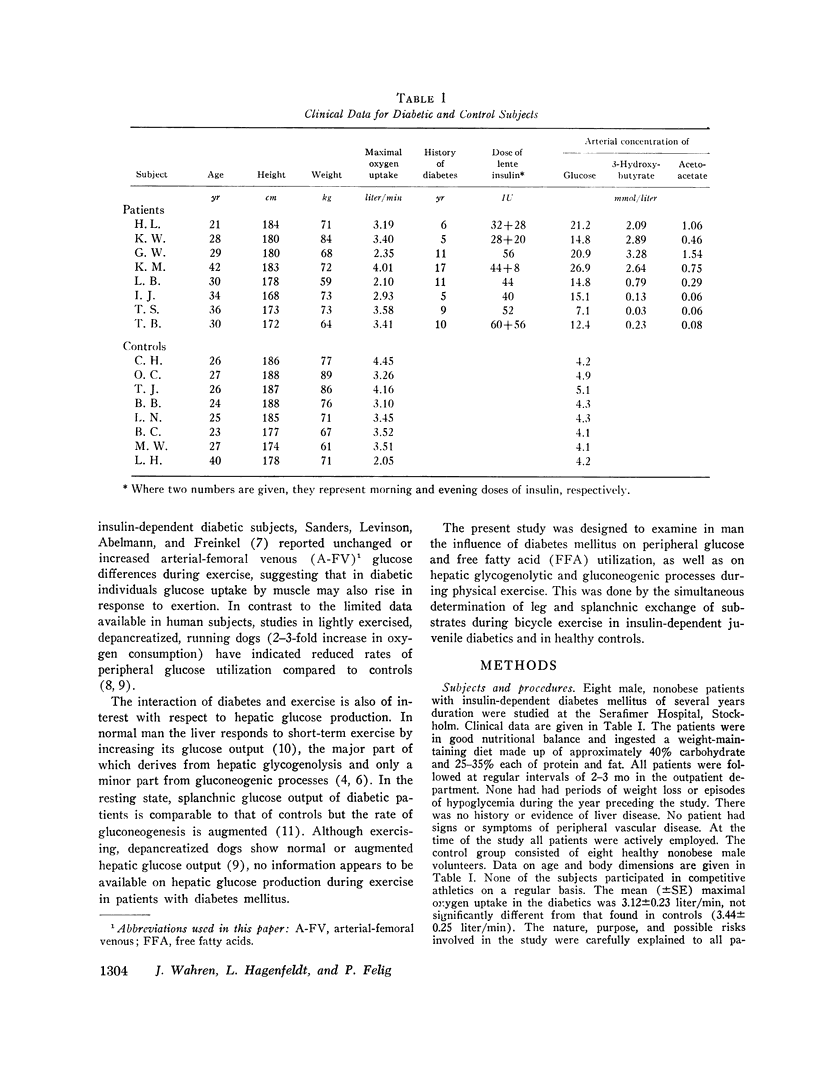
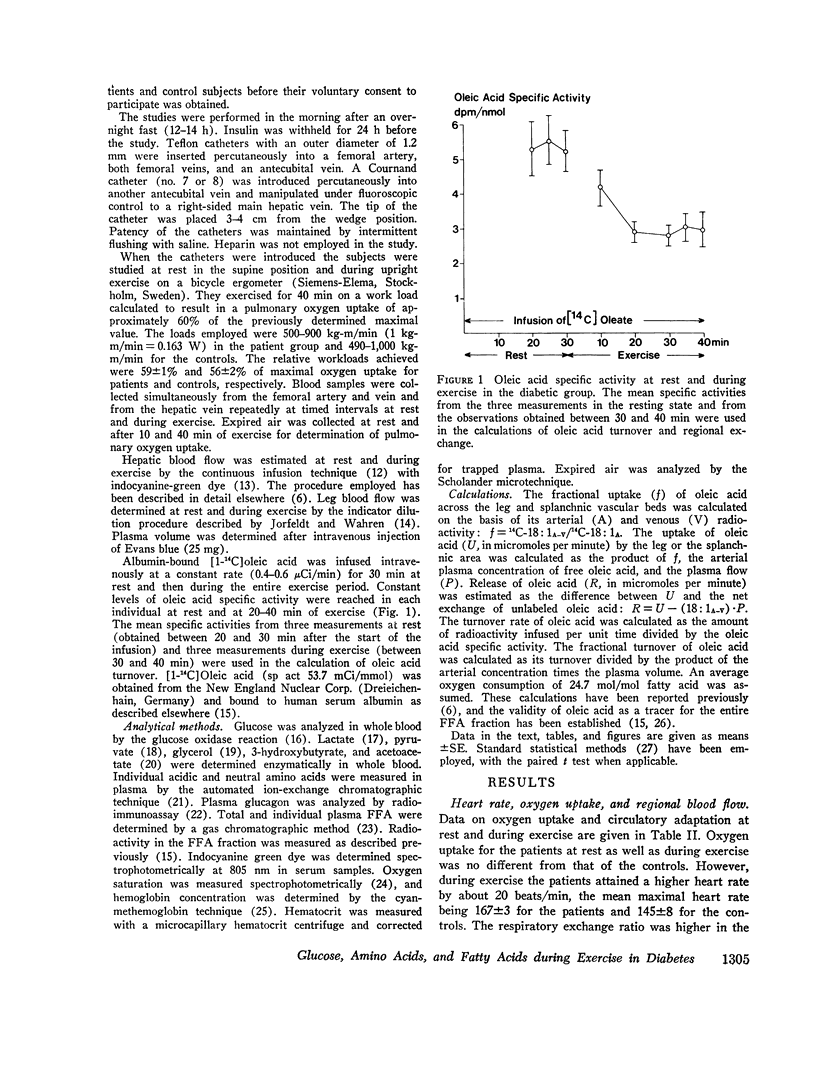
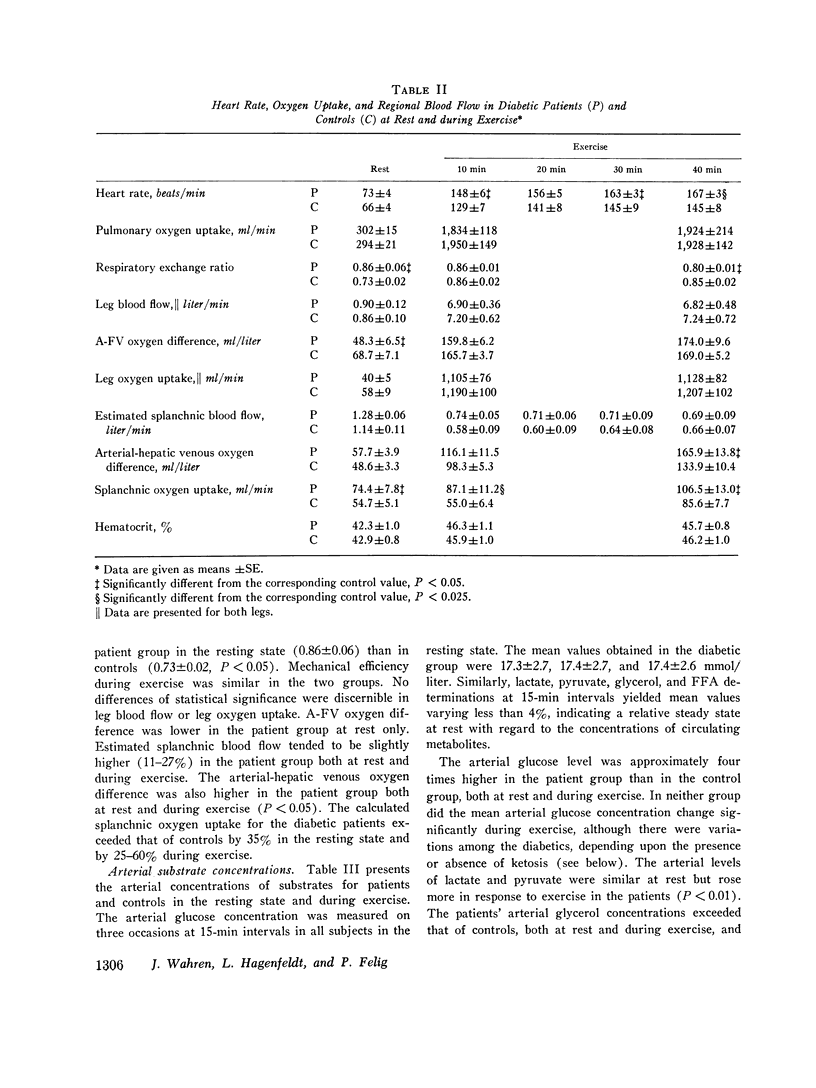
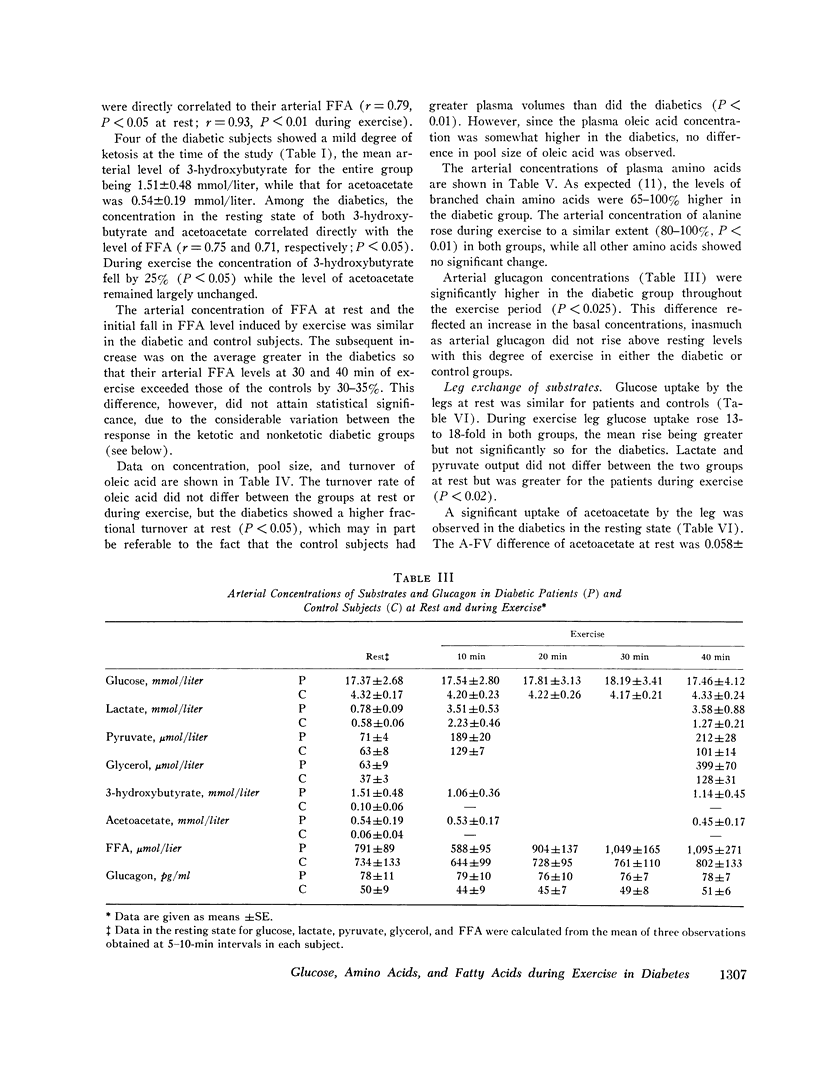
Abstract
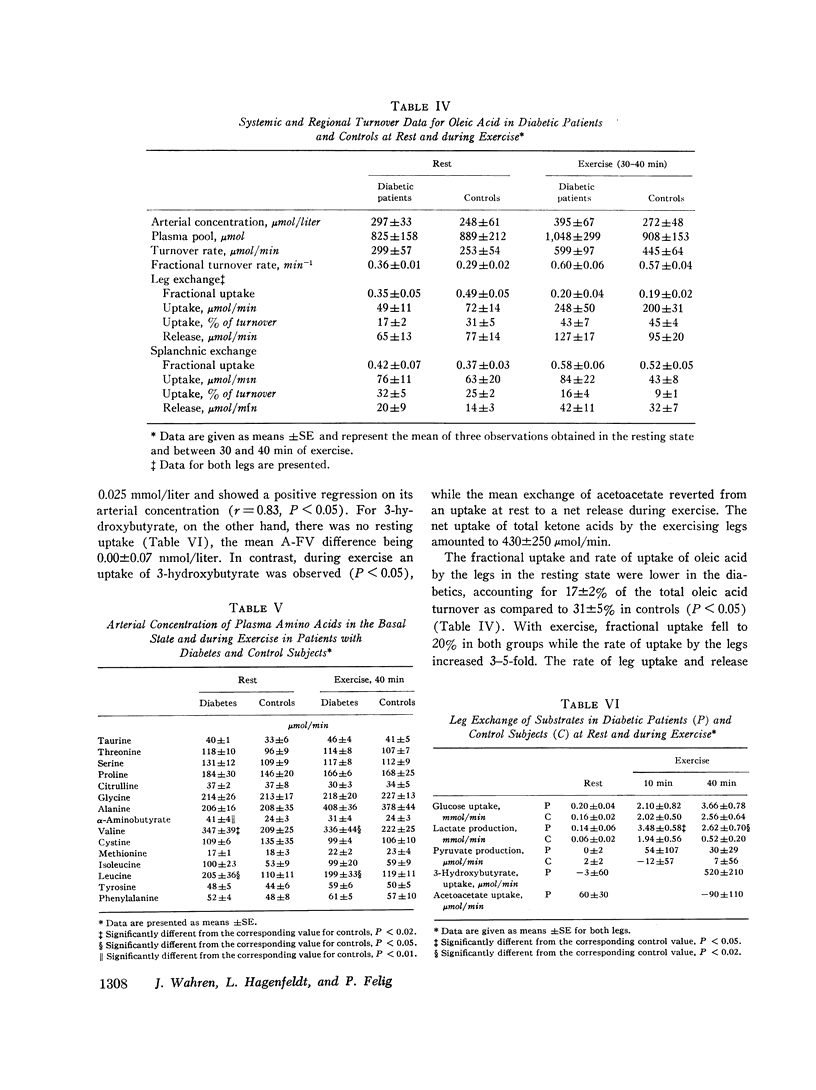
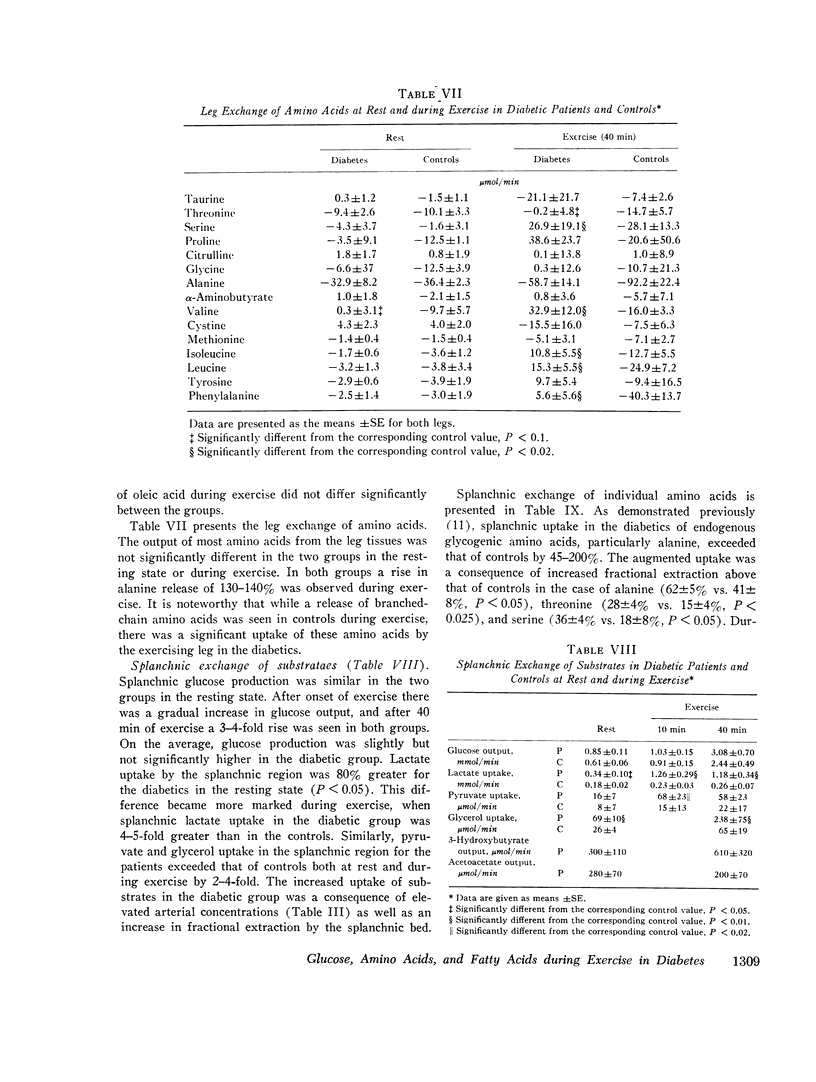
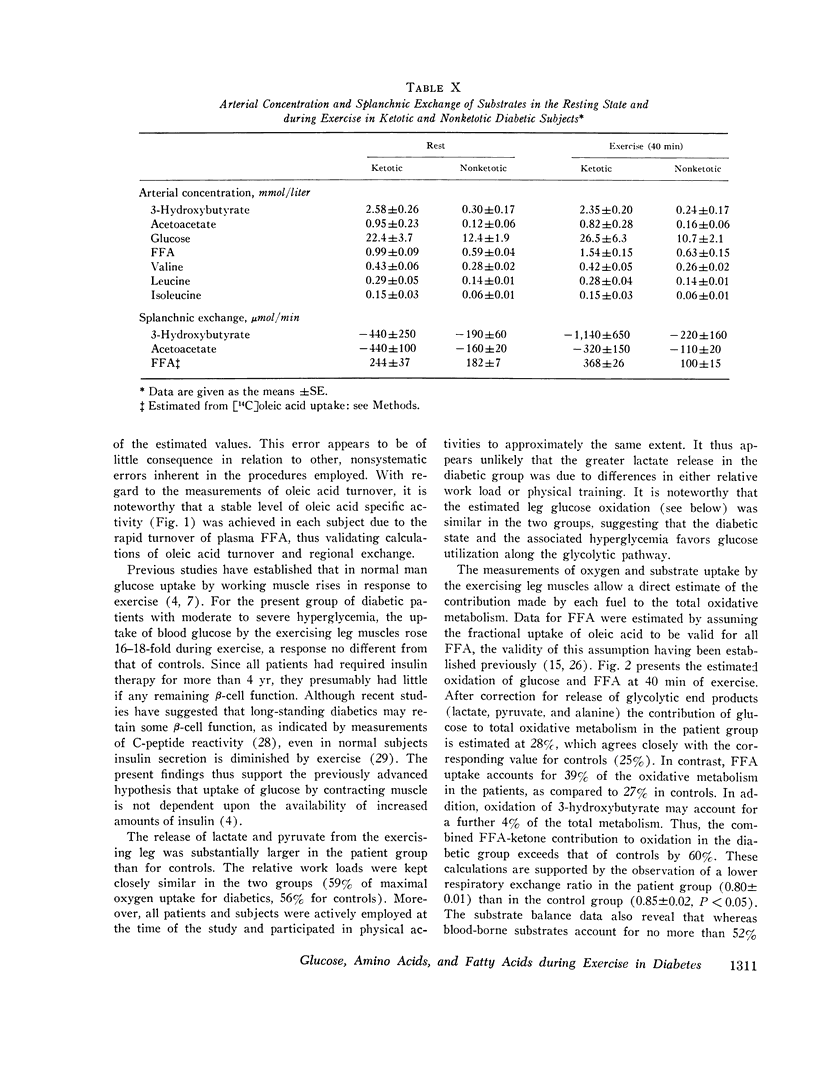
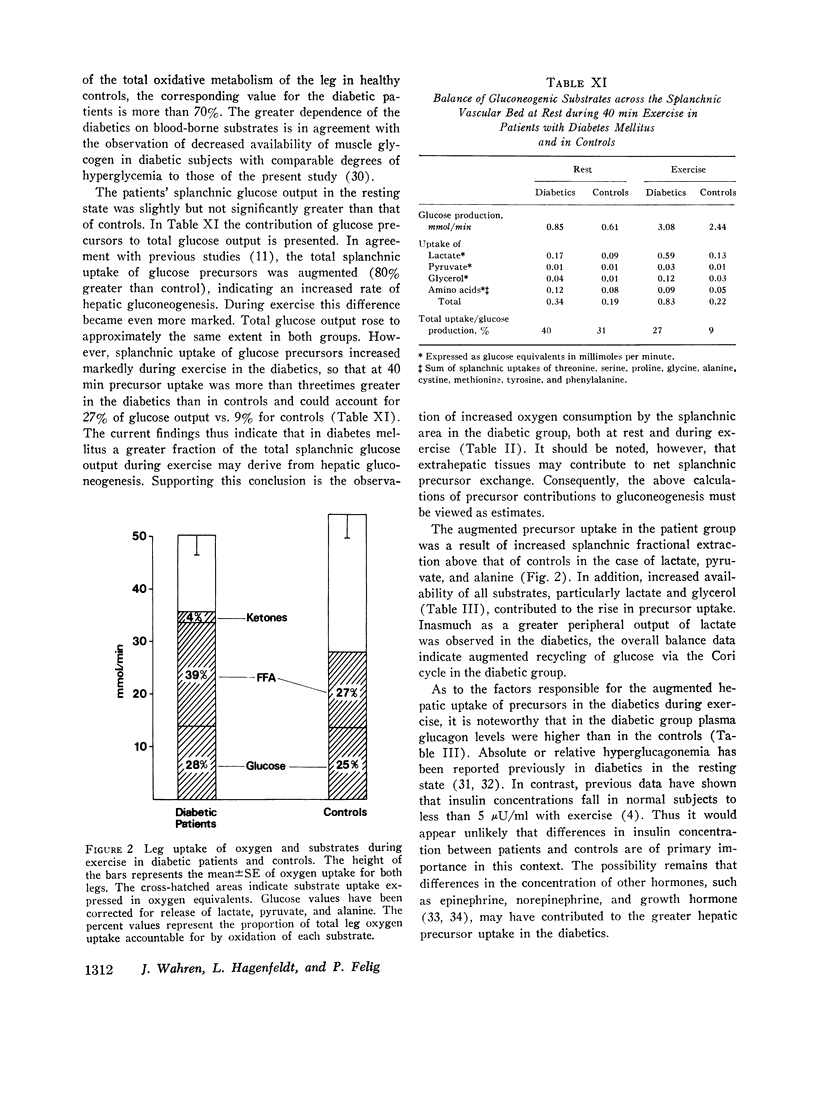
The influence of exercise on leg and splanchnic exchange of substrates was examined in eight insulin-dependent diabetics 24 h after withdrawal of insulin and in eight healthy controls studied at rest and after 40 min of bicycle ergometer exercise at 55-60% of maximal capacity. In four of the diabetic subjects, basal arterial ketone acid levels were 3-4 mmol/ liter (ketotic diabetics) and in the remainder, below 1 mmol/liter (nonketotic diabetics). ,ree fatty acid (FFA) turnover and regional exchange were evaluated with 14-C- labeled oleic acid. Leg uptake of blood glucose rose 13-18 fold during exercise in both the diabetics and controls and accounted for a similar proportion of the total oxygen uptake by leg muscles (25-28%) in the two groups. In contrast, leg uptake of FFA corresponded to 39% of leg oxygen consumption in the diabetic group but only 27% in controls. Systemic turnover of oleic acid was similar in the two groups. Splanchnic glucose output increased during exercise 3-4 fold above resting levels in both groups. In the diabetics, splanchnic uptake of lactate, pyruvate, glycerol, and glycogenic amino acids rose more than twofold above resting levels and was fourfold greater than in exercising controls. Total precursor uptake could account for 30% of the splanchnic glucose output in the diabetic group. In contrast, in the controls, total splanchnic uptake of glucose precursors was no greater during exercise than in the resting state and could account for no more than 11% of splanchnic glucose output. The augmented precursor uptake during exercise in the diabetics was a consequence of increased splanchnic fractional extraction as well as increased peripheral production of gluconeogenic substrates. The arterial glucagon concentration was unchanged by exercise in both groups, but was higher in the diabetics. In the diabetic subjects with ketosis in the resting state, exercise elicited a rise in arterial glucose and FFA, an augmented splanchnic uptake of FFA, and a 2-3 fold increase in splanchnic output of 3-hydroxybutyrate. Uptake of 3-hydroxybutyrate by the exercising leg rose more rapidly than splanchnic production, resulting in a fall in arterial levels of 3-hydroxybutyrate. It is concluded that (a) glucose uptake by exercising muscle in hyperglycemic diabetics is no different from that of controls; (b) splanchnic glucose output rises during exercise to a similar extent in diabetics and controls, while uptake of gluconeogenic substrates is markedly higher in diabetics and accounts for a greater proportion of total splanchnic glucose output; (c) exercise in diabetic patients with mild ketosis is associated with a rise in blood glucose and FFA levels as well as augmented splanchnic production and peripheral uptake of ketone bodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. B., Rosenfield R. L., Mako M. E., Steiner D. F., Rubenstein A. H. Sequential changes in beta-cell function in insulin-treated diabetic patients assessed by C-peptide immunoreactivity. N Engl J Med. 1973 May 31;288(22):1144–1148. doi: 10.1056/NEJM197305312882202. [DOI] [PubMed] [Google Scholar]
- Franckson J. R., Vanroux R., Leclercq R., Brunengraber H., Ooms H. A. Labelled insulin catabolism and pancreatic responsiveness during long-term exercise in man. Horm Metab Res. 1971 Nov;3(6):366–373. doi: 10.1055/s-0028-1094123. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L. A gas chromatographic method for the determination of individual free fatty acids in plasma. Clin Chim Acta. 1966 Feb;13(2):266–268. doi: 10.1016/0009-8981(66)90304-4. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest. 1968;21(3):263–276. doi: 10.3109/00365516809076994. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J., Pernow B., Räf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest. 1972 Sep;51(9):2324–2330. doi: 10.1172/JCI107043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A. P. Abnormal serum growth hormone response to exercise in juvenile diabetics. J Clin Invest. 1970 Aug;49(8):1467–1478. doi: 10.1172/JCI106364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A. P. Normalization of growth hormone hyperresponse to exercise in juvenile diabetics after "normalization" of blood sugar. J Clin Invest. 1971 Sep;50(9):1806–1811. doi: 10.1172/JCI106671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorfeldt L., Wahren J. Human forearm muscle metabolism during exercise. V. Quantitative aspects of glucose uptake and lactate production during prolonged exercise. Scand J Clin Lab Invest. 1970 Aug;26(1):73–81. doi: 10.3109/00365517009049217. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L., Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971 Nov;41(5):459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Löffler G., Matschinsky F., Wieland O. Uber den Mechanismus der gesteigerten Ketonköperbildung. II. Redox-Status des DPN der isolierten Rattenleber bei Durchströmung mit Fettsäuren. Biochem Z. 1965 Jun 3;342(1):76–84. [PubMed] [Google Scholar]
- Maehlum S., Pruett E. D. Muscular exercise and metabolism in male juvenile diabetics. II. Glucose tolerance after exercise. Scand J Clin Lab Invest. 1973 Oct;32(2):149–153. doi: 10.3109/00365517309084342. [DOI] [PubMed] [Google Scholar]
- Roch-Norlund A. E., Bergström J., Castenfors H., Hultman E. Muscle glycogen in patients with diabetes mellitus. Glycogen content before treatment and the effect of insulin. Acta Med Scand. 1970 Jun;187(6):445–453. [PubMed] [Google Scholar]
- Rowell L. B., Kraning K. K., 2nd, Evans T. O., Kennedy J. W., Blackmon J. R., Kusumi F. Splanchnic removal of lactate and pyruvate during prolonged exercise in man. J Appl Physiol. 1966 Nov;21(6):1773–1783. doi: 10.1152/jappl.1966.21.6.1773. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Masoro E. J., Spencer M. J. Splanchnic metabolism in exercising man. J Appl Physiol. 1965 Sep;20(5):1032–1037. doi: 10.1152/jappl.1965.20.5.1032. [DOI] [PubMed] [Google Scholar]
- SANDERS C. A., LEVINSON G. E., ABELMANN W. H., FREINKEL N. EFFECT OF EXERCISE ON THE PERIPHERAL UTILIZATION OF GLUCOSE IN MAN. N Engl J Med. 1964 Jul 30;271:220–225. doi: 10.1056/NEJM196407302710502. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Wrenshall G. A. Exercise, insulin and glucose turnover in dogs. Endocrinology. 1969 Jul;85(1):165–171. doi: 10.1210/endo-85-1-165. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971 Dec;50(12):2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Evaluation of alpha-cell function by infusion of alanine in normal, diabetic and obese subjects. N Engl J Med. 1973 Mar 8;288(10):487–490. doi: 10.1056/NEJM197303082881003. [DOI] [PubMed] [Google Scholar]



