Abstract
Context
Older patients with depression and executive dysfunction represent a population with significant disability and high likelihood of failing pharmacotherapy.
Objective
To examine whether Problem Solving Therapy (PST) reduces disability more than Supportive Therapy (ST) in older patients with depression and executive dysfunction, and whether this effect is mediated by improvement in depressive symptoms.
Design
Randomized controlled trail, with participant recruitment from 12/02-11/07 and follow-up for 36 weeks.
Setting
Weill Cornell and University of California, San Francisco.
Participants
Adults (>59 years) with major depression and executive dysfunction.
Intervention
12 sessions of either PST modified for older depressed adults with executive impairment, or ST.
Main Outcome Measure
Disability as quantified by the World Health Organization Assessment Schedule II (WHODAS II)-12 item form.
Results
653 individuals were referred to this study, 221 of whom met criteria and were randomized to PST or ST. PST and ST led to comparable improvement of disability in the first 6 weeks of treatment, but a more prominent reduction in PST participants at weeks 9 and 12. The difference between PST and ST was greater in patients with greater cognitive impairment and higher number of previous episodes. Reduction in disability paralleled reduction in depressive symptoms. The therapeutic advantage of PST over ST in reducing depression was in part due to greater reduction of disability by PST. While disability increased during the 24 weeks following the end of treatment, the advantage of PST over ST-treated patients was retained.
Conclusions
This study suggests that PST is more effective than ST in reducing disability in older patients with major depression and executive dysfunction, and its benefits were retained after the end of treatment. The clinical value of this finding is that PST may be a treatment alternative in an older patient population likely to be resistant to pharmacotherapy.
INTRODUCTION
Disability is a primary concern of patients, families, clinicians, and policy makers (1). The World Health Organization Global Burden of Disease Initiative identified unipolar depression as the leading cause of disability worldwide (2). Depression accounts for 10.7% of variance in disability and is responsible for more than one in ten years lived with disability (2). Longitudinal studies of community residing elders show a strong relationship between depression and new onset disability (3–7), with the likelihood of becoming disabled increasing with each additional symptom of depression (4). Moreover, as the number of depressive symptoms increases, the likelihood of recovering from a physical disability decreases (4). Even subsyndromal depressive symptoms are associated with disability in older persons (8). Similar relationships between depression and disability have been observed in psychiatric and primary care patients (9–13).
Executive dysfunction and its underlying neurobiological abnormalities are common in late-life depression, contribute to disability, and increase the risk for poor response to antidepressants. Approximately 40% of elderly patients with major depression have impairment in some executive functions (14, 15). Similarly, structural abnormalities of depressed older adults are mainly localized in frontal subcortical structures (16–18), whose integrity is essential for the performance of executive functions. Both executive dysfunction (19–24) and its underlying white matter lesions (16, 25, 26) are associated with disability in depressed elders. The relationship of executive dysfunction to disability is clinically intuitive since individuals with executive dysfunction have difficulties in goal-setting, planning, initiating and sequencing behavior, and terminating behavior when their goals are accomplished (27, 28). Finally, clinical, neuropathological, and structural and functional neuroimaging studies suggest that executive dysfunction and its underlying pathophysiology predicts slow, poor, and unstable response of geriatric depression to antidepressant agents (16, 29–36), necessitating novel treatment development.
Responding to the need for effective treatments for geriatric depression with executive dysfunction, we elected to study a non-pharmacological intervention (37). The concept underlying the intervention was that imparting skills and enabling patients to deal with problems resulting from depressive symptoms and executive dysfunction would reduce both their disability and their depressive symptoms by improving their daily experiences. Accordingly, problem solving therapy (PST) was selected as the basis for this intervention. PST was originally developed as a treatment for depression (38). It relies on a learning model and imparts to patients an approach for identifying problems central to their lives and a method for selecting solutions and making concrete plans for problem resolution. PST has been found effective in older adults suffering from depression (39), experiencing disability (40, 41), and in patients with schizophrenia, a disorder accompanied by executive dysfunction (42, 43).
We recently reported that modified PST is more efficacious than Supportive Therapy (ST) in reducing depressive symptoms and in leading to remission of geriatric depression with executive dysfunction (44). The PST modification used in this study retained the five original steps in selecting problems and action plans (45). However, patients were oriented towards important, yet simple and accessible problems. Further, therapists were more directive than in the original version, and provided structure on selecting triggers for action plans, sequencing actions, and terminating action upon accomplishment of goals.
This paper focuses on disability using an instrument that captures several aspects of function including self-care, household and work activities, getting around, understanding and communicating, getting along with others, and participating in social activities. It tests the hypothesis that PST is more efficacious than ST in reducing disability while these treatments are offered, and that the differential gains of the PST group made during treatment are retained during the subsequent 24 weeks. A second hypothesis postulated that the salutary effects of PST over ST on disability are mediated by improvement in depressive symptoms and signs. Exploratory analysis focused on potential moderators of differences in efficacy between PST- and ST-treated subjects.
METHODS
Participants
This analysis used data of a randomized controlled trial which compared the efficacy of a modified version of PST to Supportive Therapy (ST) of participants, recruited by Weill-Cornell and University of California San Francisco research groups between 12/02 and 11/07. Study procedures were approved by the institutional review boards of both universities.
Individuals responding to advertisements or referred by clinicians were interviewed by raters trained at each site and credentialed by the Cornell Advanced Center for Services Research. Selection criteria consisted of age 60 years or older, SCID-R/DSM-IV (46) diagnosis of major non-psychotic depression, a 24-item Hamilton Depression Rating Scale (HDRS) (47) score ≥20, a Mini Mental State Examination (MMSE) (48) score ≥24, an Initiation/ Perseveration Domain score of the Mattis Dementia Rating Scale (DRS-IP) (49) score ≤33, and a Stroop Word Color Test score ≤25 (50). The DRS-IP and Stroop were selected because they have been associated with poor response to antidepressants (16, 29–36) and because of the simplicity of their administration. Individuals were excluded if they were receiving psychotherapy or antidepressants, reported intent to attempt suicide in near future, had any Axis I psychiatric disorder or substance abuse other than unipolar depression or generalized anxiety disorder, antisocial personality (DSM-IV), dementia, history of head trauma, acute or severe medical illness (i.e., delirium, metastatic cancer, decompensated organ failure, major surgery, recent stroke or myocardial infarction), drugs known to cause depression, and inability to perform any activities of daily living even with assistance.
Participants were assigned to PST or ST within each site using random numbers in blocks of five participants. Raters were unaware of participants’ randomization status. Therapists were aware of participants’ randomization status but not of study hypotheses.
Systematic Assessment
Diagnosis was assigned in research conferences by agreement of two clinician investigators after review of clinical history, the SCID-R and all other research data obtained by trained interviewers.
Measures were selected with documented validity and reliability in older adults. All instruments were rated using the clinical judgment of interviewers and clinician investigators, rather than verbatim participant responses, given difficulties of older adults in accurately identifying psychiatric symptoms (51). Disability was quantified by the interviewer-administered World Health Organization Disability Assessment Schedule II-12 item (WHODAS) (52). The WHODAS yields a composite score of disability after assessing the domains of: Understanding and communicating; getting around; self-care; getting along with others; household and work activities; and participation in society. This instrument is compatible with the international classification system and has been validated in 16 sites and 13 countries, including the United States, and found cross culturally applicable (52). Each of its six domains had factor loading ranging from 0.82 to 98 and its items also load on a general disability factor.
Severity of depression was assessed with the 24-item HDRS. Overall cognitive impairment was assessed with the MMSE. Executive functions were assessed with the DRS-IP, Stroop Color-Word, Wisconsin Card Sort Test (WCST) (53), Trails B of the Trail Making Test (54), and the Frontal Systems Behavior Scale (FrSBe) (55). Measures related to psychopathology included age of onset for first episode of major depression (SCID-R), neuroticism (subscale of the NEO) (56), and history of antidepressant use (Composite Antidepressant Treatment Intensity Scale modified to include the available antidepressants) (57).
After baseline assessment, the HDRS and WHODAS were assessed weekly until week 12 and again at 24 and 36 weeks. Payment for transportation and arrangements, when necessary, were provided to all meetings. Compensation for time spent in assessments was offered, but not for treatment sessions.
Treatment
Treatment was offered by four doctorate-level clinical psychologists and four licensed social workers with at least five years of post licensure experience. No therapist had prior experience with formal PST or ST protocols. Each therapist offered both treatments after training, which consisted of a two-day workshop and supervision of three PST and three ST training cases. Fidelity to treatment manuals was monitored by independent experts in both PST and ST who reviewed and rated 20% of randomly selected audiotaped sessions. Experts used the PST Adherence Scale to rate the quality and adherence to PST (58) and the California Psychotherapy Assessment Scale to rate ST (59). The average session ratings for each therapist were “excellent” in both PST and ST, and ranged between “excellent and exceptional”. No differences in quality of ratings were found for any therapist or for either treatment.
Problem Solving Therapy
Twelve weekly individual PST sessions were offered according to the manual of Arean, Raue, and Julian (UCSF, unpublished manuscript, 2003). The first five weeks are devoted to train participants in the 5-step problem-solving model and subsequent sessions enhanced PST skills. Participants are guided to set goals, propose ways for reaching them, create action plans, and evaluate the accomplishment of their goals. They are also instructed to apply the problem-solving model to additional problems between sessions. In the last two sessions, participants create a relapse prevention plan using the PST model.
Supportive Therapy
Twelve weekly individual ST sessions were offered according to Sach’s manual (Cornell University, unpublished manuscript, 2000). ST is similar to person-centered psychotherapy, and therapists create a comfortable, non-judgmental environment by demonstrating genuineness, empathy, and acceptance of patients without imposing any judgments on their decisions. This approach aids patients in addressing problems without direct input from therapists. Participants are encouraged to talk about their depression and any contributing life events. Therapists do not engage in any therapeutic strategy other than active listening and offering support focusing on participants’ problems and concerns.
Data Analysis
All participants who completed baseline assessments (the intent-to-treat sample) were included in data analyses. Profiles of pretreatment and weekly WHODAS scores over 12 weeks (Disability during treatment) and, separately, between 12 and 36 weeks (Disability after treatment) were compared for the two treatment groups (PST and ST) using mixed-effects models for longitudinal data to account for the repeated measurements over time. These models included time-trend parameter(s), treatment group, site, site-treatment interaction, and time-treatment interaction. Moderation was assessed by checking the interaction of baseline variables with treatment effects in the mixed-effects model described above. Mediation was assessed by examining the effects of lagged HDRS scores to predict WHODAS scores, again using a mixed-effects regression model. For the 0–12 week analyses, the preceding week’s HDRS scores were used (leaving off the first week’s data, which has no lagged mediator) to predict current WHODAS score. For the 12–36 week analysis, HDRS scores at 6, 12, and 24 weeks were used to predict WHODAS scores at 12, 24, and 36 weeks, respectively. The “12” week outcomes were taken to be the averages of the 10, 11 and 12 week outcomes to a) reduce variation and b) reduce the influence of missing data. The mediation effect was quantified by calculating proportion of treatment effect explained by the mediator (60). The same approach was used to assess the meditational effect of WHODAS on HDRS outcome. Analyses were conducted using SAS (version 9.1, SAS Institute, Cary NC).
RESULTS
Recruitment and Drop-out From Treatment
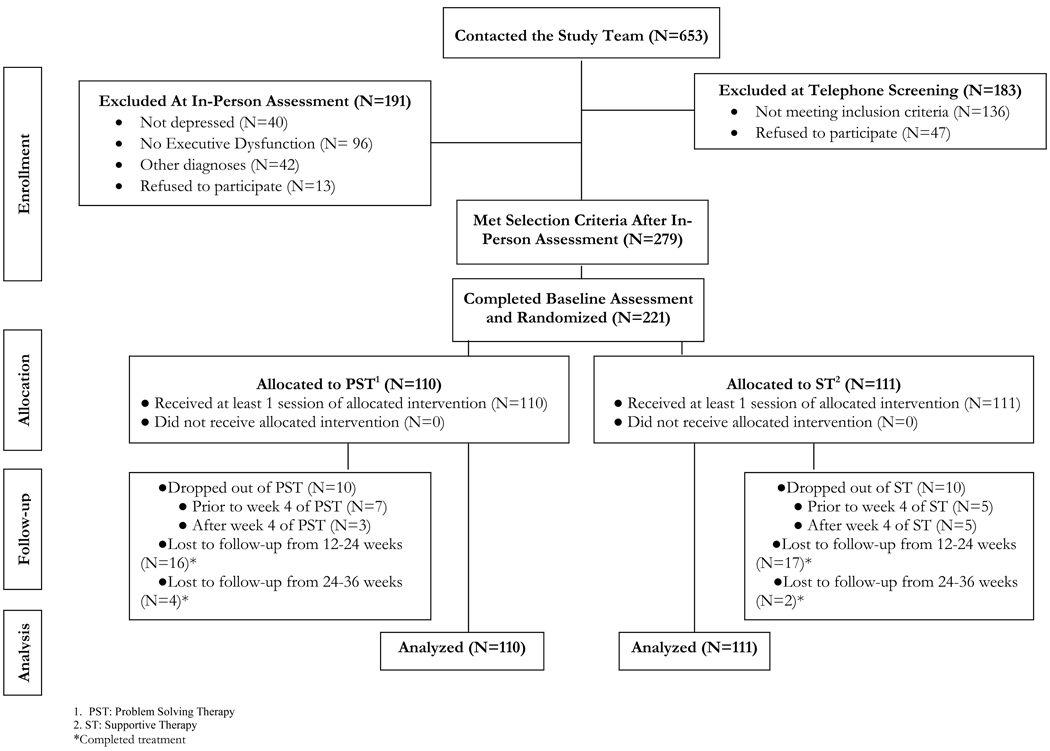
A total of 653 older persons were screened. Of these, 279 individuals met selection criteria (Figure 1). Of the 279 subjects, 221 completed the baseline assessment and were randomized to PST (N=110) or ST (N=111). Of the 221 randomized participants, 201 (91%) completed the 12-week treatment trial (Figure 1). Among those who dropped out of treatment (N=20), 10 were receiving PST and 10 ST. Nonetheless, 5 of the 20 participants who dropped out of treatment completed the 12 week assessment; 4 had received PST and 1 ST. In the end, 206 of participants received the 12 week assessment, 173 received the 24 week assessment and 167 received the 36 week assessment. The mean number of sessions attended by the PST group was 10.5 (SD=3.1) and 10.7 (SD=3.04) by the ST group; 87.5% and 89.1% of all sessions. The median number of sessions for each group was 12.
Figure 1. Flow of Subjects into the Treatment Trial.
Participant Characteristics
The participants’ demographic and clinical characteristics were reported elsewhere (44). Briefly, the randomized participants (N=221) were aged 73.0 years (SD: 7.8) and had 15.3 years (SD: 2.8) of education. Their depression (HDRS mean: 24.3, SD: 4.3), disability (WHODAS mean: 26.6, SD: 7.3), and executive function test scores (DRS-IP mean: 32.2, SD: 3.7; Stroop Color Word mean: 22.1, SD: 8.2, Wisconsin Perseverative Errors mean: 14.5, SD: 9) were in the mild to moderate severity range. Approximately 27% of participants had history of antidepressant drug treatment. Less than 2% were on benzodiazepines or sleep aides; no one was taking a cognitive enhancer. There were no significant differences in demographic or clinical variables between the two treatment arms and study sites. Further, there were no significant differences in demographics, depression severity, executive function, medical burden or disability among participants who had been on antidepressants and those who had not.
Treatment Outcomes (0–12 Weeks)
Course of Disability During Treatment (0–12 weeks)
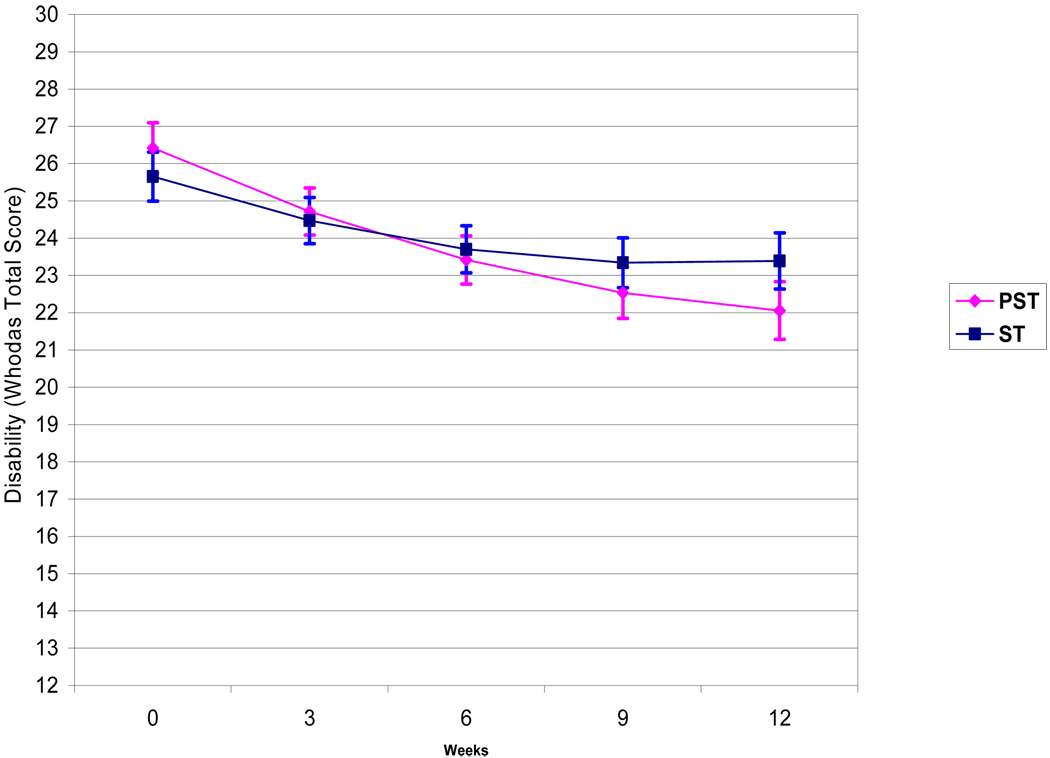
In a mixed effects model consisting of treatment group (PST vs. ST), time, time squared, treatment site (Cornell vs. UCSF), and treatment by time interaction, PST participants had significantly greater reduction in disability (total WHODAS scores) over 12 weeks than ST participants (Table 1, Figure 2). Treatment site (UCSF vs. Cornell) did not significantly contribute to WHODAS variance over time. Reduction in disability in the PST group was greater than in the ST group by approximately 0.18 points per week.
Table 1.
Comparisons of the Course of Disability During 12 Weeks of Treatment (PST vs. ST) and After Treatment Completion (12–36 weeks) in 221 Older Adults with Major Depression and Executive Dysfunction.
| Variables | Estimate | t | df | p |
|---|---|---|---|---|
| Model 1: Disability (WHODAS) aduring Treatment (0 – 12 weeks) | ||||
| Intercept | 22.9328 | 26.43 | 238 | <0.0001 |
| Treatment (PST vs. ST)b | −1.4744 | −1.39 | 204 | 0.1661 |
| Sitec | 1.0793 | 1.22 | 216 | 0.2256 |
| Timed | 0.08879 | 0.87 | 230 | 0.3855 |
| Time2 | 0.02267 | 2.99 | 194 | 0.0032 |
| Treatment × Timed | −0.1824 | −2.51 | 202 | 0.0128 |
| Model 2: Disability (WHODAS) After Treatment (12 – 36 weeks) | ||||
| Intercept | 4.3338 | 2.74 | 227 | 0.0067 |
| Treatment | −2.1414 | −2.46 | 178 | 0.0147 |
| Site | 0.1446 | 0.19 | 180 | 0.8531 |
| Time | 0.0642 | 2.15 | 142 | 0.0329 |
| Baseline WHODAS | 0.7209 | 13.50 | 185 | <0.0001 |
| Treatment X Time | 0.0134 | 0.31 | 142 | 0.7574 |
World Health Organization Disability Assessment Schedule II (12 items)
Treatment: 0=Supportive Therapy (ST); 1= Problem Solving Therapy (PST)
Site: 0=UCSF; 1=Cornell
Time variable was centered at 12 weeks
Time2 = Time × Time
Figure 2. Disability (WHODAS) Scores Over 12 Weeks of Treatment with Problem Solving Therapy (PST) vs. Supportive Therapy (ST) in 221 Older Adults with Major Depression and Executive Dysfunction.
The curves are based on the Least Squares Means of the Mixed Effects Model: Time + Treatment + Site + Time Square + Treatment × Time (Treatment × Time: t=0.31, df=1, 202, p=0.0128) with 1 Standard Error above and below the mean. PST: Problem Solving Therapy, ST: Supportive Therapy.
Moderators of Treatment Efficacy (0–12 Weeks)
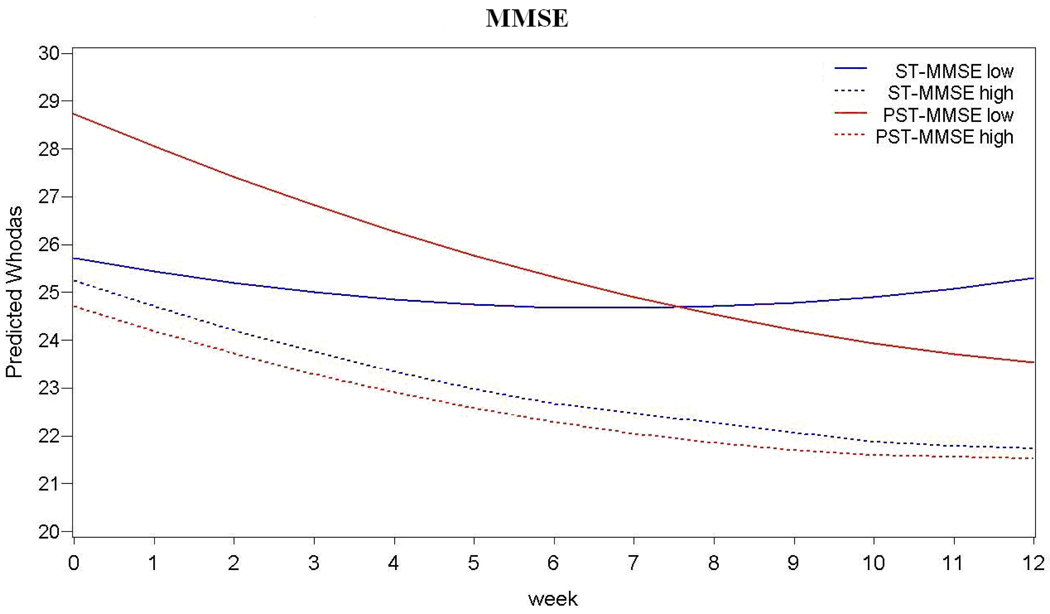
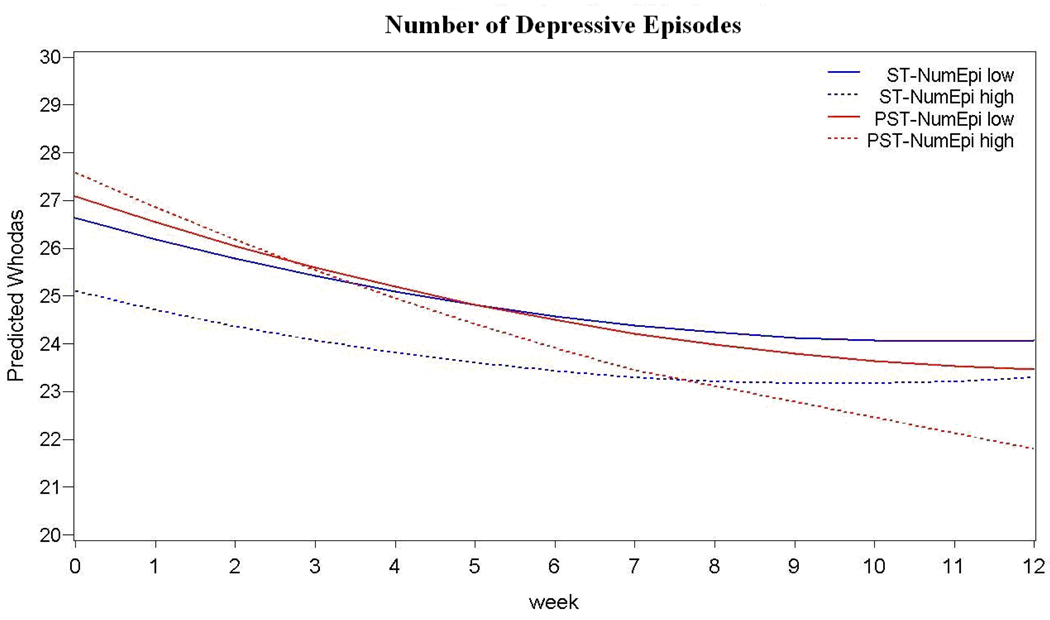
PST was associated with greater reduction of disability than ST in patients with higher number of depressive episodes and greater cognitive impairment (i.e., lower MMSE scores) (Table 2, Figure 3).
Table 2.
Moderation of Problem Solving Therapy Efficacy (vs. Supportive Therapy) Disability in 221 Older Adults with Major Depression and Executive Dysfunction
| Evaluated Moderators |
Mean | SD | OUTCOME: WHODAS 0–12 WEEKS |
OUTCOME: WHODAS 12– 36 WEEKS |
||||
|---|---|---|---|---|---|---|---|---|
| Fa | df | P | Fa | df | P | |||
| Age | 72.97 | 7.74 | 0.03 | 198 | 0.855 | 1.3 | 145 | 0.256 |
| Education (years) | 15.24 | 2.79 | 2.67 | 195 | 0.104 | 2.1 | 153 | 0.15 |
| Baseline Variables | ||||||||
| Severity of Depression (HDRS) | 24.32 | 4.27 | 1.14 | 192 | 0.286 | 0 | 158 | 0.967 |
| Age of Depression Onset (years) | 55.88 | 22.35 | 0.1 | 140 | 0.752 | 1.39 | 106 | 0.242 |
| Number depressive episodes | 2.23 | 2.21 | 4.15 | 153 | 0.043 | 1.21 | 118 | 0.274 |
| Mini Mental State Examination | 27.80 | 1.69 | 4.51 | 200 | 0.035 | 1.44 | 151 | 0.233 |
| DRS-IPb | 32.24 | 3.71 | 0 | 213 | 0.988 | 0.1 | 160 | 0.748 |
| Stroop Color-Word | 22.03 | 8.22 | 0.07 | 201 | 0.793 | 0.16 | 141 | 0.694 |
| Wisconsin Preservative Errorsc | 14.58 | 9.12 | 0 | 130 | 0.967 | 1.08 | 107 | 0.302 |
| Trails B | 137.59 | 63.50 | 0.01 | 174 | 0.92 | 2.22 | 128 | 0.138 |
| Frontal Systems Behavior Scale Clinician Rated | 39.89 | 9.04 | 0.08 | 169 | 0.773 | 0.01 | 140 | 0.929 |
| Neuroticism (NEO) | 14.98 | 5.14 | 1.94 | 192 | 0.166 | 0.04 | 148 | 0.846 |
| Anxiety Factor | 4.54 | 2.00 | 0 | 196 | 0.994 | 2.27 | 150 | 0.134 |
Effect estimate reflecting the change in the difference between the HDRS slopes of the PST and the ST groups when the moderator's score is increased by 1 point;
Initiation/Perseveration Subscale of Mattis Dementia Rating Scale;
Wisconsin Card Sort Test
PST = problem solving therapy; ST = supportive therapy; MWU = Mann-Whitney U test; HDRS = Hamilton Depression Rating Scale; DRS-IP = initiation/preservation scale of the Mattis Dementia Rating Scale; WHODAS = World Health Organization Disability Assessment Schedule II; NEO = neuroticism scale of the Neuroticism, Extroversion, Openness Scale; Anxiety Factor = Sum of HDRS Items: Agitation, Hypochondriasis, Psychic Anxiety, and Somatic Anxiety
Figure 3. Moderators of Problem Solving Therapy Efficacy (vs. Supportive Therapy) on Disability in 221 Older Adults with Major Depression.
Mini Mental State Exam (MMSE) scores at baseline (F=4.51, df=1, 200, p=0.035) and Number of Depressive Episodes (F=4.15, df=1, 153, p=0.043) moderate the effects of treatment on disability. MMSE low and MMSE high scores are 1 Standard Deviations below and above the mean, respectively (Mean=27.8; SD=1.71); Low Number of Depressive Episodes =1 and High Number of Depressive Episodes =4.
Mediators of Treatment Efficacy (0–12 Weeks)
To examine whether depression severity mediates treatment effects on disability, a mixed effects model was used in which depression severity (HDRS score) during each week was used as a predictor of disability (WHODAS score) during the immediately following week. The model consisted of treatment group (PST vs. ST), site (Cornell vs. UCSF), time, treatment group by time interaction, and HDRS score. HDRS scores predicted the effect on disability in the following week (F=27.32, df=1, 1934, P<0.0001). In the whole group, for every point reduction in depression (i.e. HDRS of 1 point) at each week, there was a statistically significant reduction in disability of 0.12 points in WHODAS in the following week. However, HDRS did not explain any of the PST vs. ST treatment difference on WHODAS.
To examine whether disability mediates treatment effects on depression, a mixed effects model was used in which disability (WHODAS score) during each week was used as a predictor of depression severity during the immediately following week, i.e. treatment group (PST vs. ST), site (Cornell vs. UCSF), time, time squared, treatment group by time interaction, and WHODAS score. WHODAS scores predicted the effect on depression in the following week (F=48.66, df=1, 1973, P<0.0001). In the whole group, for every point reduction in disability (i.e. WHODAS of 1 point) at each week, there was a statistically significant reduction in depression of 0.14 points in HDRS in the following week. WHODAS explained 10% of the PST vs. ST treatment difference on HDRS.
Outcomes After Completion of Treatment
Course of Disability (12 to 36 weeks)
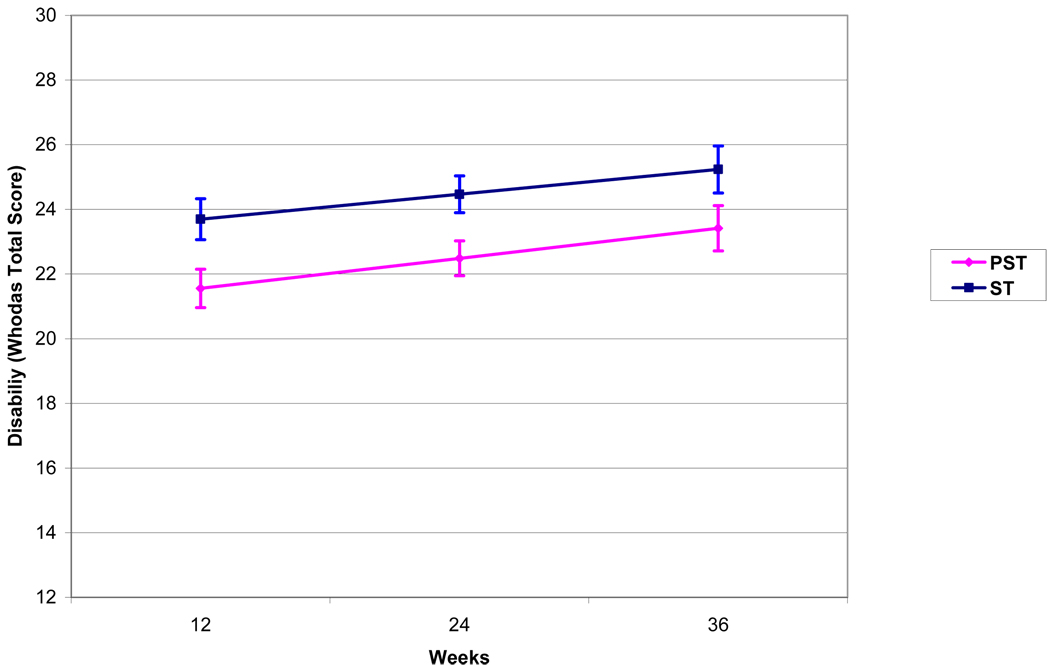
To study the course of disability (WHODAS) after the end of treatment, a mixed effects analysis was performed using a model consisting of treatment group (PST vs. ST), site (Cornell vs. UCSF), time (12, 24, and 36 weeks), treatment by time interaction, and baseline disability. Mixed effects models demonstrated no significant difference between PST and ST groups in the course of disability after treatment (group by time interaction: t=0.16, df=1, 142, p=0.66). Participants in both PST and ST demonstrated an increase in disability between 12 and 36 weeks (Time: t=2.15, df=1, 142, p=0.03; Least squares means: PST: 12 weeks=21.56, 24 weeks=22.49, 36 weeks=23.42; ST: 12 weeks=23.70, 24 weeks=24.47, 36 weeks=25.23). Treatment site (UCSF vs. Cornell) did not significantly contribute to WHODAS variance over time (between 12 and 36 weeks) (t=0.19, df=1, 180, p=0.86) (Figure 4).
Figure 4. Course of Disability After Treatment Completion (12–36 Weeks) in 206 Older Adults with Major Depression and Executive Dysfunction.
Curves are based on the Least Squares Means of the Mixed Effects Model: Time + Treatment + Site + Baseline WHODAS + Treatment × Time (Treatment × Time: t=0.31, df=1, 142, p=0.7574) with 1 Standard Error above and below the mean. PST: Problem Solving Therapy, ST: Supportive Therapy.
Moderators After Completion of Treatment (12–36 Weeks)
No demographic or clinical characteristics assessed at entry to the study moderated the course of disability between 12 and 36 weeks, a period in which no treatment was offered. (Table 2).
Relationship of Depression and Disability After Completion of Treatment (12–36 Weeks)
To examine whether depression severity predicted disability after the end of treatment, we studied the relationships of depression severity (HDRS scores) at week 12 and 24 to disability (WHODAS) scores at weeks 24 and 36. A mixed effects model was constructed consisting of treatment group (PST vs. ST), site (Cornell vs. UCSF), time, HDRS scores. HDRS scores at weeks 12 and 24 predicted WHODAS scores at week 24 and 36 respectively (F=3.84, df=1, 103, P=0.002). Specifically, for every point change in HDRS at week 12 and 24, there was a change of 0.18 in WHODAS at week 24 and 36, respectively.
To examine whether disability predicted depression after the end of treatment, we studied the relationships of disability (WHODAS scores) at week 12 and 24 to depression severity (HDRS) scores at weeks 24 and 36. A mixed effects model was constructed consisting of treatment group (PST vs. ST), site (Cornell vs. UCSF), time, WHODAS scores. WHODAS scores at weeks 12 and 24 predicted HDRS scores at week 24 and 36 respectively (F=54.74, df=1, 244, P<=0.0001). Specifically, for every point change in WHODAS of 1 point) at week 12 and 24, there was an HDRS change of 0.39 points in HDRS at week 24 and 36, respectively.
DISCUSSION
The main finding of this study is that PST was more effective than ST in reducing disability in older patients with major depression and executive dysfunction. The advantage of PST over ST was most pronounced in patients with greater cognitive impairment and those with history of more depressive episodes, an often difficult to treat population. Disability increased in both the PST and the ST group during the two years after the end of treatment but the PST group retained the advantage over ST made during the treatment period and experienced less disability during follow-up. The salutary effect of PST on disability in depressed, executive impaired older adults is particularly important since such patients experience significant disability and are likely to have poor or slow response to pharmacotherapy (16, 29–36).
This is the first study, to our knowledge, to demonstrate that PST can reduce disability in older patients with major depression and executive dysfunction. However, we have reported that PST was superior to ST in reducing depressive symptoms and signs and in leading to higher rates of response and remission in the same sample (44). The benefit of PST over ST on disability was approximately the same as the benefit on depression. These findings are consistent with earlier studies documenting that PST benefits depressed elderly patients without cognitive impairment (39) and with significant medical burden (40, 41, 61). Furthermore, PST led to behavioral gains in schizophrenic patients with executive dysfunction (42, 43).
This study has several limitations. First, each therapist administered both PST and ST, a design that may have introduced a therapist bias on efficacy. An alternative design, each therapist offering a single treatment only, would have drastically increased the sample size to control for therapist-specific effects (62). Further, a nested design does not exclude therapist bias because some therapists may assume that they offer the control treatment and view it as less efficacious. The study offered equally intensive training and certification procedures for PST and ST. Moreover, all PST and ST sessions were audiotaped and a random 20% of sessions were reviewed by independent experts. Participants in this study had mild executive dysfunction. It is unclear whether PST is helpful in patients with severe executive dysfunction, or in those with executive dysfunction part of a dementia syndrome. Moreover, the absence of a depressed group without executive dysfunction prevents knowing whether executive dysfunction influences the efficacy of PST and ST. Finally, the sample selection process may have biased our results. Participants in the study had an average of 15 years of education, and one fifth of those who met selection criteria did not enter the study because of refusal and/or poor adherence to rating procedures. However, 91% of those who started treatment remained in treatment until the end of the 12-week period. Therefore, the results of this trial may be generalizable to educated older adults with ability to remain in treatment. Another limitation might be the reliance on an interviewer-rated instrument for disability rather than a performance-based instrument. Performance-based instruments are time consuming and difficult to use in a study requiring frequent assessments in order to capture the timetable of disability change. Furthermore, performance instruments may be influenced by the lack of energy and motivational disturbances of depression. The study paid for transportation and, when necessary, provided transportation arrangements. Therefore, its findings can only be generalized to individuals with access to treatment. Home-based care and utilization of telemedicine may make PST-type approaches available to an increasing number of patients.
The construct of disability is complex. While associated with medical and psychiatric burden, disability is a distinct dimension of health with unique prognostic significance (19). In this study, disability was assessed with an interviewer-administered instrument (WHODAS II), which provides a comprehensive evaluation of disability (6 domains) associated with health conditions but not of functional states unrelated to health, e.g. restriction in participation due to race, gender, religion, and socioeconomic factors. Finally, the WHODAS II treats all disorders at parity when determining the level of disability. The 12-item WHODAS II is suitable for frequent administration and its strong psychometric properties and factor structure justifies its use as a measure of global disability (52, 63).
Participants in this study had moderate disability at entry; 27, the score approximating the mean baseline WHODAS-II score of our subjects can be obtained by having severe impairment (scores of 4) in 1 item, moderate impairment (scores of 3) in 4 items, mild impairment (score of 2) in 4 items, and no impairment (score of 1) in 3 items. Disability declined in both PST and ST treated patients. This was not surprising since both PST and ST are active treatments. The mean difference in WHODAS-II scores between PST and ST at the end of the 12 week treatment was 2.3 points, equal to approximately one standard deviation of normal elderly subjects. However, even the PST-treated subjects had mild disability (WHODAS-II mean: 21.8) at the end of the 12 week treatment phase. The remaining disability may be accounted in part by the residual executive dysfunction at the end of the trial, an observation consistent with earlier literature (64).
The advantage of PST over ST emerged after the 6th week of treatment, and the advantage of PST was retained during the follow-up period even though disability increased in both groups after the end of treatment. The design of this study does not permit identification of the exact mechanisms underlying the reasons and timing of PST efficacy. Indeed, there was a mild improvement in executive functions during the 12 week trial. However, change in executive functions during treatment was similar in the PST and ST arms and did not explain the PST therapeutic advantage. Another question is whether the disability measure was influenced by the patients’ depressive symptoms and that change in disability mainly reflected change in reporting bias. Indeed, depression scores predicted subsequent change in disability, and disability scores predicted subsequent change in depression. However, the differential treatment effect on disability may not be fully accounted by depression-related reporting bias. Reduction in depression did not mediate the differential effect of PST (over ST) on disability, even though reduction in disability mediated the differential effect of PST (over ST) on depression. Developing skills contributing to the individual’s specific behavioral limitations is inherent in PST and appears to be consistent with the timetable of improvement of PST compared to the ST group. The first few weeks of PST are devoted to learning the problem solving technique while, in the latter part of treatment, patients continue to use the PST approach alone or with their therapists in problems with a negative impact on their lives. Therefore, the timing of PST efficacy parallels the course of PST skill development, behavioral activation, self-efficacy, and hopefulness (38). Identifying the mechanisms and retaining the elements by which PST decreases disability and depression may simplify its administration and make it accessible to large numbers of patients.
Although PST led to a greater reduction of disability than ST, both treatments reduced disability during the 12 week treatment phase. While used as a comparison condition in this study, ST itself is a treatment with established efficacy in patients with a wide range of severity of depression (65–68). Therapeutic alliance and support are elements common to PST and ST and may have accounted both for the high retention in treatment and the beneficial effect on disability and mood (69).
This study noted a reciprocal relationship of disability and depression both during the 12-week treatment phase and after treatment completion. This observation is consistent with findings of community-based populations. Among high-functioning elderly adults, depressive symptoms were associated with an increased risk for disability onset after adjusting for baseline sociodemographic factors, physical health, and cognitive functioning (3). Similarly, increases in disability over time predict emergence of depressive symptoms (70–72).
The therapeutic advantage of PST over ST on disability was not mediated by reduction in depressive symptoms and signs. Therefore, the second hypothesis was not confirmed. However, reduction of disability did mediate improvement in depressive symptomatology during the 12-week treatment phase. This observation suggests that the higher efficacy of PST over ST in reducing depressive symptomatology is in part due to greater reduction in disability perhaps through skill development and behavioral activation.
While anxiety, neuroticism, and behavioral symptoms of executive dysfunction did not influence treatment efficacy, PST conferred greater benefits than ST to patients with greater cognitive impairment and history of a large number of depressive episodes. Cognitively impaired patients with recurrent depression are difficult to treat and may require skill development in addition to empathy and support offered by both PST and ST. Observing that PST reduced disability in non-demented patients with cognitive impairment encourages studies of PST modified to address the needs of depressed patients with mild dementia.
In conclusion, this study suggests that PST is effective in reducing disability in older patients with major depression and executive dysfunction. The difference between PST and ST was particularly prominent in patients with greater cognitive impairment and high number of previous episodes. Reduction in disability paralleled reduction in depressive symptoms. The therapeutic advantage of PST over ST in reducing depression was in part due to the greater reduction of disability by PST. While disability increased during the two years following the end of treatment, the gains made by PST treated patients were retained. Thus PST may be a promising treatment for an older patient population with significant disability, likely to fail antidepressant drug therapy. Next steps following these findings may include approaches aimed to sustain the effects of PST, e.g. booster sessions, and interventions to improve access to PST by disabled community populations, including home care, and telemedicine.
Acknowledgments
Grant support: NIMH grants R01 MH064099, R01 MH063982, K24 MH074717, P30 MH085943 , and the Sanchez Foundation.
Footnotes
Disclosures: Dr. Alexopoulos received grant support from Forest; served as a consultant to Scientific Advisory Board of Forest, Sanofi-Aventis, and Novartis; and has been a member of speakers’ bureaus sponsored by Cephalon, Forest, Lilly, Bristol Meyers Squibb, Glaxo, Janssen and being a stockholder of Johnson and Johnson. No other authors report competing interests. Dr. Alexopoulos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ClinicalTrials.gov Identifier: NCT00052091
REFERENCES
- 1.Lehman AF, Alexopoulos GS, Goldman H, Jeste D, Ustun B. Mental Disorders and disability: time to reevaluate the relationship? In: Kupfer DJ, First MB, Regier DA, editors. A Research Agenda for DSM-IV. Washington, DC: American Psychiatric Association; 2002. pp. 201–218. [Google Scholar]
- 2.Murray CJL, Lopez AD, editors. The Global Burden of Disease: A comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Cambridge, MA: Harvard University Press on behalf of the World Health Organization and the World Bank; 1996. [Google Scholar]
- 3.Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994;84:1796–1799. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronin-Stubbs D, de Leon CF, Beckett LA, Field TS, Glynn RJ, Evans DA. Six-year effect of depressive symptoms on the course of physical disability in community-living older adults. Arch Intern Med. 2000;160(20):3074–3080. doi: 10.1001/archinte.160.20.3074. [DOI] [PubMed] [Google Scholar]
- 5.Dalle Carbonare L, Maggi S, Noale M, Giannini S, Rozzini R, Lo Cascio V, Crepaldi G ILSA Working Group. Physical disability and depressive symptomatology in an elderly population: a complex relationship. The Italian Longitudinal Study on Aging (ILSA) Am J Geriatr Psychiatry. 2009;17(2):144–154. doi: 10.1097/jgp.0b013e31818af817. [DOI] [PubMed] [Google Scholar]
- 6.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 7.Andreescu C, Chang CC, Mulsant BH, Ganguli M. Twelve-year depressive symptom trajectories and their predictors in a community sample of older adults. Int Psychogeriatr. 2008;20(2):221–236. doi: 10.1017/S1041610207006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry LC, Allore HG, Bruce ML, Gill TM. Longitudinal association between depressive symptoms and disability burden among older persons. J Gerontol A Biol Sci Med Sci. 2009;64(12):1325–1332. doi: 10.1093/gerona/glp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffens DC, Hays JC, Krishnan KR. Disability in geriatric depression. Am J Geriatr Psychiatry. 1999;7(1):34–40. [PubMed] [Google Scholar]
- 10.Brenes GA, Penninx BW, Judd PH, Rockwell E, Sewell DD, Wetherell JL. Anxiety, depression and disability across the lifespan. Aging Ment Health. 2008;12(1):158–163. doi: 10.1080/13607860601124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair PA, Lyness JM, King DA, Cox C, Caine ED. Depression and self-reported functional status in older primary care patients. Am J Psychiatry. 2001;158(3):416–419. doi: 10.1176/appi.ajp.158.3.416. [DOI] [PubMed] [Google Scholar]
- 12.Lyness JM, King DA, Cox C, Yoediono Z, Caine ED. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc. 1999;47(6):647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 13.Karp JF, Skidmore E, Lotz M, Lenze E, Dew MA, Reynolds CF., 3rd Use of the late-life function and disability instrument to assess disability in major depression. J Am Geriatr Soc. 2009;57(9):1612–1619. doi: 10.1111/j.1532-5415.2009.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- 15.Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, Mesholam RI, Gur RE. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18(5):529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 17.Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher-Tascio M, Hrabe J, Ardekani BA, Alexopoulos GS. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16(4):255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- 18.Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, Reynolds CF, 3rd, Aizenstein H. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology. 2008;33(11):2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Vrontou C, Kakuma T, Meyers BS, Young RC, Klausner E, Clarkin J. Disability in geriatric depression. Am J Psychiatry. 1996;153:877–885. doi: 10.1176/ajp.153.7.877. [DOI] [PubMed] [Google Scholar]
- 20.Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. Executive dysfunction and disability in elderly patients with major depression. Am J Geriatr Psychiatry. 2001;9(3):269–274. [PubMed] [Google Scholar]
- 21.Kiosses DN, Alexopoulos GS, Murphy C. Symptoms of striatofrontal dysfunction contribute to disability in geriatric depression. Int J Geriatric Psychiatry. 2000;15:992–999. doi: 10.1002/1099-1166(200011)15:11<992::aid-gps248>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Moorhouse P, Song X, Rockwood K, Black S, Kertesz A, Gauthier S, Feldman H. Consortium to investigate vascular impairment of cognition. Executive dysfunction in vascular cognitive impairment in the consortium to investigate vascular impairment of cognition study. J Neurol Sci. 2010;288(1–2):142–146. doi: 10.1016/j.jns.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, Rubin SM, Yaffe K, Satterfield S, Kritchevsky SB Health ABC study. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 25.Steffens DC, Bosworth HB, Provenzale JM, MacFall JR. Subcortical white matter lesions and functional impairment in geriatric depression. Depress Anxiety. 2002;15(1):23–28. doi: 10.1002/da.1081. [DOI] [PubMed] [Google Scholar]
- 26.Steffens DC, Pieper CF, Bosworth HB, MacFall JR, Provenzale JM, Payne ME, Carroll BJ, George LK, Krishnan KR. Biological and social predictors of long-term geriatric depression outcome. Int Psychogeriatr. 2005;17(1):41–56. doi: 10.1017/s1041610205000979. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos GS, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Murphy CF. Anterior cingulate dysfunction in geriatric depression. Int J Geriatr Psychiatry. 2008;23(4):347–355. doi: 10.1002/gps.1939. [DOI] [PubMed] [Google Scholar]
- 28.Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry. 2005;13(3):244–249. doi: 10.1176/appi.ajgp.13.3.244. [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, Etwaroo GR, Klimstra S, Foxe JJ. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18(3):217–221. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29(12):2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57(3):285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 33.Kalayam B, Alexopoulos G. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 34.Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29(12):2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 35.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010;18(2):128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed Patients. Am J Geriatr Psychiatry. 2007;15(7):553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulos GS, Raue PJ, Kanellopoulos D, Mackin S, Arean PA. Problem solving therapy for the depression-executive dysfunction syndrome of late life. Int J Geriatr Psychiatry. 2008;23(8):782–788. doi: 10.1002/gps.1988. [DOI] [PubMed] [Google Scholar]
- 38.D'Zurilla TJ, Nezu AM. Problem-solving therapy: A social competence approach to clinical intervention. New York: Singer; 1999. [Google Scholar]
- 39.Arean PA, Perri MG, Nezu AM, Schein RL, Christopher F, Joseph TX. Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. J Consult Clin Psychol. 1993;61(6):1003–1010. doi: 10.1037//0022-006x.61.6.1003. [DOI] [PubMed] [Google Scholar]
- 40.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist. 2008;48(3):311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 41.Rovner BW, Casten RJ. Preventing late-life depression in age-related macular degeneration. Am J Geriatr Psychiatry. 2008;16(6):454–459. doi: 10.1097/JGP.0b013e31816b7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarrier N, Sharpe L, Beckett R, Harwood S, Baker A, Yusopoff L. A trial of two cognitive behavioural methods of treating drug-resistant residual psychotic symptoms in schizophrenic patients. II. Treatment-specific changes in coping and problem-solving skills. Soc Psychiatry Psychiatr Epidemiol. 1993;28(1):5–10. doi: 10.1007/BF00797826. [DOI] [PubMed] [Google Scholar]
- 43.van der Gaag M, Kern RS, van den Bosch RJ, Liberman RP. A controlled trial of cognitive remediation in schizophrenia. Schizophr Bull. 2002;28(1):167–176. doi: 10.1093/oxfordjournals.schbul.a006919. [DOI] [PubMed] [Google Scholar]
- 44.Areán PA, Raue PJ, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem Solving Therapy and Supportive Therapy in Older Adults with Major Depression and Executive Dysfunction. Am J Psychiatry. 2010 June 1; doi: 10.1176/appi.ajp.2010.09091327. (E Pub ahead of press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nezu AM, Nezu CM, Perri MG. Problem-solving therapy for depression: Theory research, and clinical guidelines. New York: Wiley; 1989. [Google Scholar]
- 46.Spitzer RL, Williams JBW, Gibbons M. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Washington DC: Am Psych Assoc Press, Inc; 1995. [Google Scholar]
- 47.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 49.Mattis S. Dementia Rating Scale. Odessa: Psychological Assessment Resources; 1989. [Google Scholar]
- 50.Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12(3):323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 51.Wetherell JL, Petkus AJ, McChesney K, Stein MB, Judd PH, Rockwell E, Sewell DD, Patterson TL. Older adults are less accurate than younger adults at identifying symptoms of anxiety and depression. J Nerv Ment Dis. 2009;197(8):623–626. doi: 10.1097/NMD.0b013e3181b0c081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epping-Jordan JA, Ustun TB. The WHODAS-II: leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- 53.Lineweaver TT, Bond MW, Thomas RG, Salmon DP. A normative study of Nelson's (1976) modified version of the Wisconsin Card Sorting Test in healthy older adults. Clin Neuropsychol. 1999;13(3):328–347. doi: 10.1076/clin.13.3.328.1745. [DOI] [PubMed] [Google Scholar]
- 54.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 55.Stout JC, Ready RE, Grace J, Malloy PF, Paulsen JS. Factor analysis of the frontal systems behavior scale (FrSBe) Assessment. 2003;10(1):79–85. doi: 10.1177/1073191102250339. [DOI] [PubMed] [Google Scholar]
- 56.McCrae RR, John OP. An introduction to the five-factor model and its applications. J Pers. 1992;60(2):175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 57.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Feder M, Einhorn A, Rosendahl E. Recovery in geriatric depression. Arch Gen Psychiatry. 1996;53(4):305–312. doi: 10.1001/archpsyc.1996.01830040039008. [DOI] [PubMed] [Google Scholar]
- 58.Hegel MT, Dietrich AJ, Seville JL, Jordan CB. Training residents in problem-solving treatment of depression: a pilot feasibility and impact study. Fam Med. 2004;36(3):204–208. [PubMed] [Google Scholar]
- 59.Marmar C, Weiss D, Gaston L. Toward the validation of the California Therapeutic Alliance Rating System. Psychological Assessment. 1989;1:46–52. [Google Scholar]
- 60.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11(2):167–178. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 61.Gellis ZD, McGinty J, Misener E, Bruce M. Problem solving therapy for late life depression in home care: a randomized field trial. Am J Geriatr Psychiatry. 2007;15(11):968–978. doi: 10.1097/JGP.0b013e3180cc2bd7. [DOI] [PubMed] [Google Scholar]
- 62.Bhaumik DK, Roy A, Aryal S, Hur K, Duan N, Normand S, Brown H, Gibbons RD. Sample size determination for studies with repeated continuous outcomes. Psychiatr Ann. 2008;38(12):765–771. doi: 10.3928/00485713-20081201-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrews G, Kemp A, Sunderland M, Von Korff M, Ustun TB. Normative data for the 12 item WHO Disability Assessment Schedule 2.0. PLoS One. 2009;4(12):e8343. doi: 10.1371/journal.pone.0008343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2003;11:1–7. [PubMed] [Google Scholar]; 64 Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, Leese M, McCrone P, Harris T, Moore M, Byng R, Brown G, Barthel S, Mander H, Ring A, Kelly V, Wallace V, Gabbay M, Craig T, Mann A. Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study. Health Technol Assess. 2009;13(22):iii–iv. ix–xi, 1–159. doi: 10.3310/hta13220. [DOI] [PubMed] [Google Scholar]
- 65.Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Davila-Roman VG, Steinmeyer BC, Hogue CW., Jr Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(4):387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freeman MP, Davis MF. Supportive psychotherapy for perinatal depression: preliminary data for adherence and response. Depress Anxiety. 2010;27(1):39–45. doi: 10.1002/da.20596. [DOI] [PubMed] [Google Scholar]
- 67.Brajkovic L, Jevtovic S, Bilic V, Bras M, Loncar Z. The efficacy of a brief supportive psychodynamic therapy in treating anxious-depressive disorder in Daily Hospital. Coll Antropol. 2009;33(1):245–251. [PubMed] [Google Scholar]
- 68.Oei TPS, Shuttlewood GJ. Specific and nonspecific factors in psychotherapy: A case of cognitive therapy for depression. Clinical Psychology Review. 1996;16(2):83–103. [Google Scholar]
- 69.Bruce ML. Depression and disability in late life: directions for future research. Am JGeriatr Psychiatry. 2001;9(2):102–112. [PubMed] [Google Scholar]
- 70.Lenze EJ, Rogers JC, Martire LM, Mulsant BH, Rollman BL, Dew MA, Schulz R, Reynolds CF., 3rd The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry. 2001;9(2):113–135. [PubMed] [Google Scholar]
- 71.Weinberger MI, Raue PJ, Meyers BS, Bruce ML. Predictors of new onset depression in medically ill, disabled older adults at 1 year follow-up. Am J Geriatr Psychiatry. 2009;17(9):802–809. doi: 10.1097/JGP.0b013e3181b0481a. [DOI] [PMC free article] [PubMed] [Google Scholar]