Abstract
We transplanted kidneys from α1,3-galactosyltransferase knockout (GalT-KO) pigs into six baboons using two different immunosuppressive regimens, but most of the baboons died from severe acute humoral xenograft rejection. Circulating induced antibodies to non-Gal antigens were markedly elevated at rejection, which mediated strong complement-dependent cytotoxicity against GalT-KO porcine target cells. These data suggest that antibodies to non-Gal antigens will present an additional barrier to transplantation of organs from GalT-KO pigs to humans.
Preformed antibodies directed against a specific oligosaccharide (Galαl-3Galβ1-4GlcNAc; Gal) on the vascular endothelium of porcine blood vessels1-4 have precluded successful pig-to–higher-order primate xenotransplantation. Extensive pig-to–nonhuman primate experiments have shown that Gal-specific antibodies not only cause hyperacute rejection (which occurs in a few hours), but also initiate acute humoral xenograft rejection (AHXR; which occurs in a few days to a few weeks)5-8. The recent production of cloned pigs lacking the galactosyltransferase enzyme (GalT-KO)9,10 raised hope that the organs from these animals would not only be spared from hyperacute rejection, but also would not be subject to AHXR. Here, we report our initial results of testing GalT-KO porcine kidneys in a life-supporting transplant model in baboons. The baboon recipients were immunosuppressed with either a multiagent immunosuppressive protocol, previously used to test transgenic porcine organs that express human decay accelerating factor (hDAF)11, or alternatively with a simpler ‘tolerance-friendly’ (a protocol with minimal immunosuppression) treatment strategy of immunosuppression that has been used clinically12.
We performed six kidney transplants in baboons using GalT-KO pigs as donors. We treated three of the baboons with a multiagent regimen that included a short course of thymoglobulin (ATG) followed by daily doses of tacrolimus, mycophenolate mofetil and steroids. We induced immunosuppression in the other three with a single high dose of ATG followed by monotherapy with tacrolimus (‘light therapy’; Table la and Supplementary Methods online).
Table 1.
| Table 1a Immunosuppressive protocols | |||
|---|---|---|---|
| Immunosuppressants | Immunosuppressive protocols |
||
| Multiagent | Light | ||
| Thymoglobulin (intravenous) | 2.5 mg/kg (day −2), 1.5–2.5 mg/kg (days 0–7), targeting lymph count <0.5 × 109 | 25 mg/kg, once (day –1) | |
| Tacrolimus (per os) | Dose | 4–5 mg/kg twice daily (from day 0) | 3–4 mg/kg twice daily (from day 0) |
| Trough level | 20–30 ng/ml | 10–15 ng/ml | |
| Methylprednisolone (intravenous) | 1.0 mg/kg (days 0–2), tapered by 0.05 mg/kg/d to 0.2 mg/kg | 1.0 g, once (day –1) | |
| MMF(per os) | 25–30 mg/kg twice daily (from day 8) | None | |
| Cobra venom factor (intravenous) | 0.05 mg/kg (day –2), 0.02 mg/kg every 36 h (days 1–14) | None | |
|
Table 1b Immunosuppressive protocol, antibody levels, survival, cause of death and terminal graft histology for individual animals | ||||||||
| Preformed antibodies to non-Gal antigensa |
Induced antibodies to non-Gal antigensa |
|||||||
| Animal ID | Protocol | IgG | IgM | IgG | IgM | Survival (d) | Cause of death | Graft histology |
| 20-02 | Light | 2.9 | 1.1 | 19.5 | 1.6 | 8 | Renal failure | AHXR III |
| 64-03 | Light | 3.4 | 1.0 | 88.1 | 2.5 | 11 | Renal failure | AHXR III |
| 67-03 | Lightb | 2.9 | 1.1 | 8.6 | 1.1 | 10 | Sepsis | AHXR I |
| 19-02 | Multiagent | 5.1 | 1.0 | 30 | 1.5 | 16 | Renal failure | AHXR III |
| 66-03 | Multiagentc | 2.7 | 1.1 | 7.2 | 3.0 | 13 | Renal failure | AHXR III |
| 65-03 | Multiagentd | 3.4 | 1.3 | 6.8 | 1.2 | 9 | Gastrointestinal bleeding | ACXR I, AHXR I |
Antibody data presented are the ratio of relative mean fluorescence (RMF); underline indicates high values of induced antibodies to non-Gal antigens.
This recipient received 50 mg/kg ATG for induction therapy.
This recipient received 21 d of desensitization pretreatment (plasmapheresis and intravenous immunoglobulin five times, two doses of Rituxan and daily tacrolimus and MMF).
This recipient received cyclophosphamide instead of ATG as induction therapy with splenectomy and Rituxan. MMF, mycophenolate mofetil; ACXR, acute cellular xenograft rejection.
Both endothelial cells and lymphocytes, isolated from the GalT-KO donor pigs, had undetectable Gal expression measured by FACS when compared with the negative controls (Supplementary Fig. 1 online), confirming that the GalT-KO donor pigs were truly Gal-negative.
All recipient baboons had low levels of preformed non-Gal–specific IgG and IgM (Table lb) before transplantation. Furthermore, sera collected from the recipient baboons before transplantation showed similar levels of complement-dependent cytotoxicity (CDC) against GalT-KO porcine lymphocytes as controls (Fig. la). None of the GalT-KO porcine grafts in this study developed hyperacute rejection.
Figure 1.
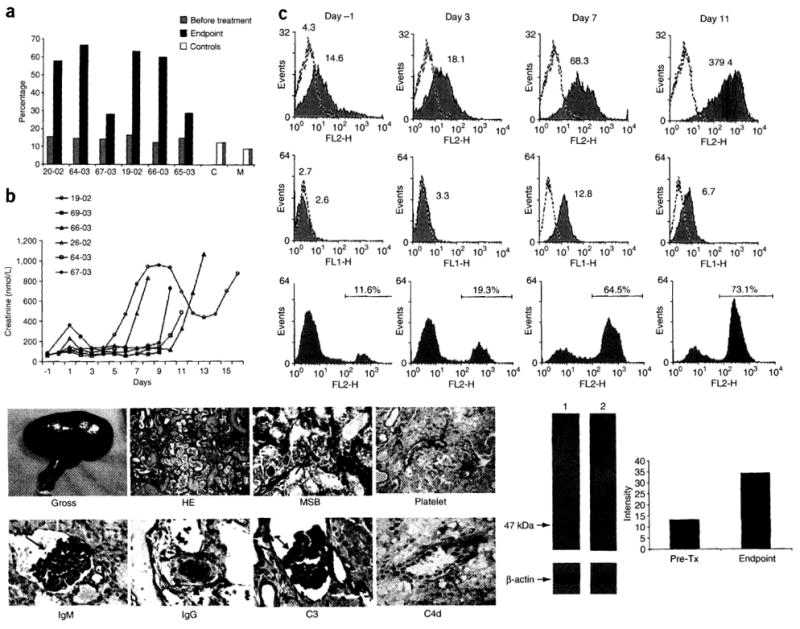
Elicited antibodies to non-Gal antigens lead to AHXR of GalT-KO kidney grafts. (a) CDC against GalT-KO porcine lymphocytes measured from sera collected before transplantation and on the day of death (Endpoint). Sera from 20-02, 64-03, 19-02 and 66-03 showed a marked elevation in CDC titers at the endpoint, when the xenografts developed severe AHXR. Target cells incubated with medium alone, without baboon serum and rabbit complement, served as medium control (M) and cells incubated with rabbit complement alone served as complement control (C). (b) Changes in serum creatinine levels of each recipient after transplantation. (c) Sequential measurement of antibodies to non-Gal antigens in the circulation and CDC to porcine GalT-KO endothelial cells in a representative recipient (64-03): IgG levels (upper panels) and IgM levels (middle panels). Both IgG and IgM levels were measured by FACS using GalT-KO porcine lymphocytes as targets. Cells were incubated with media and then stained with the secondary antibody serving as a control (dashed lines). Values represent geometric mean fluorescence intensity. There was marked elevation of both IgG and IgM antibodies to non-Gal antigens after GalT-KO kidney grafting. Lower panels show CDC against porcine GalT-KO endothelial cells. CDC increased after transplantation, and reached a peak level at the endpoint. (d) Representative gross and pathological changes of a GalT-KO kidney graft with severe AHXR in baboon 20-02: gross finding of the terminal graft (Gross); H&E staining of the terminal graft showing severe AHXR (HE); MSB staining of the terminal graft showing intravascular thrombosis (red stain with arrows, MSB). Immunopathology staining of the terminal graft showed positive platelet deposition (arrow, Platelet), massive IgM deposition (arrow, IgM), massive IgG deposition (arrow, IgG), massive C3 deposition (arrow, C3) and positive C4d deposition (arrow, C4d). (e) Representative photo of reactivity of baboon serum (19-02) against GalT-KO pig endothelial cells in western blots. Lane 1, incubated with baboon serum before transplantation (pre-Tx) and stained for human IgG, IgM and IgA antibodies; Lane 2, incubated with baboon serum at the endpoint stained with human IgG, IgM and IgA antibodies (left panel). Intensity comparison between pre-transplantation and endpoint as determined by western blot (right panel). We performed signal quantification with ImageJ software (US National Institutes of Health).
Table lb summarizes the clinical events and terminal graft histology for each animal. Using either immunosuppressive protocol, survival was limited between 8 and 16 d. Four baboons developed renal failure resulting from severe AHXR (Fig. lb), despite the fact that peripheral lymphocyte counts were well controlled at a level of less than 0.5 × 109 cells/1 (Supplementary Fig. 2 online). AHXR was coincident with acute thrombocytopenia and proteinuria followed by marked elevations in serum creatinine, reduction of urine output and increase of non-Gal–specific antibody (Fig. 1c and Supplementary Fig. 2 online). Terminal pathology analysis from these four animals showed classic features of severe AHXR, characterized by massive interstitial hemorrhage, infarction, necrosis, thrombosis and loss of tubules with polymorph infiltration and massive deposition of IgG, IgM, C3, C4d (despite complement depletion with cobra venom factor) and platelets (Fig. 1d). There was no notable change in the ELISA-measured levels of either Gal-specific IgG or IgM in the sera of recipient baboons during the entire follow-up transplant period (Supplementary Fig. 3 online). The other two baboons (67-03 and 65-03) died from Gram-negative bacterial sepsis or gastrointestinal bleeding on postoperative day 10 and 9, respectively. The pathology of renal grafts in these two cases showed only mild rejection (Grade I AHXR and/or AHXR; Table 1b).
Despite low levels of preformed antibodies to non-Gal antigens before transplantation, the porcine-specific antibodies were markedly induced after grafting. In three of the four baboons that developed severe AHXR, circulating non-Gal IgG antibodies against donor lymphocytes increased 7–26-fold from baseline to the time of rejection (Table lb and Fig. 1c). There was also a 1.5–2.5-fold increase of anti-non-Gal IgM levels in these three animals (Table lb and Fig. 1c). The fourth baboon (66-03) had only a moderate increase in non-Gal–specific IgG levels at the endpoint, but there was a threefold increase in non-Gal–specific IgM levels (Table 1b). Finally, the sera collected at the time of AHXR in these four baboons showed strong CDC (57.5–66.8%) against lymphocytes from GalT-KO pigs (Fig. 1a). Similar results also were obtained when vascular endothelial cells were targets (Fig. 1c). The xenografts of baboons 67-03 and 65-03, which died from infection (65-03) or gastrointestinal bleeding (67-03) when AHXR or ACXR was only Grade I, had minimal elevations in non-Gal–specific IgG antibody levels and no increase in non-Gal–specific IgM levels (Table 1b). At the endpoint, the sera collected from these two baboons showed low titers of CDC (< 30%) against GalT-KO porcine lymphocytes (Fig. 1a). The magnitude of the response of induced antibodies to non-Gal antigens, including CDC to GalT-KO lymphocytes or endothelial cells, was well associated with severity of rejection, suggesting that induced antibodies against non-Gal epitopes were responsible for AHXR.
To determine whether there were specific molecules that induced production of antibodies to non-Gal proteins during rejection, we performed western blotting using baboon sera against GalT-KO pig endothelial cells (Supplementary Methods online). Incubation of baboon preoperative sera with the porcine endothelial cell lysate showed multiple bands (Fig. 1e). When incubated with sera collected at the endpoint of rejection, one of these bands at molecular weight of 47 kDa was enhanced in all baboons except 66-03, in which the results were obscured by intravenous immunoglobulin the baboon received (Fig. 1e). These results indicate that a protein of 47 kDa isolated from porcine endothelial cells may be a major protein antigen that induced non-Gal antibody production during rejection.
Our results were notably different from those reported by others13,14 who transplanted kidneys and hearts from GalT-KO miniature pigs. In those studies, elevated levels of antibodies against non-Gal antigens were not reported. Different immunosuppressive protocols, thymic radiation, cotransplantation of donor thymus, two kinds of GalT-KO pigs or different sources of the baboons may have contributed to the difference in the outcome. The inclusion of a CD154-specific monoclonal antibody in the immunosuppressive protocol used in these studies might effectively inhibit elicited antibodies13,14. The clinical use of this antibody, however, has been hampered by its thrombotic tendencies15. Furthermore, a high complication rate and mortality in their kidney transplant recipients were noted in their report13. We previously used the same multiagent immunosuppressive protocol used in this study combined with the neutralization of Gal-specific antibodies in an hDAF pig-to-baboon kidney transplant model11. A median 13-d survival (range, 7–75 d) was achieved in this study. These data suggest that, in the presence of an induced antibody response, genetic depletion of Gal antigens does not provide a major benefit in xenograft survival.
In conclusion, kidneys from GalT-KO pig donors are not hyper-acutely rejected by baboons under different immunosuppressive regimens. The problem posed by AHXR, however, has eluded solution. In our studies, AHXR was caused by induced antibodies to non-Gal antigens, and it seems essential to prevent this. Results from current studies in nonhuman primates are not promising enough to justify clinical trials. The future of xenotransplantation should emphasize further modification of donors, such as the combination of hDAF, GalT-KO, deletion of non-Gal antigens and adding a gene to inhibit coagulation dysregulation, rather than using more potent immunosuppressive agents in recipients.
Supplementary Material
Acknowledgments
The authors thank Revivicor Inc. for providing GalT-KO pigs, Fujisawa Canada for the donation of tacrolimus, Sangstat for providing Thymoglobulin and Bayer Canada for providing IVIG. We appreciate editorial assistance from C. Abbott and secretarial support from S. Mutch and T. Mangan. This work was supported by the Ontario Research Development Challenge Fund; Multi-Organ Transplant Program, London Health Sciences Centre; Fujisawa Canada, US National Institutes of Health grant R0lDK64207, AI38899 (to T.S.); and an unrestricted gift to the Thomas E. Starzl Transplantation Institute from the Eberly Family Fund for Transplant Innovation.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare competing financial interests (see the Nature Medicine website for details).
Note: Supplementary information is available on the Nature Medicine website.
References
- 1.Galili U, et al. J Exp Med. 1985;162:573–582. doi: 10.1084/jem.162.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oriol R, et al. Transplantation. 1993;56:1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DK, et al. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 4.Collins BH, et al. Xenotransplantation. 1994;1:36–46. [Google Scholar]
- 5.Ye Y, et al. Transplantation. 1994;58:330–337. [PubMed] [Google Scholar]
- 6.Xu Y, et al. Transplantation. 1998;65:172–179. doi: 10.1097/00007890-199801270-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lin SS, et al. Transplantation. 2000;70:1667–1674. doi: 10.1097/00007890-200012270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bhatti FN, et al. Transplant Proc. 1999;31:958. doi: 10.1016/s0041-1345(98)01855-7. [DOI] [PubMed] [Google Scholar]
- 9.Lai L, et al. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 10.Phelps CJ, et al. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, et al. Transplantation. in the press. [Google Scholar]
- 12.Starzl TE, et al. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K, et al. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 14.Kuwaki K, et al. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 15.Knechtle S. Immunol Rev. 2003;196:237–246. doi: 10.1046/j.1600-065x.2003.00086.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


