Abstract
Objective
To determine the incidence, clinical presentation, and outcome and confounding factors associated with the development of a brain abscess in solid organ transplant recipients.
Design
A 14-year retrospective survey.
Setting
A single, multiorgan, academic transplantation center.
Patients
A total of 2380 liver transplant recipients, 1650 kidney transplant recipients, and 598 heart, heart-lung, or lung transplant recipients of all ages (pediatric and adult) were included. All patients were given cyclosporine-based immunosuppression during this period.
Main Outcome Measure
A brain abscess was determined to be present if there was histological and/or microbiological confirmation of a brain lesion seen by a computed tomographic scan. A brain abscess was considered suspicious if radiographic findings were seen in the clinical setting of neurologic symptoms and fever without histological or microbiological confirmation.
Results
A brain abscess developed in a total of 28 patients (0.61%) of the total study population. The frequency of brain abscess according to organ type was as follows: 0.63%, liver; 0.36%, kidney; and 1.17%, heart and heart-lung. The overall mortality was 86%. Complicating factors associated with fungal (Candida and Aspergillus sp) abscess formation included major subsequent operations, retransplantations, antirejection therapy, associated bacteremia or viremia, and multiorgan failure. The lung was the primary site of dissemination in 18 patients. Low-dose prophylactic amphotericin was ineffective in preventing a fungal brain abscess in 10 high-risk patients. Because of the ineffective therapy and the deadly nature of established fungal abscesses, full-dose antifungal therapy and reduced immunosuppression were warranted on identification of a high-risk clinical setting. Nonfungal abscesses (Nocardia and Toxoplasma sp) occurred in healthy graft recipients long after transplantation. The existing medical therapy is usually effective in these patients, provided that rapid tissue diagnosis is established.
Conclusion
The epidemiological features of brain abscess formation after solid organ transplantation suggest 2 populations of patients exist that differ in timing, clinical setting, and response to therapy. For the chronically immunosuppressed outpatient, an established abscess should be empirically treated with sulfonamides until tissue diagnosis is confirmed. On the other hand, the acutely immunosuppressed posttransplant recipient, with defined risk factors, should receive full-dose therapy with amphotericin B and concomitantly lowered immunosuppression.
A brain abscess is a focal suppurative process of variable size that develops within the brain parenchyma and is surrounded by an inflammatory exudate. In the immunocompetent host, brain abscesses are usually bacterial. They consist of a central area of necrotic debris and leukocytes with a fibroblastic capsule surrounded by cerebritis and perivascular infiltrates.
In the immunocompromised host, however, fungal brain abscesses predominate and usually originate as hematogenous dissemination from a primary site of invasion. Microscopically, these are manifested by an arteritis with fungal thrombosis and obliteration of the vessel lumen producing ischemic or hemorrhagic infarcts.1 This pattern of encephalomalacia may result in solitary or multiple abscesses. Mortality among patients with brain abscesses who have not undergone transplantation can be as high as 25% in some series.2 This study encompasses a 14-year period with a minimum 2-year follow-up of 4628 solid organ transplant recipients who have been receiving cyclosporine-based immunosuppression after transplantation at the University of Pittsburgh Medical Center, Pittsburgh, Pa. The clinical spectrum of brain abscesses in the liver, kidney, and heart, lung, and heart-lung transplant recipients is characterized.
This study (1) defines the incidence and severity of brain abscesses in this population, (2) identifies patients at risk for the development of a brain abscess, (3) describes the clinical pattern and evolution of a brain abscess, (4) examines the cause of the brain abscesses according to allograft type, (5) assesses the efficacy of available treatment options, and (6) proposes other effective treatment methods.
PATIENTS AND METHODS
PATIENT POPULATION
A 14-year retrospective review of all adult and pediatric patients who underwent liver (n=2380), kidney (n=1650), and heart, heart-lung, or lung (n=598) transplantation was done to determine the frequency and outcome of brain abscesses in a solid organ transplantation population who were receiving cyclosporine therapy. All organ recipients were classified according to United Network Organ Sharing guidelines and selected based on medical urgency.
SURGICAL TECHNIQUE
Established techniques for multiple organ procurement were used in all cases. Standard surgical techniques for orthotopic liver, heart, double lung, heart-lung, and heterotopic kidney transplantations were performed.3 No single lung transplantations were done during this interval
IMMUNOSUPPRESSION
Liver and kidney recipients were given cyclosporine, 8 to 12 mg/kg per day, for induction therapy, then adjusted to maintain cyclosporine concentrations of 750 to 1000 ng/mL as measured by fluorescence polarization immunoassays (TDx, Abbott Diagnostic, Chicago, Ill). Methylprednisolone sodium succinate (1 g for patients weighing ≥30 kg and 500 mg for those weighing <30 kg) was also given for induction therapy, followed by a 7-day methylprednisolone taper for adults. The daily maintenance prednisone dose was 10 mg for recipients weighing less than 30 kg and 20 mg for those weighing 30 kg and more. Acute cellular rejection was treated with 1 g of methylprednisolone sodium succinate and/or a corticosteroid taper and adjustment of the cyclosporine dose. Resistant rejections were treated with monoclonal antibodies or muromonab-CD3 (Orthoclone OKT3, Ortho Pharmaceutical Corp, Raritan, NJ), 5 mg/d (adult dose) or 2.5 mg/d (pediatric dose), for 7 to 14 days.
Heart, lung, and heart-lung transplant recipients all received a preoperative loading dose of cyclosporine orally, 17.5 mg/kg, and azathioprine sodium intravenously, 4 mg/kg; intraoperatively, they received 1 g of methylprednisolone sodium succinate intravenously. Postoperatively, cyclosporine was adjusted to maintain a 12-hour trough with blood concentrations between 500 and 1000 ng/mL (TDx). The heart transplant recipients received a corticosteroid taper to a daily-maintenance prednisone dose of 15 to 20 mg. The heart-lung transplant recipients did not receive corticosteroids for the first 14 days post-operatively to allow the tracheal anastomosis to heal. During this time, they received a 3- to 5-day course of rabbit antithymocyte globulin and/or azathioprine sodium, 1 to 2 mg/kg per day. Acute cellular rejection was treated with 1 g of methylprednisolone sodium succinate and a taper for the first rejection episode and with rabbit antithymocyte globulin or muromonab-CD3 for subsequent episodes. The postoperative course of all patients was observed up to January 1, 1995.
ANTIBIOTIC PROPHYLAXIS
Selective bowel decontamination was not used in any of the transplant recipients during this period. Acyclovir and a combination of trimethoprim-sulfamethoxazole in adjusted doses were used as prophylaxis for herpesvirus and pneumocystic infections. Prophylactic Systemic antibiotics were used for 3 to 5 postoperative days. Liver transplant recipients, for the last 7 years of the study, received a low dose of amphotericin B, 0.25 to 0.30 mg/kg per day, as a prophylactic antifungal therapy when (1) operations lasted longer than 12 hours, (2) the primary nonfunction of the graft required retransplantation, and (3) multiple subsequent operations were necessary. Therapy was generally continued for at least 2 weeks or for the duration of the patient's stay in the intensive care unit after identification of the patient as high risk.
DEFINITION OF A BRAIN ABSCESS
A pyogenic brain abscess classically consists of a central area of necrotic debris and leukocytes surrounded by a dense fibroblastic capsule. Beyond this is a zone of cerebritis, neovascularity, and edema. This appears on a computed tomographic (CT) scan as a hypodense region surrounded by an enhancing ring of uniform thickness with a peripheral area of hypodensity representing edema.3
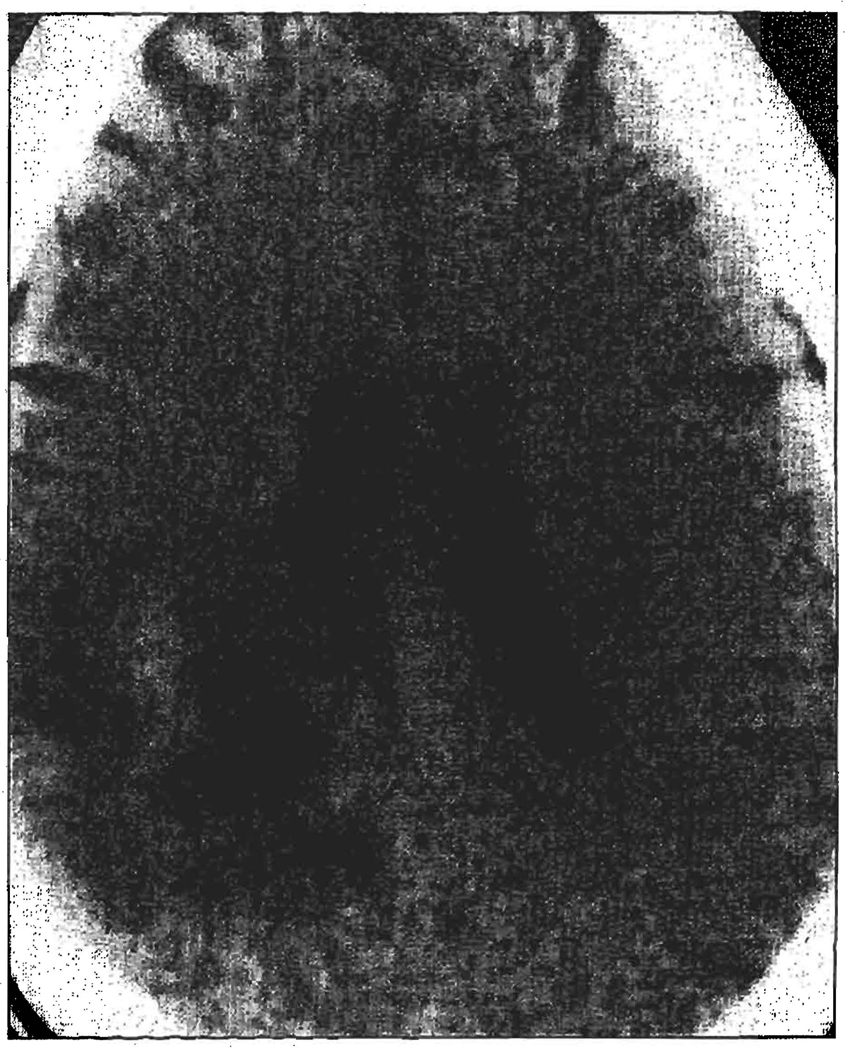
The cause of a brain abscess in patients who have undergone transplantation is usually fungal. These abscesses have disseminated hematogenously from other sites and lead to arteritis with fungal thrombosis of the cerebral vessel lumen (Figure 1). Solitary or multiple brain abscesses may arise from the resulting encephalomalacia. These areas of ischemic or hemorrhagic infarction are often not well encapsulated and can appear on a CT scan as nonspecific, low-density lesions with minimal or no contrast enhancement (Figure 2).
In this study, a brain abscess was determined to be present if there was histological and/or microbiological confirmation of a brain lesion as seen by the CT scan. Specimens were obtained during a stereotactic or open biopsy or at postmortem autopsy. A brain abscess was considered suspicious if radiographic findings were seen in the clinical setting of neurologic symptoms and fever even if histological or microbiological confirmation was absent. Positive CT findings included solitary or multiple low-density lesions with minimal contrast enhancements or areas of ischemic or hemorrhagic infarction as previously described.
STATISTICAL ANALYSIS
This is a retrospective review of all solid organ transplant recipients in whom a brain abscess developed during a 14-year period. The clinical course of those in whom a brain abscess developed is described. A statistical comparison was impossible because of the large number of patients without a brain abscess. The results are given in descriptive form.
RESULTS
GENERAL
During a 14-year period, there were 28 patients with brain abscesses among 4628 transplant recipients for an overall prevalence of 0.61 %. Brain abscesses occurred in 0.63% of liver, 0.36% of kidney, and 1.17% of heart and/or lung transplant recipients. The average age (± SE) of affected patients was 36.5±2.8 years (range, 14 days to 66 years) with a male-female ratio of 1:1.
Fever (in 26 patients), seizures (in 12 patients), and deterioration of mental status with progression to coma (in 12 patients) were the most common signs. Cytological cerebrospinal fluid abnormalities were slight and nonspecific, and all 22 cerebrospinal fluid cultures were sterile. Eleven electroencephalograms were performed, showing epileptiform foci in 4 and slow waves or no cortical activity in another 4; the electroencephalographic recordings were normal for 3 patients. Contrast-enhanced CT scans of the head showed a single abscess in 8 patients and multiple abscesses in 13 patients. Characteristic CT scan findings of enhancing lesions were noted in 16 patients, and hemorrhagic infarctions were noted in 5 patients; 4 patients had a normal CT scan finding. All anatomical areas of the brain were represented in abscess formation.
Brain abscesses were confirmed by histological and/or microbiological isolation from autopsy (17 patients), brain biopsy (7 patients), or maxillary sinus biopsy (2 patients) specimens. In 2 patients, the diagnosis of a brain abscess was clinical, based on a primary site of Aspergillus sp lung infection and brain lesions on a CT scan. The diagnosis was made in 2 of the patients post mortem. All infections were caused by a fungus or Nocardia or Toxoplasma. There were no typical pyogenic brain abscesses. With the exception of 3 patients with mixed isolates, all patients had abscesses with a single isolate. Hematogenous spread from the lung occurred in 18 of 28 cases, while an adjacent sinus infection caused the abscess in 2 patients. Other sites of secondary dissemination, aside from the liver, included the heart, thyroid, spleen, kidneys, muscles, skin, pleura, and pericardium.
LIVER
Liver recipients in whom brain abscesses developed (n= 15) required augmented immunosuppression; 9 patients received at least one 7-day course of muromonab-CD3, and 11 received at least one additional corticosteroid bolus and/or taper. Associated bacteremia occurred in 11 recipients, and cytomegalovirus viremia occurred in 5 recipients. The retransplantation ratio was 1.8 livers per patient, and 11 patients had poor initial graft function. All 15 patients were ventilator dependent beyond 2 weeks; 12 had renal failure and were hemodialysis dependent. In addition, 9 liver recipients underwent at least 1 abdominal reexploration exclusive of transplantations. The reasons for the subsequent operation were intra-abdominal hemorrhage in 7 patients, biliary reconstruction in 1 patient, and peritonitis in 1 patient. Mortality was due to sepsis in all but 2 cases, in which it was directly related to hemorrhagic complications of neuro-surgical procedures (Table 1).
Table 1.
Clinical Characteristics of Brain Abscesses by Causative Agent*
| Causative Agent |
Allograft Type | Initial Graft Function |
Primary Site of Infection |
Additional Antirejection Treatments |
Associated Infection | Time From Transplantation to Diagnosis, d |
Outcome |
|---|---|---|---|---|---|---|---|
| Aspergillus sp (22) | Liver (13); kidneys (5); and heart and heart-lung (4) | Poor (16), good (6) | Lungs (17) and sinus (1) | Corticosteroids (9); muromonab-CD3 (15); and rabbit antithymocyte globulin (2) | Bacteremia (17); viremia (8); cytomegalovirus (9); and Epstein-Barr virus (1) | 24† | Dead (21), alive (1) |
| Toxoplasma sp (2) | Liver (1) and heart (1) | Poor (0), good (2) | Blood (2) | Corticosteroids (0) and muromonab-CD3 (0) | Bacteremia (0) and viremia (0) | 26‡ | Dead (2), alive (0) |
| Nocardia sp (2) | Heart (2) | Poor (0), good (2) | Chest wall (2) | Corticosteroids (0) and muromonab-CD3 (0) | Bacteremia (1) and viremia (0) | 267‡ | Dead (0), alive (2) |
| Mucorales sp (1) | Liver (1) | Poor (1), good (0) | Sinuses (1) | Corticosteroids (1) and muromonab-CD3 (0) | Bacteremia (1) and viremia (0) | 28 | Dead (1) |
| Candida sp (1) | Kidney (1) | Poor (1), good (0) | Lung (1) | Corticosteroids (5) and muromonab-CD3 (0) | Bacteremia (1) and viremia (0) | 134 | Alive (1) |
The number in parentheses indicated the number of patients.
Median.
Mean.
Aspergillus sp were the most common organism found in the liver recipients (found in 13 of the 15 patients), and these organisms were seen a median of 24 days after transplantation. A mucormycosis infection occurred in 1 ventilator-dependent liver transplant recipient with poor initial graft function, an associated bacterial infection, and prolonged leukopenia who required additional antirejection therapy. It involved the left frontal and adjacent maxillary sinus with direct extension to brain tissue. The patient died of sepsis 37 days posttransplantation despite receiving antifungal therapy. A brain abscess caused by Toxoplasma sp developed 62 weeks post-transplantation in one patient with a functional graft and no other major risk factors (Table 2).
Table 2.
Clinical Characteristics of Brain Abscesses by Allografted Organ Type
| Organ Type* | Transplantation Frequency |
No. of Major Subsequent Operations |
Organism Frequency* | No. of Patients Who Were |
Outcome* | |
|---|---|---|---|---|---|---|
| Ventilator Dependent |
Hemodialysis Dependent |
|||||
| Liver (15) | 1.8 Livers per patient | 9 | Aspergillus sp (13); Mucorales sp (1); and Toxoplasma sp (1) | 14 | 12 | Dead (14), alive (1) |
| Heart and heart-lung (7) | 1.1 Hearts per patient | 3 | Aspergillus sp (4); Nocardia sp (2); and Toxoplasma sp (1) | 4 | 4 | Dead (5), alive (2) |
| Kidneys (6) | 1.3 Kidneys per patient | 5 | Aspergillus sp (5) and Candida sp (1) | 6 | 5 | Dead (5), alive (1) |
The number in parentheses indicates the number of patients.
HEART
Brain and chest wall abscesses caused by Nocardia sp developed in 2 heart recipients with good primary graft function and no serious rejection episodes an average of 38 weeks after transplantation. Both recovered after undergoing an aspiration biopsy and receiving antibiotic therapy (Table 1).
A brain abscess caused by Toxoplasma sp developed 12 weeks posttransplantation in a heart recipient who was otherwise healthy and had an uneventful postoperative course (Table 2). Unfortunately, he died before specific diagnosis and treatment of the brain abscess were accomplished.
A brain abscess caused by Aspergillus sp occurred in 4 heart or heart-lung recipients; these recipients had poor graft function (3 patients), bacteremia (3 patients), and cytomegalovirus or Epstein-Barr virus viremia (1 patient), were ventilator (all 4 patients) and hemodialysis (3 patients) dependent, and required additional treatments of muromonab-CD3 and rabbit antithymocyte globulin (2 patients). All 4 patients died with medical management alone.
KIDNEYS
Four of 6 kidney transplant recipients in whom a brain abscess developed had failed allografts, with a retransplant ratio of 1.3 kidneys per patient. Five had major subsequent operations, 2 for allograft nephrectomy and 1 each for intra-abdominal hemorrhage, gastrointestinal bleeding, and colonic perforation. All 6 received additional corticosteroid boluses or taper, and 1 received murornonab-CD3 for acute cellular rejection (Table 1).
Associated bacteremia was present in 5 of the 6 patients, and cytomegalovirus viremia was noted in 2 patients. All 6 patients were ventilator dependent for prolonged periods, and 5 were hemodialysis dependent. A brain abscess caused by Aspergillus sp disseminated from primary pulmonary invasion in 4 patients and by direct extension from a mastoid sinusitis in 1 patient. No primary site of invasion was identified in 1 patient (Table 2).
MEDICAL AND SURGICAL THERAPY
Despite the administration of low-dose prophylactic amphotericin B (0.25–0.3 mg/kg per day) fungal brain abscesses (caused by Aspergillus or Candida sp or by mucormycosis) developed in 10 of 24 patients (9 liver and 1 kidney recipient). We estimate that this figure represents approximately 20% of the total patients who received prophylactic amphotericin B. Of the 24 patients, 19 received therapeutic doses (ie, 0.75–1 mg/kg per day) of intravenous amphotericin B after diagnosis, 1 received intravenous and intrathecal amphotericin B, and 4 were treated with intravenous amphotericin B and by brain abscess drainage.
The remaining 4 recipients with nonfungal brain abscesses (caused by Nocardia and Toxoplasma sp) were treated with intravenous and oral antibiotic combinations; in the 2 patients with a brain abscess caused by Nocardia sp, excision of the chest wall lesions was performed.
SURVIVAL
Of 28 patients, 24 died, for an overall mortality of 86%. Two (8.3%) of the 24 patients with a fungal brain abscess in this study survived, while 2 (50%) of the patients with a nonfungal brain abscess survived. Both patients with an abscess caused by Toxoplasma sp died, while both patients infected with Nocardia sp survived. An analysis of the 4 survivors produced the following findings. A 14-day-old pediatric liver recipient with aspergillosis was treated with intrathecal and intravenous amphotericin B and was still alive at the 64-month follow-up visit. However, this patient has residual neurologic deficits and mental retardation.
A second liver recipient in whom a brain abscess caused by Candida sp developed was treated with drainage and intravenous amphotericin B and was alive without residual neurologic deficits at the 146-month follow- up visit. Two heart recipients with an abscess caused by Nocardia sp were treated with local chest wall excision and a combination of trimethoprim-sulfamethoxazole and aminoglycoside antibiotics; they also survived their infection.
COMMENT
Our pathologic definition of a brain abscess included all patients with infected localized intracerebral mass lesions. This dovetails well with the classification scheme of Conti and Rubin,4 who categorized central nervous system infections after transplantation into 4 clinical syndromes: acute meningitis, subacutechronic meningitis, meningoencephalitis, and focal brain dysfunction due to a localized mass lesion (ie, a brain abscess).
Two clinical patterns emerged from our survey of this transplantation population that may change the therapeutic approach for these patients. First, a pyogenic bacterial brain abscess, commonly seen in immunocompetent patients, did not occur in this population. This is most likely due to the fact that posttransplantation immunosuppression primarily impairs T-cell rather than B-cell function. It has been observed in our series and other series5–7 that Aspergillus sp is the most common causative organism, followed by Toxoplasma gondii and Nocardia asteroides.
Second and most importantly, there are 2 completely disparate groups within the transplantation population with regard to the timing, susceptibility, and predisposition for brain abscesses. This fact has important diagnostic and therapeutic implications. One group, which transcends allograft boundaries, includes those recipients who, shortly (median, 24 days in our series) after receiving induction therapy, have a postoperative course severely complicated by medical, surgical, and immunologic events. Fungi (ie, Aspergillus, Candida, and Mucorales sp) caused all of the abscesses in this setting and were resistant to any form of therapy. The second group consisted of the chronically immunosuppressed although otherwise healthy recipients; abscesses caused by non fungal organisms (ie, Nocardia and Toxoplasma sp) developed long (mean, 264 days) after transplantation. There may be some tendency to stratification by allograft type in this second group, particularly with Nocardia sp, which exclusively involved the heart transplant recipients in this series.
FUNGAL BRAIN ABSCESSES
Fungal brain abscesses in the transplantation population most often arise by hematogenous dissemination from the lung.8–12 Aspergillus sp are common respiratory pathogens with a 10% incidence of dissemination to the brain,13 most of which occur during the first 3 months after transplantation,5,14 An acute brain infarction or bleeding may be the earliest clue to the presence of a brain abscess caused by Aspergillus sp because of the organism's propensity to invade both large and small cerebral vessels, producing thrombosis and consequently hemorrhagic or ischemic infarcts. 1,8,15,16 Associated fungal meningitis is rare and, therefore, cerebrospinal fluid smears and cultures for organisms are usually negative.4,8,17 A CT scan usually reveals multiple low-density, nonenhancing lesions that are located most frequently in the cerebrum and the basal ganglia.4,17–19 The most reliable method of diagnosis remains biopsy with a histological examination and a culture. It is rarely treated successfully even when diagnosed after death,8,20,21 possibly because of the virulence of the organism and its tendency to occlude blood vessels with consequent difficulty in the delivery of antifungal agents to infected brain tissue.
Less commonly, abscesses caused by Candida and Mucorales sp arose under similar clinical circumstances.
The systemic administration of treatment with fulldose amphotericin B seems to be inadequate because of suboptimal penetration across the blood-brain barrier. Craniotomy with excision and/or drainage22–26 for diagnostic and therapeutic purposes should play an active role in the treatment of these patients. The role of newer antifungal agents, such as liposomal amphotericin B or itraconazole, is still unclear.
The mortality of 91.7% for patients with fungal brain abscesses in this series is similar to the 85% to 100% mortality reported in the English-language literature.1,23,27–31 Even the administration of low-dose prophylactic amphotericin B in 10 patients who were deemed to be at higher risk did not completely prevent the development of fungal brain abscesses. However, the denominator of high-risk patients who received this low-dose therapy was difficult to determine, so it cannot be stated that this form of therapy was entirely ineffective. Nevertheless, it seems that established central nervous system abscess formation is almost always fatal and that the survivors have a risk for serious neurologiC sequelae.
The low frequency of fungal brain abscesses among all transplant recipients, and their complete absence in those chronically immunosuppressed although otherwise healthy outpatients, suggests that the virulence of these organisms depends on proximate circumstances being present before a cerebral fungal abscess can occur. However, once multiple complicating factors (ie, subsequent operations, a retransplantation, hemodialysis, or ventilator dependence) are present, and especially when pulmonary colonization occurs, then fungemia may be imminent. Because treatment with low-dose amphotericin B did not prevent the development of a brain abscess in these cases. we would advocate a trial of full-dose amphotericin B therapy for those patients deemed to be at high risk and who have pulmonary colonization with Aspergillus sp. In conjunction, a reduction or cessation of immunosuppression will allow reinstitution of immune mechanisms and hopefully prevent abscess formation during this short-term medical period.
NONFUNGAL BRAIN ABSCESS
Nonfungal brain abscesses in transplant recipients also have unifying features: (l) They tend to occur in healthier patients at a long interval after transplantation when the patients are essentially recovered. In this sense, they resemble the typical opportunistic pneumonias (ie, Legionella and Pneumocystis sp) and delayed cryptococcal meningitis. (2) The onset is fairly insidious but pursues a rather fulminant course once objective symptomatology is present. (3) There may be some stratification by allograft type with heart recipients being at highest risk. (4) Effective medical therapy does exist and should be empirical until diagnostic or therapeutic drainage provides a specific organism.
A brain abscess caused by Nocardia sp usually takes the form of a single rather than multiple brain abscesses or meningitis.3 Because cerebrospinal fluid and serological tests have not been found to be helpful, diagnosis is often made by presumption, particularly in patients with known nocardial lung or soft tissue infections and abnormal neurologic and/or CT scan findings.4,32 A brain abscess caused by Toxoplasma sp frequently presents as an intracranial mass lesion and seizures, although the absence of symptoms is not uncommon.33,34 Unlike abscesses caused by Nocardia sp, positive results of serological tests and tissue examinations are diagnostic. Reports in the literature have described Toxoplasma sp brain dissemination from a donated heart,35 spread from activation of latent infection in experimental animals, and transmission by transfusion of whole blood cells.36
Sulfonamides have been established to be effective if rapidly instituted in patients with a nonfungal brain abscess because the minimum inhibitory concentration is readily achievable with good penetration across the blood-brain barrier. Computed tomography-guided stereotactic aspiration or an open crapiotomy and drainage are recognized as adjunctive to medical therapy.37–39
Our overall mortality for patients with a nonfungal brain abscess was 50%. Both heart recipients with a brain abscess caused by Nocardia sp in our series survived the infection, which is in stark contrast with the mortality of 75% to 90% reported in the literature.40,41 This is probably due to early diagnosis and prompt treatment of the infected patients.
CONCLUSION
The epidemiological features of brain abscess formation after solid organ transplantation suggest 2 populations of at-risk patients who are distinct in time, clinical setting, and therapeutic approach. For the chronically immunosuppressed outpatient, an established brain abscess should be empirically treated to manage Nocardia and Toxoplasma sp infections until tissue diagnosis is available. On the other hand, the acutely immunosuppressed posttransplantation recipient with high medical acuity is at high risk for the formation of a fungal brain abscess. There is no effective therapy, but frequent surveillance for pulmonary colonization should be performed. Should Aspergillus sp be found in these patients, it should be treated with therapeutic doses of amphotericin B. In both types of abscess formation, surgical intervention to confirm an empirical diagnosis or to drain amenable lesions is valuable.
Figure 1.
Arteritis with fungal thrombosis of the cerebral vessel lumen.
Figure 2.
A computed tomographic scan of a brain abscess.
REFERENCES
- 1.Walsh JJ, Hier OB, Caplan LR. Aspergillosis of central nervous system: clinicopathological analysis of 17 patients. Ann Neural. 1985;18:574–582. doi: 10.1002/ana.410180511. [DOI] [PubMed] [Google Scholar]
- 2.Seydoux C, Francioll P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394–401. doi: 10.1093/clind/15.3.394. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Shapiro R, Simmons RL. Atlas of Organ Transplantation. Philadelphia, Pa: Grower Medical Publishing; 1992. [Google Scholar]
- 4.Conti DJ, Rubin RH. Infection of the central nervous system. Neurol Clinics. 1988;6:241–258. [PubMed] [Google Scholar]
- 5.Boon AP, Adams DH, Buckels JAC, McMaster P. Neuropathologic findings in autopsies alter liver transplantation. Transplant Proc. 1991;23:1471–1472. [PubMed] [Google Scholar]
- 6.Britt RH, Enzmann DR, Remington JS. Intracranial infection in cardiac transplant recipients. Ann Neurol. 1981;9:107–119. doi: 10.1002/ana.410090203. [DOI] [PubMed] [Google Scholar]
- 7.Martinez AJ, Barmada MA. Main complications in children and adults. Mod Pathol. 1993;6:25–32. [PubMed] [Google Scholar]
- 8.Young RC, Bennett JE, Vogel CL, Carborne PP, DeVita VT. Aspergillosis: the spectrum of the disease in 98 patients. Medicine (Baltimore) 1970;49:147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Meyer RD, Young LS, Armstrong D, et al. Aspergillosis complicating neoplastic disease. Am J Med. 1973;54:6–15. doi: 10.1016/0002-9343(73)90077-6. [DOI] [PubMed] [Google Scholar]
- 10.Shaw FW, Warthen HJ. Aspergillosis of bone. South Med J. 1936;29:1070–1071. [Google Scholar]
- 11.Prystowsky SK, Vogelstein B, Ettinger OS, et al. Invasive aspergillosis. N Engl J Med. 1976;295:655–658. doi: 10.1056/NEJM197609162951206. [DOI] [PubMed] [Google Scholar]
- 12.Epstein NE, Hollingsworth R, Black K, Farmer P. Fungal brain abscesses in 2 immunosuppressed patients. Surg Neurol. 1991;35:286–289. doi: 10.1016/0090-3019(91)90006-u. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan K. Brain abscess. Med Clin North Am. 1985;69:345–360. [PubMed] [Google Scholar]
- 14.Wajszczuk CP, Dummer JS, Ho M, et al. Fungal infections in liver transplant recipients. Transplantation. 1985;40:347–353. doi: 10.1097/00007890-198510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohandas S, Ahuja GK, Sood VP, Virmani V. Aspergillosis of the central nervous system. J Neural Sci. 1978;38:229–233. doi: 10.1016/0022-510x(78)90069-2. [DOI] [PubMed] [Google Scholar]
- 16.Torre CJ, Lopez OL, Kusne S, et al. CNS aspergillosis in organ transplantation: a clinicopathologic study. J Neurol Neurosurg psychiatry. 1993;56:188–193. doi: 10.1136/jnnp.56.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Yu V, Gayowski T. CNS lesions in adult liver transplant recipients: clinical review with implications for management. Medicine. 1994;73:110–118. doi: 10.1097/00005792-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Estol CT, Pessin MS, Martinez AJ. Cerebrovascular complications after orthotopic liver transplant: a clinicopathologic study. Neurology. 1991;41:815–819. doi: 10.1212/wnl.41.6.815. [DOI] [PubMed] [Google Scholar]
- 19.Polo JM, Fabrega E, Casafont F, et al. Treatment of cerebral aspergillosis after liver transplantation. Neurology. 1992;42:1817–1819. doi: 10.1212/wnl.42.9.1817. [DOI] [PubMed] [Google Scholar]
- 20.Burton JR, Zachery JB, Bessin R, et al. Aspergillosis in 4 renal transplant recipients: diagnosis and effective treatment with amphotericin B. Ann intern Med. 1972;77:383–388. doi: 10.7326/0003-4819-77-3-383. [DOI] [PubMed] [Google Scholar]
- 21.Aisner J, Schimpff SC, Wiernik PH. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann Intern Med. 1977;86:539–543. doi: 10.7326/0003-4819-86-5-539. [DOI] [PubMed] [Google Scholar]
- 22.Parker JC, McCloskey JJ, Lee RS. Human cerebral candldosis: a postmortem evaluation of 19 patients. Hum Pathol. 1981;12:23–28. doi: 10.1016/s0046-8177(81)80238-9. [DOI] [PubMed] [Google Scholar]
- 23.Goodman ML, Coffey RJ. Stereotactic drainage of aspergillus brain abscess with long-term survival: case report and review. Neurosurgery. 1989;24:96–99. doi: 10.1227/00006123-198901000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Bradley SF, McGuire NM, Kauffman CA. Sino-orbital and cerebral aspergillosis: cure with medical therapy. Mykosen. 1987;30:379–385. [PubMed] [Google Scholar]
- 25.Green M, Wald ER, Tzakis A, Todo S, Starzl TE. Aspergillosis of the CNS in a pediatric liver transplant recipient case report and review. Rev Infect Dis. 1991;13:653–657. doi: 10.1093/clinids/13.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camarata PJ, Dunn DL, Farney AC, Parker RG, Seljeskog EL. Continual intracavitary administration of ampho 8 as an adjunct in the treatment of aspergillus brain abscess: case report and review of literature. Neurosurgery. 1992;31:575–579. doi: 10.1227/00006123-199209000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Hamill H, Oney LA, Crane LR. Successful therapy for rhinoocerebral mucormycosis with associated bilateral brain abscesses. Arch Intern Med. 1983;13:581–583. [PubMed] [Google Scholar]
- 28.Karandanis D, Shulman JA. Factors associated with mortality in brain abscesses. Arch Intern Med. 1975;135:1145–1150. [PubMed] [Google Scholar]
- 29.Levy RM, Rosenbloom S, Perrett LV. Neuroradiologic findings in AIDS: a review of 200 cases. Am J Radiol. 1986;147:977–983. doi: 10.2214/ajr.147.5.977. [DOI] [PubMed] [Google Scholar]
- 30.Woods KF, Hanna BJ. Brain stem mucormycosis in a narcotic addict with eventual recovery. Am J Med. 1986;80:126–128. doi: 10.1016/0002-9343(86)90062-8. [DOI] [PubMed] [Google Scholar]
- 31.Lowe J, Bradley J. Cerebral and orbital aspergillus infection due to invasive aspergillosis of�ethmoid sinus. J Clin Pathol. 1986;39:774–778. doi: 10.1136/jcp.39.7.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger EG, Norsa L, Kenney M, et al. Nocardiosis of the central nervous system. J Neurosurg. 1954;11:226–233. doi: 10.3171/jns.1954.11.3.0226. [DOI] [PubMed] [Google Scholar]
- 33.Ruskin J, Remington JS. Toxoplasmosis in the compromised host. Ann Intern Med. 1976;84:193–199. doi: 10.7326/0003-4819-84-2-193. [DOI] [PubMed] [Google Scholar]
- 34.Snow RB, Lavyne MH. CNS toxoplasmosis in a patient with AIDS. Infect Surg. 1983;9:669–679. [Google Scholar]
- 35.Syning FW, McLeod R, Maddox JC, et al. Probable transmission of Toxoplasma gondii by organ transplantation. Ann Intern Med. 1979;90:47–49. doi: 10.7326/0003-4819-90-1-47. [DOI] [PubMed] [Google Scholar]
- 36.Frenkel JK. Toxoplasma in and around us. Biosci. 1973;23:343–352. [Google Scholar]
- 37.Hall WA, Martinez AJ, Dummer JS, Lunsford LD. Nocardial brain abscess: diagnostic and therapeutic use of stereotactic aspiration. Surg Neurol. 1987;28:114–118. doi: 10.1016/0090-3019(87)90083-8. [DOI] [PubMed] [Google Scholar]
- 38.Kirmani N, Tuazon C, Ocuin J, et al. J Neurosurg. 1978;49:924–928. doi: 10.3171/jns.1978.49.6.0924. [DOI] [PubMed] [Google Scholar]
- 39.Lunsford LD, Nelson PB. Stereotactic aspiration of a brain abscess using the therapeutic CT scanner: a case report. Acta Neurochir. 1982;62:25–29. doi: 10.1007/BF01402208. [DOI] [PubMed] [Google Scholar]
- 40.Frazier AR, Rosenow EG, Roberts GO. Nocardiosis: a review of 25 cases during 24 months. Mayo Clin Proc. 1975;50:657–663. [PubMed] [Google Scholar]
- 41.Weiss MH, Jane JA. Nocardia asteroides brain abscess successfully treated by enucleation: case report. J Neurosurg. 1969;30:83–86. doi: 10.3171/jns.1969.30.1.0083. [DOI] [PubMed] [Google Scholar]