is unlikely that smoking was a major contributor to the initial rise in incidence of EAC. Cigarette smoking is an established risk factor for numerous GI cancers, yet similar historical patterns in incidence are not observed for other malignancies such as non-cardia gastric cancer and esophageal squamous cell cancer.
Gastric cardia cancer and EAC share many risk factors. The rise in incidence of gastric cardia cancer preceded the rise in EAC by a decade; the incidence of gastric cardia cancer plateaued roughly two decades ago, and perhaps a similar plateau will be observed for EAC incidence in the near future. However, comparisons with gastric cardia cancer are not straightforward, as adjustment for improved subsite classification over time suggests a much earlier plateau (25).
While the data from the present study are from a single state, the incidence curves for EAC, ESCC, and cardia and non-cardia gastric cancer mirror the overall SEER incidence data. Underdiagnosis of esophageal cancer in the pre-endoscopy era could result in an exaggerated increase in EAC incidence, although this effect should also have been observed for ESCC. Historically, esophageal cancer is one of the most accurately classified tumors, based on studies comparing antemortem diagnoses to autopsy findings (7).
The incidence of EAC began to rise in the late 1960s, and has continued to increase through the present. It is likely that a change in local or systemic exposures predated this rise by several years. Changing trends in obesity do not appear to be responsible for the initial rise in EAC incidence, whereas declining rates of infection with H. pylori are temporally associated. Future investigations into new or altered exposures from the mid-20th century may reveal important information with regard to the pathogenesis of esophageal adenocarcinoma.
ACKNOWLEDGMENT
The authors would like to thank the Connecticut Tumor Registry for their generous assistance in providing the data for this study.
Funding: Dr. Abrams is supported in part by a Career Development Award from the National Cancer Institute (K07 CA132892). Dr. Sharaiha is supported by a training grant from the National Cancer Institute (T32 CA009529).
References
- 1.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. Journal of the National Cancer Institute. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945–1952. doi: 10.1158/1055-9965.EPI-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng T, Mayne ST, Holford TR, et al. Time trend and age-period-cohort effects on incidence of esophageal cancer in Connecticut, 1935–89. Cancer Causes Control. 1992;3:481–492. doi: 10.1007/BF00051361. [DOI] [PubMed] [Google Scholar]
- 4.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 5.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329–338. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteman DC, Parmar P, Fahey P, et al. Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Roush GC, Holford TR, Schymura MJ, White C. Cancer Risk and Incidence Trends: The Connecticut Perspective. Washington, DC: Hemisphere Publishing Corporation; 1987. [Google Scholar]
- 8.Heston JF, Kelly JA, Meigs JW, Flannery JT. Forty-five years of cancer incidence in Connecticut, 1935–79. NCI Monogr. 1986;70:1–31. [PubMed] [Google Scholar]
- 9.US Population Data Downloads. [cited 2010 April 1];2010 Available from: http://seer.cancer.gov/popdata/download.html.
- 10.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2009 Sub (1973–2007) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2007 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2010 April; based on the November 2009 submission ed.
- 11.Hansson LE, Sparen P, Nyren O. Increasing incidence of both major histological types of esophageal carcinomas among men in Sweden. Int J Cancer. 1993;54:402–407. doi: 10.1002/ijc.2910540309. [DOI] [PubMed] [Google Scholar]
- 12.Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer. 1990;62:440–443. doi: 10.1038/bjc.1990.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [cited 2010 April 1];NHANES MMWR and Short Reports, Body Weight/Obesity. 2010 Available from: http://www.cdc.gov/nchs/nhanes/nhanesmmwrs_obesity.htm.
- 14.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4:566–572. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168:219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- 17.Rehnberg-Laiho L, Rautelin H, Koskela P, et al. Decreasing prevalence of helicobacter antibodies in Finland, with reference to the decreasing incidence of gastric cancer. Epidemiol Infect. 2001;126:37–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson LA, Murphy SJ, Johnston BT, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–739. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- 19.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–1359. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 20.Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Multivariate analysis of the association of acid and duodeno-gastro-oesophageal reflux exposure with the presence of oesophagitis, the severity of oesophagitis and Barrett's oesophagus. Gut. 2008;57:1056–1064. doi: 10.1136/gut.2006.119206. [DOI] [PubMed] [Google Scholar]
- 21.Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol. 2010;105:1007–1013. doi: 10.1038/ajg.2009.734. quiz 6, 14. [DOI] [PubMed] [Google Scholar]
- 22.Pei Z, Yang L, Peek RM, Jr, Levine SM, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005;11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 25.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst. 2004;96:1383–1387. doi: 10.1093/jnci/djh265. [DOI] [PubMed] [Google Scholar]