Abstract
PON3 is a member of the paraoxonase gene family that includes PON1 and PON2. For example, PON3 and PON1 share approximately 60% identity at the amino acid level. Recent studies have demonstrated that PON3 is present in human and rabbit HDL but not in mouse HDL. Mouse PON3 appears to be cell-associated and is expressed in a wide range of tissues such as liver, adipose, macrophage, and the artery wall. In vitro studies have shown that PON3 can prevent LDL oxidation and destroy bacterial quorum-sensing molecules. Previous studies also showed that human PON3 transgenic mice were protected from obesity and atherosclerosis in both the C57BL/6J wild-type and LDLR knockout genetic background. Administration of adenovirus expressing the human PON3 gene into apoE −/− mice also decreased atherosclerotic lesion formation. In order to further understand the functions of PON3 in physiology and disease, we performed in situ hybridization analysis to examine Pon3 gene expression patterns in newborn and adult mice, in various tissues, including atherosclerotic lesions of apoE −/− mice. Our results show relatively high levels of Pon3 mRNA labeling in the adrenal gland, submaxillary gland, lung, liver, adipose, pancreas, large intestine, and other tissues of newborn mice. In the adult mouse, Pon3 mRNA levels were much lower in the corresponding tissues as mentioned above for the newborn mouse. Sections of the aortic root from the hearts of both wild-type and apoE −/− mice displayed moderate levels of Pon3 mRNA labeling. Pon3 mRNA was also detected in the atherosclerotic lesion areas at the aortic root of apoE −/− hearts. Our data revealed that mouse Pon3 is expressed in a wide range of tissues, and that its expression is temporally controlled.
Keywords: PON3, In situ hybridization, Developmental regulation, Mouse
1 Introduction
The paraoxonase gene family contains 3 members, PON1, PON2, and PON3, located as a cluster on mouse chromosome 6 and human chromosome 7. The three human PON proteins share abouty 60% identity in amino acid sequence. Human PON1 is expressed primarily in the liver and is found associated with HDL particles in the blood (Blatter et al. 1993; Hassett et al. 1991). Human PON3 is expressed primarily in the liver, with lower expression levels seen in other tissues (Reddy et al. 2001; Shamir et al. 2005). Human PON2, on the other hand, is ubiquitously expressed and is found in a variety of tissues (Ng et al. 2001). In addition, whereas human PON1 and PON3 associate with HDL in the circulation, PON2 protein is not associated with HDL or LDL, but appears to remain intracellular, associated with membrane fractions of the cell (Ng et al. 2001). Our recent studies showed that mouse PON3, unlike human PON3, is not detectable in circulation or HDL (Ng et al. 2007; Shih et al. 2007), suggesting mouse PON3 is a cell-associated protein like PON2. A recent study also suggests that human PON1, PON2, and PON3 are localized to the endoplasmic reticulum (Rothem et al. 2007). We and others have shown that all three PON proteins exhibit lactonase activities with many common substrates (Draganov et al. 2005; Ozer et al. 2005; Yang et al. 2005). For example, all three PONs very efficiently metabolize 5-hydroxy-eicosatetraenoic acid 1,5-lactone and 4-hydroxy-docosahexaenoic acid, which are products of both enzymatic and nonenzymatic oxidation of arachidonic acid and docosahexaenoic acid, respectively, and may represent the endogenous substrates of PONs. All PONs, especially PON3, also have been shown to hydrolyze estrogen esters (Teiber et al. 2007), likely endogenous substrates for PONs. Furthermore, human and mouse PONs have been shown by us and other groups to hydrolyze and thereby inactivate bacterial quorum-sensing molecules, N-acyl-homoserine lactones, such as N-(3-oxododecanoyl)-L-homoserine lactone (3OC12-HSL) (Draganov et al. 2005; Ozer et al. 2005; Yang et al. 2005). These molecules are extracellular quorum-sensing signals secreted by Gram-negative bacteria such as the pathogenic bacteria, Pseudomonas aeruginosa, to regulate biofilm formation and secretion of virulence factors (Parsek and Greenberg, 2000). Our in vitro studies using PON1 knockout (KO) mouse serum (Ozer et al. 2005) and airway epithelial cells isolated from the PON2-deficient mice also demonstrated that both PON1 and PON2 are important for degradation of 3OC12-HSL (Stoltz et al. 2007). Therefore, growing evidence suggests possible roles of PONs in innate immunity against bacterial infection.
Both rabbit and human PON3 have been shown to associate with HDL in the circulation (Draganov et al. 2000; Reddy et al. 2001). Rabbit PON3, when purified from serum, inhibits copper-induced LDL oxidation in vitro (Draganov et al. 2000). Our studies have shown that LDL incubated with stably transfected cells overexpressing human PON3 has significantly less lipid hydroxides, and is less capable of inducing monocyte chemotactic activity (Reddy et al. 2001). Our studies of transgenic mice overexpressing human PON3 showed that elevated PON3 levels protect against atherosclerosis and obesity (Shih et al. 2007). Administration of adenovirus expressing the human PON3 gene into apoE −/− mice also decreased atherosclerotic lesion formation (Ng et al. 2007). Therefore, PON3 appears to protect against atherosclerosis and metabolic disorders such as obesity. In order to further understand the functions of PON3 in physiology and disease, we performed in situ hybridization analysis to examine Pon3 mRNA expression patterns in newborn and adult mice. Our results revealed that mouse Pon3 is expressed in a wide range of tissues, and that Pon3 expression is temporally controlled with higher expression levels detected in newborn mice as compared to the adult mice.
2 Methods
2.1 Tissue Fixation, Embedding, and Pretreatment
Whole body sections of C57BL/6 newborn and adult mice were used. The animals were sacrificed in a CO2 chamber. Hearts of male apoE −/− mice that were 6 months old and maintained on a chow diet were also collected. These hearts were embedded in OCT medium (OCT Compound, 4583 Tissue-Tek). Tissues were frozen and cut into 10-micron sections, mounted on gelatin-coated slides and stored at −80°C. Before in situ hybridization (ISH), they were fixed in 4% formaldehyde (freshly made from paraformaldehyde; Sigma Aldrich P6148) in phosphate buffered saline (PBS), treated with triethanolamine/acetic anhydride, washed and dehydrated with a series of ethanol. Before proceeding to the ISH with Pon3 probes, all tissues were validated with riboprobes to LDL receptor mRNA (data not shown).
2.2 cRNA Probe Preparation
A 631 bp mouse Pon3 cDNA fragment cloned into the pBluescript II KS plasmid was used for generation of anti-sense and sense cRNA transcripts. The cRNA transcripts were synthesized in vitro according to manufacturer’s conditions (Ambion) and labeled with 35S-UTP (> 1000 Ci/mmol; Amersham).
2.3 Hybridization and Washing Procedures
Sections were hybridized overnight at 55°C in 50% deionized formamide, 0.3 M NaCl, 20 mM Tris-HCl pH 7.4, 5 mM EDTA, 10 mM NaH2PO4, 10% dextran sulphate, 1 × Denhardt’s, 50 mg/ml total yeast RNA, and 50–80,000 cpm/ml 35S-labeled cRNA probe. The tissue was subjected to stringent washing at 65°C in 50% formamide, 2 × SSC, 10 mM DTT and washed in PBS before treatment with 20 mg/ml RNAse A at 37°C for 30 min. Following washes in 2 × SSC and 0.1 × SSC for 10 min at 37°C, the slides were dehydrated, exposed to Kodak BioMaxMR X-ray film, then dipped in Kodak NTB nuclear track emulsion and exposed in light-tight boxes with desiccant at 4°C.
2.4 Imaging
Photographic development was carried out in Kodak D-19. Slides were counter-stained lightly with cresyl violet and analyzed using both brightfield and darkfield optics. Sense (control) cRNA probes (identical to the mRNAs) always gave background levels of hybridization signal.
3 Results
3.1 Pon3 mRNA Expression in Newborn Mice
Pon3 mRNA distribution in wild-type newborn (P1) mouse whole body sections was examined. X-ray film autoradiography provided evidence for the presence of relatively high Pon3 mRNA concentrations in the adipose tissue, submaxillary gland, liver, lung, pancreas, large intestine, adrenal cortex (Fig. 1), and stomach (Fig. 2). Figure 2 shows higher magnification images of liver and the adjacent stomach. High levels of Pon3 mRNA were detected in the hepatocytes of liver and in the glandular epithelium of stomach (Fig. 2). Higher magnification images following emulsion autoradiography showed high levels of Pon3 mRNA in the adipose tissue (Fig. 3), whereas skeletal muscle and bone remain unlabeled. Emulsion autoradiography detected high levels of Pon3 mRNA in the bronchiole epithelium of lung (Fig. 4). High levels of Pon3 mRNA were also detected in the acinar cells of pancreas, but not in the pancreatic islet (Figs. 5 and 10a).
Fig. 1.
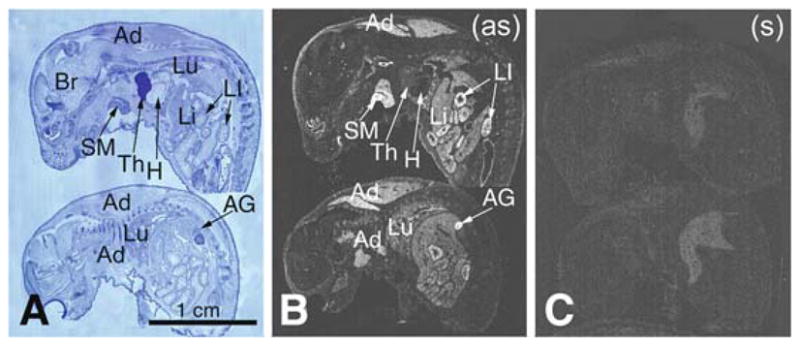
Pon3 mRNA distribution in wild-type newborn (P1) mouse whole body sections. (a) Anatomical view of whole body sections following staining with cresyl violet seen under bright-field illumination. (b) X-ray film autoradiography detection of Pon3 mRNA seen as bright labeling. (c) Control hybridization in an adjacent section comparable to (b). Abbreviations: Ad – adipose tissue; AG – adrenal gland; Br – brain; H – heart; Li – liver; LI – large intestine; Lu – lung; SM –submaxillary gland; Th – thymus; (as) – antisense; (s) – sense
Fig. 2.
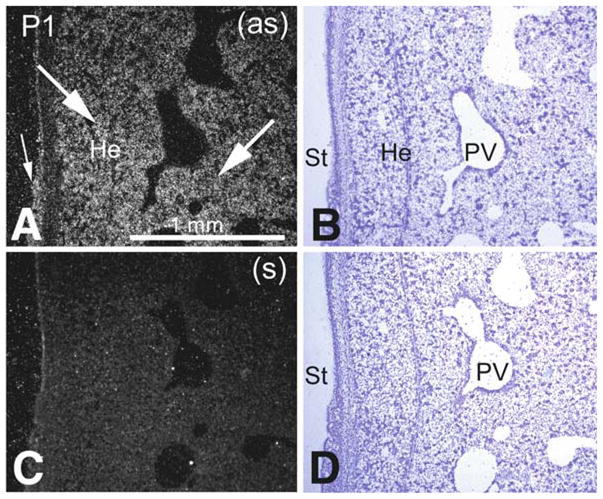
Pon3 mRNA expression in wild-type newborn (P1) mouse liver. (a) Emulsion autoradiography detection of Pon3 mRNA in hepatic tissue (arrows) and glandular epithelium (small arrow) of stomach seen as bright labeling under darkfield illumination. (b) Cresyl violet staining of the same section shown in (a) seen under brightfield illumination. (c) Control hybridization in an adjacent section comparable to (a). (d) Cresyl violet staining of the same section shown in (c) seen under brightfield illumination. Abbreviations: He – hepatocyte; PV – portal vein, branch; St – stomach; (as) – antisense; (s) – sense
Fig. 3.
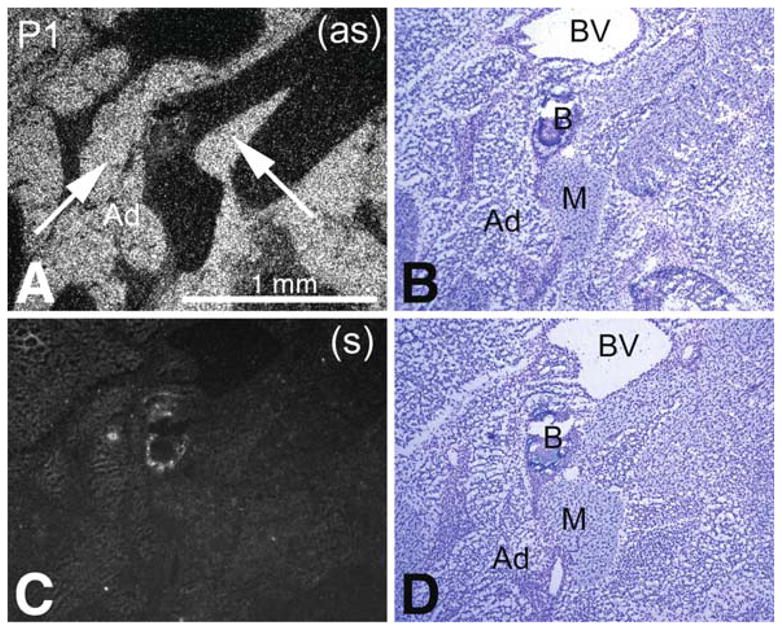
Pon3 mRNA expression in wild-type newborn (P1) mouse adipose tissue. (a) Emulsion autoradiography detection of Pon3 mRNA seen as bright labeling under darkfield illumination. Labeling is seen in adipose tissue (arrows), whereas skeletal muscle and bone remain unlabeled. (b) Cresyl violet staining of the same section shown in (a) seen under brightfield illumination. (c) Control hybridization in an adjacent section comparable to (a). (d) Cresyl violet staining of the same section shown in (c) seen under brightfield illumination. Abbreviations: Ad – adipose tissue; B – bone; BV – blood vessel; M – skeletal muscle; (as) – antisense; (s) – sense
Fig. 4.
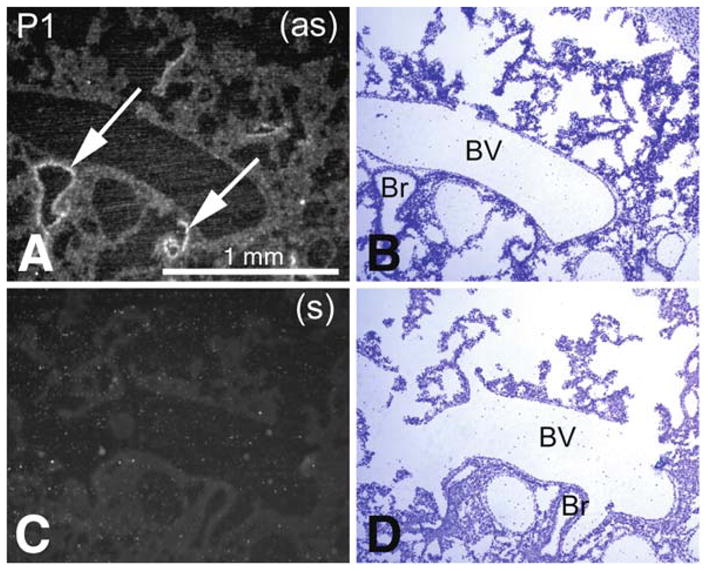
Pon3 mRNA expression in wild-type newborn (P1) mouse lung. (a) Emulsion autoradiography detection of Pon3 mRNA in bronchiole epithelium (arrows) seen as bright labeling under brightfield illumination. (b) Cresyl violet staining of the same section shown in (a) seen under brightfield illumination. (c) Control hybridization in an adjacent section comparable to (a). (d) Cresyl violet staining of the same section shown in (c) seen under brightfield illumination. Abbreviations: Br – bronchiole; BV – blood vessel; (as) – antisense; (s) – sense
Fig. 5.
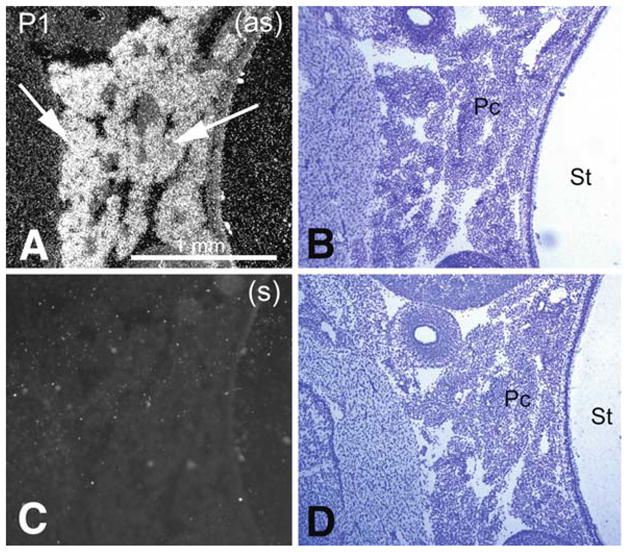
Pon3 mRNA expression in wild-type newborn (P1) mouse pancreas. (a) Emulsion autoradiography detection of Pon3 mRNA in acini of the pancreatic tissue (arrows) seen as bright labeling under brightfield illumination. (b) Cresyl violet staining of the same section shown in (a) seen under brightfield illumination. (c) Control hybridization in an adjacent section comparable to (a). (d) Cresyl violet staining of the same section shown in (c) seen under brightfield illumination. Abbreviations: Pc – pancreas; St – stomach; (as) – antisense; (s) – sense
Fig. 10.
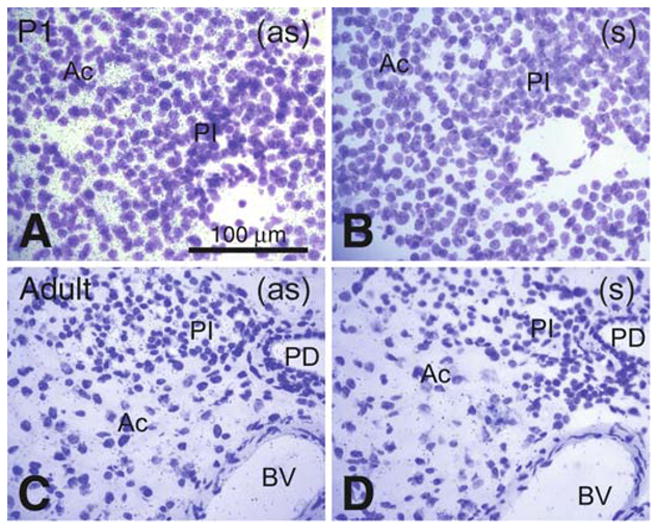
Comparative Pon3 mRNA expression in wild-type newborn (P1) and adult mouse pancreas. (a) Emulsion autoradiography detection of Pon3 mRNA in newborn mouse pancreatic acinar cells seen as black precipitate (silver grains) on a cresyl violet background under brightfield illumination. Pancreatic islets seem to be unlabeled. (b) Control hybridization in an adjacent section comparable to (a). (c) Low level Pon3 mRNA labeling in the pancreas of the adult mouse; the pancreatic islets, blood vessels, and pancreatic duct are unlabeled. (d) Control hybridization in an adjacent section comparable to (c). Abbreviations: Ac – acinar cells, BV – blood vessel; PD –pancreatic duct; PI – pancreatic islet; (as) – antisense; (s) – sense
3.2 Pon3 mRNA Expression in Adult Mice
Highly sensitive X-ray film autoradiography provided evidence of Pon3 expression in the adrenal cortex, salivary glands, adipose tissue, lung, liver, and skin of adult mouse (Fig. 6). Emulsion autoradiography confirmed the X-ray film findings. As detailed in Figs. 7, 8, 9, and 10, the levels of Pon3 expression in adult adipose tissue, liver, lung, and pancreas appeared to be much lower when compared to their newborn mouse counterparts. Similar to the newborn (Fig. 7a), in adult mouse Pon3 mRNA was detected in the adipose tissue but not in the adjacent skeletal muscle (Fig. 7c). As seen in the newborn pancreas (Fig. 10a), Pon3 mRNA is only detected in the acinar cells of adult pancreas, but not in the pancreatic islets (Fig. 10c).
Fig. 6.
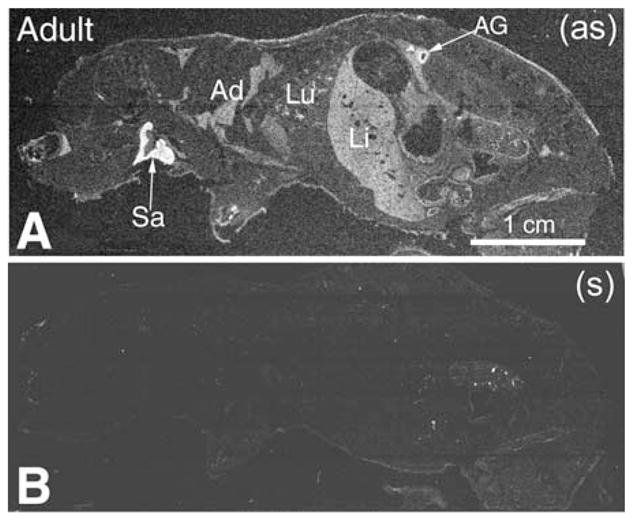
Pon3 mRNA distribution in wild-type adult mouse whole body sections. (a) X-ray film autoradiography detection of Pon3 mRNA seen as bright labeling under darkfield illumination. Higher Pon3 expression levels seem to occur in adrenal gland cortex and salivary glands (arrows). Less labeling is seen in adipose tissue, lung, and liver. (b) Control hybridization in an adjacent section comparable to (a). Abbreviations: Ad – adipose tissue; AG – adrenal gland; Li – liver; Lu – lung; Sa – salivary gland; (as) – antisense; (s) – sense
Fig. 7.
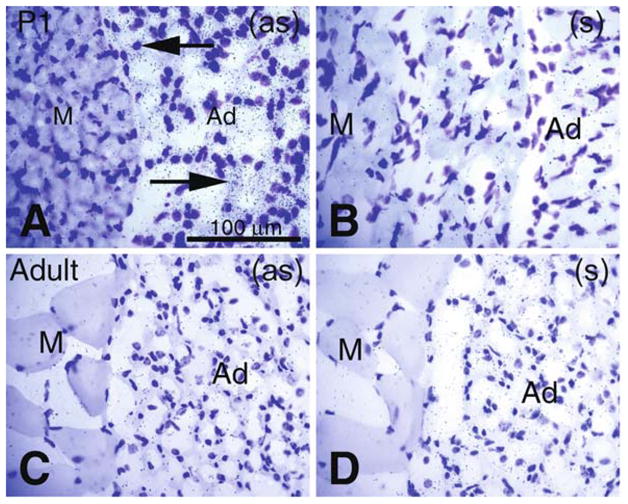
Comparative Pon3 mRNA expression in wild-type newborn (P1) and adult mouse adipose tissue. (a) Emulsion autoradiography detection of dense Pon3 mRNA in newborn mouse adipose tissue (arrows) seen as black precipitate (silver grains) on a cresyl violet background under bright-field illumination. Skeletal muscle fibers are not labeled. (b) Control hybridization in an adjacent section comparable to (a). (c) Low-level Pon3 mRNA labeling in the adipose tissue of an adult mouse. (d) Control hybridization in an adjacent section comparable to (c). Abbreviations: Ad –adipose tissue; M – skeletal muscle; (as) – antisense; (s) – sense
Fig. 8.
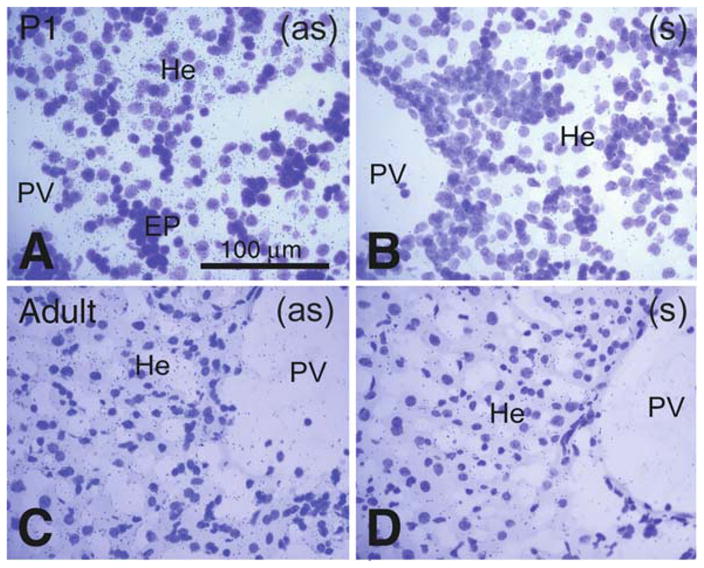
Comparative Pon3 mRNA expression in wild-type newborn (P1) and adult mouse liver. (a) Emulsion autoradiography detection of Pon3 mRNA in newborn mouse liver hepatocytes seen as black precipitate (silver grains) on a cresyl violet background under brightfield illumination. A group of hematopoietic cells looks unlabeled. (b) Control hybridization in an adjacent section comparable to (a). (c) Pon3 mRNA labeling in the liver of an adult mouse. Labeling is much less concentrated in the hepatocytes. (d) Control hybridization in an adjacent section comparable to (c). Abbreviations: EP – erythropoietic cells; He – hepatocytes; PV – portal vein; (as) – antisense; (s) –sense
Fig. 9.
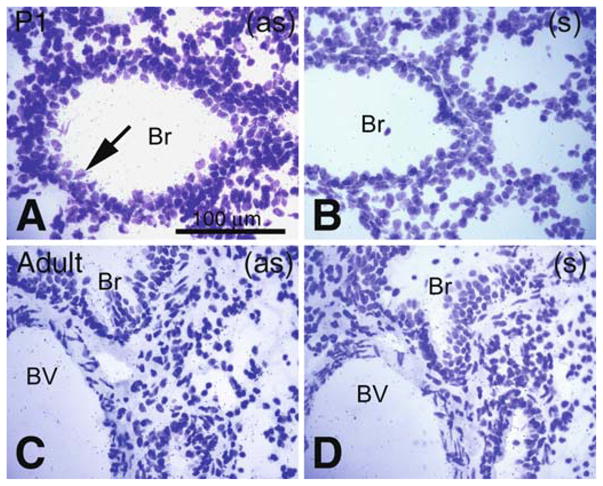
Comparative Pon3 mRNA expression in wild-type newborn (P1) and adult mouse lung. (a) Emulsion autoradiography detection of Pon3 mRNA in newborn mouse bronchiole epithelium (arrow) seen as black precipitate (silver grains) on a cresyl violet background under brightfield illumination. (b) Control hybridization in an adjacent section comparable to (a). (c) Low level Pon3 mRNA labeling in the bronchiole of an adult mouse. (d) Control hybridization in an adjacent section comparable to (c). Abbreviations: Br – bronchiole; BV – blood vessel; (as) – antisense; (s) – sense
3.3 Pon3 mRNA Expression in Wild-Type and apoE −/− Adult Mouse Hearts
Pon3 labeling was detected in the heart wall and blood vessels. Ubiquitous labeling occurred in the wild-type mouse heart, including the heart wall (cardiac muscle), aortic root, vein, and connective tissue around the heart. This labeling was absent in the sense (control) hybridization (Fig. 11). A similar pattern of ubiquitous Pon3 mRNA distribution was seen in the heart tissue of a mouse with the apoE −/− genotype (Fig. 12). Pon3 mRNA was also detected in the atherosclerotic lesion areas in the aortic root of apoE −/− mouse (Fig. 12a), whereas this labeling was absent in the sense (control) hybridization (Fig. 12c). A previous report shows that mouse Pon3 mRNA and enzyme activity is expressed by macrophages (Rosenblat et al. 2003). Therefore, the Pon3 mRNA present in the atherosclerotic lesion area of apoE −/− mouse is probably expressed by the macrophages or foam cells present in the lesion.
Fig. 11.
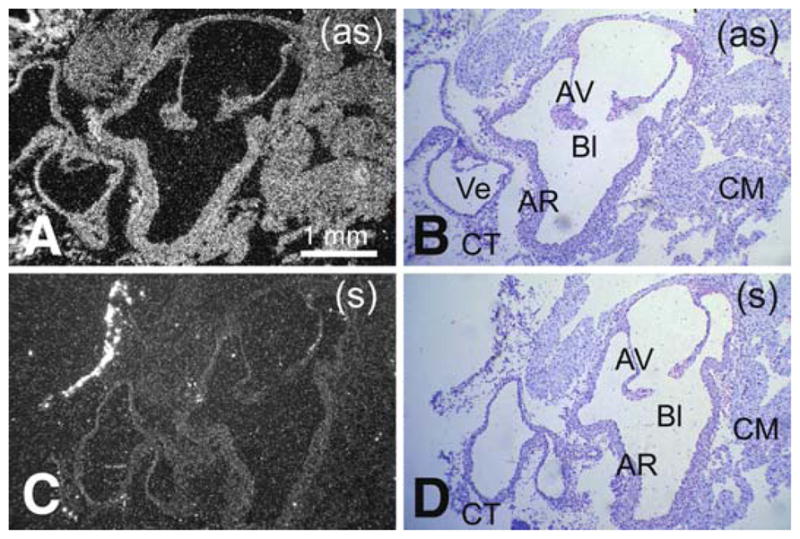
Pon3 mRNA expression in wild-type adult mouse heart. (a) Emulsion autoradiography detection of Pon3 mRNA seen as bright labeling under darkfield illumination. Labeling occurs in multiple heart regions including the aortic root, aortic valve, vein, cardiac muscle, and connective tissue. (b) Cresyl violet staining of the same section shown in (a) seen under brightfield illumination. (c) Control hybridization in an adjacent section comparable to (a). (d) Cresyl violet staining of the same section shown in (c) seen under brightfield illumination. Abbreviations: AV – aortic valve, leaflet; AR – artery root; Bl – blood; CM – cardiac muscle; CT – connective tissue; En –endocardium; Ve – vein; (as) – antisense; (s) – sense
Fig. 12.
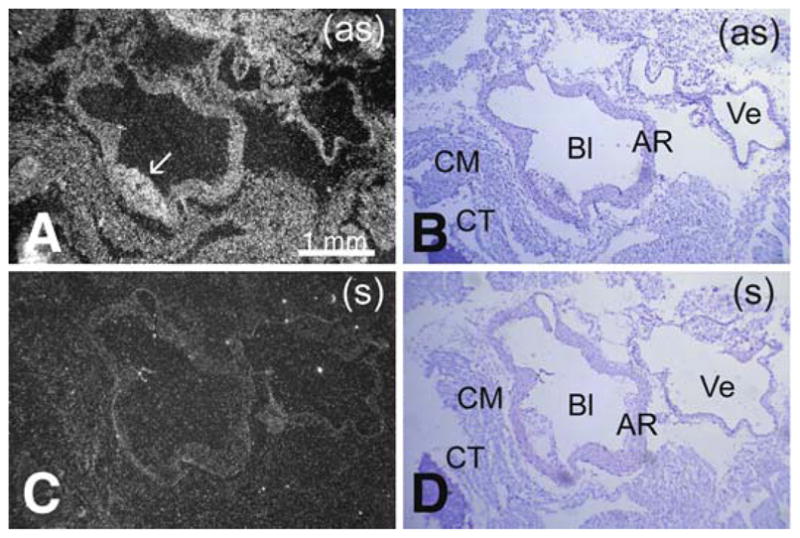
Pon3 mRNA expression in ApoE −/− adult mouse heart. (a) Emulsion autoradiography detection of Pon3 mRNA seen as bright labeling under darkfield illumination. Labeling occurs in several tissues including the aortic root, vein, cardiac muscle, and connective tissue around the heart. Labeling also occurs in the atherosclerotic lesion area (arrow). (b) Cresyl violet staining of the same section shown in (a) seen under brightfield illumination. (c) Control hybridization in an adjacent section comparable to (a). (d) Cresyl violet staining of the same section shown in (c) seen under brightfield illumination. Abbreviations: AR – aortic root; Bl – blood; CM – cardiac muscle; CT – connective tissue; Ve – vein; (as) – antisense; (s) – sense
4 Discussion
Although the anti-atherogenic function of PON3 has been established using animal models (Ng et al. 2007; Shih et al. 2007), the physiological function of PON3 is still unclear. As a first step to understanding the role of PON3 in physiology, we determined the expression pattern of mouse Pon3 mRNA in newborn and adult stages using the in situ hybridization technique. We detected high levels of mouse Pon3 mRNA in adipose tissue, submaxillary gland, adrenal cortex, liver, lung, pancreas, stomach, and large intestine of newborn mice. Pon3 mRNA was detected in these same tissues in adult mice, although the expression levels were lower than in the newborns. We also detected moderate levels of Pon3 mRNA in the heart wall (cardiac muscle), aortic root, vein, and connective tissue around the heart of adult wild-type and apoE −/− mice. In apoE −/− mouse heart, Pon3 mRNA was also present in the atherosclerotic lesion area at the aortic root. The detection of Pon3 mRNA in the aortic root and atherosclerotic lesion areas suggests that PON3 may exert its athero-protective, anti-oxidative effect locally in the artery wall.
The developmental down-regulation of mouse Pon3 expression between newborn and adult stages is interesting and different from what is known for PON1. Previously we reported that human PON1 levels plateau between 6 and 15 months of age, whereas mouse PON1 levels plateau at 3 weeks of age (Cole et al. 2003). Therefore, our data suggest different developmental regulation patterns between mouse Pon1 and Pon3 genes, with Pon1 being up-regulated and Pon3 being down-regulated, respectively, as the mice reach adulthood. It remains to be seen whether the same developmental down-regulation is also true for the human PON3 gene.
Our Pon3 mRNA tissue distribution data are in general agreement with a recent report of the immunohistochemical analysis of PON3 expression in normal mouse tissues (Marsillach et al. 2008). Both studies found PON3 expression in mouse skin, salivary gland, glandular epithelium of stomach, intestine, hepatocytes of liver, acinar cells of pancreas, heart, adipose tissue, and bronchiole epithelium of lung. Our study showed high levels of Pon3 mRNA present in the adrenal cortex which was not included in the immunohistochemical study (Marsillach et al. 2008). Unlike the immunohistochemical study, the Pon3 mRNA levels in the brain, skeletal muscle, kidney, and bone were too low to be detected in our study.
Detection of Pon3 mRNA in adipocytes suggests a possible role for PON3 in adipogenesis. In fact, our previous study showed that transgenic mice overexpressing human PON3 are protected against obesity (Shih et al. 2007), implicating PON3’s involvement in adipocyte functions. Expression of Pon3 in the epithelium of skin, lung, stomach, and intestine is intriguing. Since PON3 is known to hydrolyze bacterial quorum-sensing molecules, such as 3OC12-HSL (Draganov et al. 2005; Ozer et al. 2005; Yang et al. 2005), it is plausible that PON3’s presence in the epithelium may play a protective role against bacterial infection. PON3’s presence in the exocrine glands such as the salivary gland and the acinar cells of pancreas is very interesting given the highly secretory nature of these tissues. The secretory function of these cells relies on the capacity of the ER to fold and modify nascent polypeptides and to synthesize phospholipids for the subsequent trafficking of secretory proteins through the ER–Golgi network. Since PON3 is localized to ER (Rothem et al. 2007), we postulate that PON3 may play a role in the secretory machinery in exocrine cells.
Lastly, the high level of expression of Pon3 mRNA in the adrenal cortex of newborn and adult mice is unexpected and intriguing. The adrenal cortex mediates the stress response through the production of mineralocorticoids and glucocorticoids, including aldosterone and cortisol respectively. It is also a secondary site of androgen synthesis. Our data suggest a possible role for PON3 in steroidogenesis. We have constructed a PON3 KO mouse through gene-targeting (Stoltz et al. 2007). This mutant mouse will be useful in elucidating the function(s) of PON3 in various organs such as the adrenal gland, adipose tissue, and liver. Detailed biochemical studies are also necessary to identify the physiological substrate(s) of PON3 in various cells/tissues.
Acknowledgments
We thank Yi-Shou Shi, XuPing Wang, and Phylogeny, Inc. (Columbus, OH) for excellent technical support. This work is supported by NIH grants PO1 HL30568 (to AJL and DMS), and 2RO1 HL071776-05A1 (to DMS), and AHA grant 0755069Y (to DMS).
References
- Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem. 1993;211:871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, Tward A, Lusis AJ, Jack RM, Costa LG, et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- Draganov DI, Stetson PL, Watson CE, Billecke SS, La Du BN. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J Biol Chem. 2000;275:33435–33442. doi: 10.1074/jbc.M004543200. [DOI] [PubMed] [Google Scholar]
- Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- Hassett C, Richter RJ, Humbert R, Chapline C, Crabb JW, Omiecinski CJ, Furlong CE. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991;30:10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- Marsillach J, Mackness B, Mackness M, Riu F, Beltran R, Joven J, Camps J. Immunohistochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free Radic Biol Med. 2008;45:146–157. doi: 10.1016/j.freeradbiomed.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Ng CJ, Bourquard N, Hama SY, Shih D, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1368–1374. doi: 10.1161/ATVBAHA.106.134189. [DOI] [PubMed] [Google Scholar]
- Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, Greenberg EP, Zabner J. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol Lett. 2005;253:29–37. doi: 10.1016/j.femsle.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Greenberg EP. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci U S A. 2000;97:8789–8793. doi: 10.1073/pnas.97.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, Shih DM, Lusis AJ, Navab M, Fogelman AM. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–547. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
- Rothem L, Hartman C, Dahan A, Lachter J, Eliakim R, Shamir R. Paraoxonases are associated with intestinal inflammatory diseases and intracellularly localized to the endoplasmic reticulum. Free Radic Biol Med. 2007;43:730–739. doi: 10.1016/j.freeradbiomed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Shamir R, Hartman C, Karry R, Pavlotzky E, Eliakim R, Lachter J, Suissa A, Aviram M. Paraoxonases (PONs) 1, 2, and 3 are expressed in human and mouse gastrointestinal tract and in Caco-2 cell line: selective secretion of PON1 and PON2. Free Radic Biol Med. 2005;39:336–344. doi: 10.1016/j.freeradbiomed.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Shih DM, Xia YR, Wang XP, Wang SS, Bourquard N, Fogelman AM, Lusis AJ, Reddy ST. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res. 2007;100:1200–1207. doi: 10.1161/01.RES.0000264499.48737.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, Shih DM. Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L852–L860. doi: 10.1152/ajplung.00370.2006. [DOI] [PubMed] [Google Scholar]
- Teiber JF, Billecke SS, La Du BN, Draganov DI. Estrogen esters as substrates for human paraoxonases. Arch Biochem Biophys. 2007;461:24–29. doi: 10.1016/j.abb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Yang F, Wang LH, Wang J, Dong YH, Hu JY, Zhang LH. Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett. 2005;579:3713–3717. doi: 10.1016/j.febslet.2005.05.060. [DOI] [PubMed] [Google Scholar]


