Less frequent and less severe rejection following orthotopic liver transplants (OLT) under FK 506 has been reported earlier from our institution. 1,2 In the present study, the frequency of episodes of rejection were correlated with: (1) IV FK 506 dosage, (2) oral FK 506 dosage, (3) trough FK 506 plasma concentrations, and (4) use of steroids following primary OLT in a group of patients who experienced rejection episodes, and to compare them with a group who did not experience any rejection episodes.
MATERIAL AND METHODS
One hundred ten adults (mean ± SD: 46.2 ± 12.4 years; 58 male, 52 female) with primary OLT were selected. First, a 90-day posttransplant period was chosen for study purposes, since the majority of rejection (approximately 90%) generally occurs in this time period. Six patients who underwent retransplants (n = 5) or died (n = 1) within 10 days after transplant were excluded from the analysis.
FK 506
Protocol
All patients were commenced on IV FK 506 (0.15 mg/kg per day) in two divided doses. This dose was larger than the current FK 506 dose (which is 0.1 mg/kg per day), and was given over 24-hour continuous infusion. Subsequent adjustments in doses were made depending on the trough levels, hepatic function, renal function, side effects from the drug, history of rejection, or presence of infection.
Steroids
High Prophylaxis Steroid Group
Of the 104 patients available for analysis, 53 patients received 1 g of methylprednisone after perfusion of the liver, then 200 mg in four divided doses was given on the first postoperative day. The dose was reduced by 40 mg every day until a 20 mg/d baseline maintenance dose was reached.
Low Prophylaxis Steroid Group
The remaining 51 patients received only 20 mg/d of methylprednisone. Subsequent adjustment in baseline steroid dose was made on an individual basis in relation to the clinical course of the patient.
Rejection
The diagnosis of rejection was made by rises in serum bilirubin and hepatic enzymes in the absence of biliary complications, ischemic damage, or development of hepatitis (hepatitis B, non-A, non-B, or cytomegalovirus [CMV]). In addition, all the patients had protocol biopsies at 10 to 14 days posttransplant, and subsequently if clinically indicated.
Statistical Methodology
For each patient, daily doses of IV FK 506, oral FK 506, FK 506 trough levels, and daily dose of steroids were recorded. A format test of the relationship of the following four variables to organ rejection was carried out: (1) IV FK 506, (2), oral FK 506, (3) FK 506 trough plasma levels, and (4) baseline steroids; a proportional hazards model with time-varying covariates was used. Time-independent covariate of initial prophylaxis steroid dose and IV FK 506 were also examined. The data were also examined graphically to see whether any obvious pattern existed.
RESULTS
Effects of FK 506 Dosage (IV + oral), FK 506 Trough Plasma Levels, and Baseline Steroids
Of 104 patients, 44 experienced episodes of rejection within the 90-day posttransplant period. The median FK 506 dose at the time of rejection was 0.207 mg/kg per day while, at the same time, in the nonrejection group, it was 0.199 mg/kg per day. The median FK 506 level prior to rejection was 1.05 ng/mL compared with 0.9 ng/mL for the nonrejecting patients. Similarly, baseline mean steroid dose at the time of rejection was 14.8 mg/d (SD = 7.6) for patients who experienced rejection, and 16.03 mg/d (SD = 10.7) for those who had no rejection episodes in the same time period (Table 1).
Table 1.
Amount of Oral FK 506, FK 506 Levels, and Baseline Steroid Dose at the Time of Rejection in 44 Patients Over 60 Patients Who Did Not Reject at the Same Time
| FK 506 Dose (mg/kg per day), Median |
FK 506 Level (ng/mL), median |
Prednisone (mg/d), Mean (SD) |
|
|---|---|---|---|
| Rejection (n = 44) | 0.207 | 1.05 | 14.80 (7.6) |
| Nonrejection (n = 60) | 0.199 | 0.90 | 16.07 (10.7) |
| P value | NS | NS | NS |
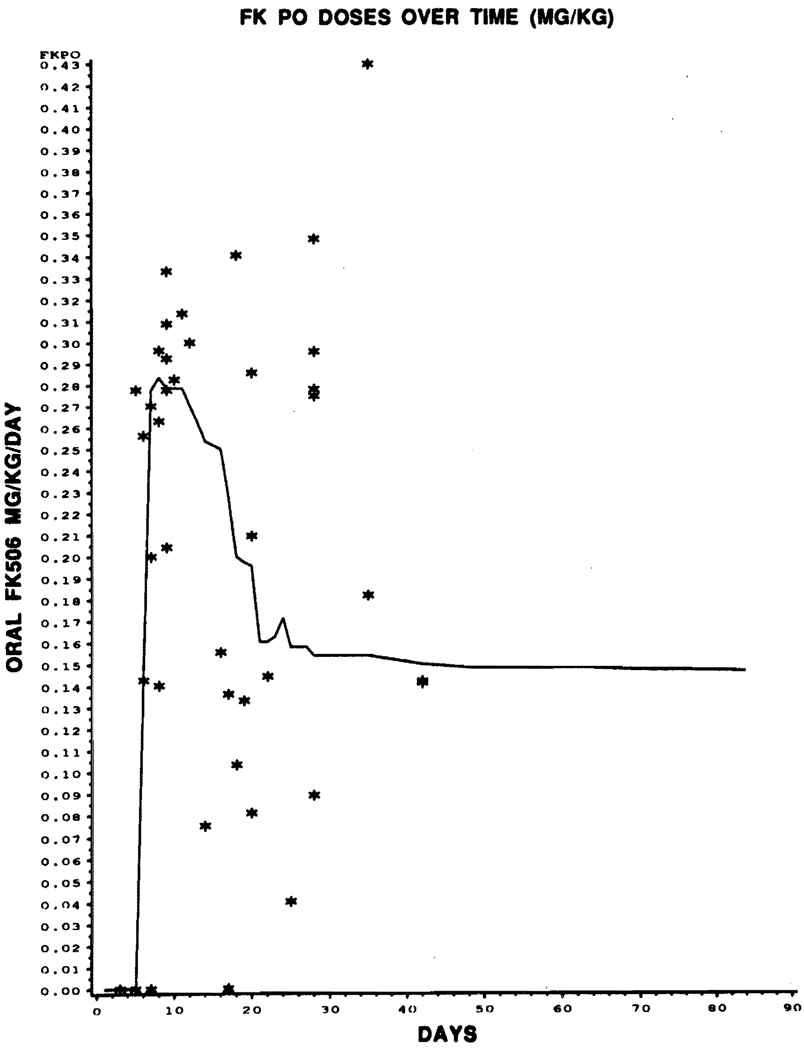
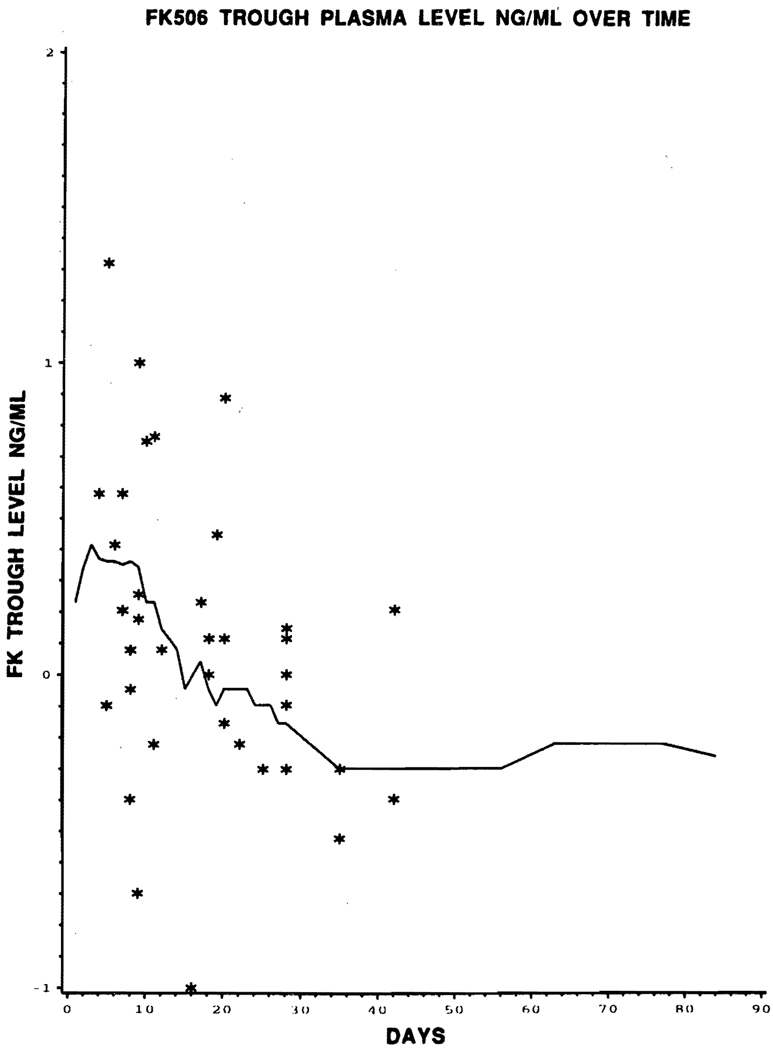
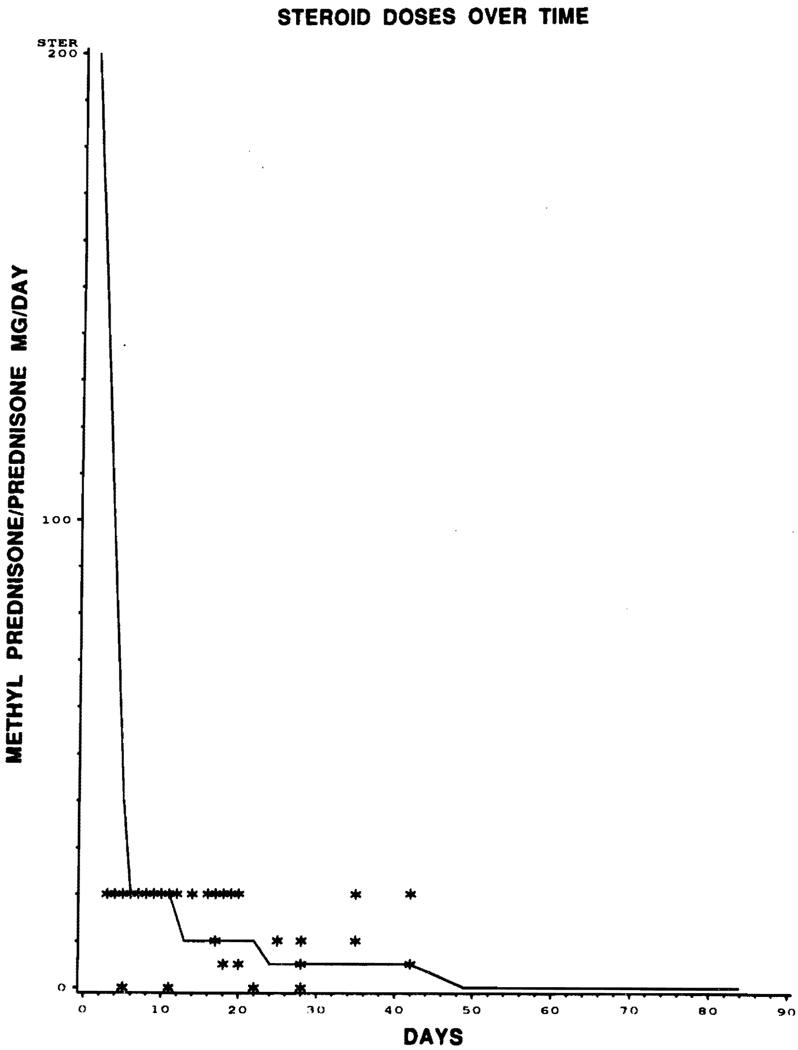
The proportional hazards models with time-varying covariates gave no indication of any relationship between the incidence of rejection and the values of FK 506 dose (IV and oral), FK 506 level, or baseline prednisone, for any of the four covariates just prior to rejection (P = .6); nor was any relationship observed graphically. In Figs 1, 2, and 3, the covariate level of each of the 44 patients experiencing rejections is graphed against the time to rejection (plotted with asterisks), with lines superimposed joining the median covariate values over time for nonrejectors.
Fig 1.
Graphical representation of episodes of rejection (*) with oral FK 506 dose over time. Superimposed line joins the median covariate of oral FK 506 dose over time for nonrejector.
Fig 2.
Graphic representation of episodes of rejection (*) with FK 506 trough plasma level (ng/mL) over time (days). Superimposed line joins the median covariate of FK 506 level over time for nonrejector.
Fig 3.
Graphic representation of episode of rejection (*) with prednisone dose (mg/d) over time (days). Superimposed line joins the median covariate of prednisone dose over time for nonrejector.
Effect High-Dose Steroid Prophylaxis Versus Low-Dose IV FK 506
Exploration of the data did reveal fewer episodes of rejection in the group of patients who received a high prophylactic dose of steroids on day 1 to 5 (17 of 53) (Table 2) compared with low prophylaxis (27 of 51). This difference, by chi-square test of association, is not significant (P = .08) as reported before.2 However, if a proportional hazards model is fit to the data with (time-independent) covariates of initial steroids (over days 1 to 5) (Table 2) and IV FK 506, then the initial steroid level is significantly related to rejection (P < .05), while initial IV FK 506 dose is not.
Table 2.
High Versus Low-Dose Steroid Prophylaxis (Days 1 to 5)
| Did not Reject (%) | Rejection (%) | Total | |
|---|---|---|---|
| High-dose steroid prophylaxis | 36 (68) | 17 (32) | 53 |
| Low-dose steroid prophylaxis | 24 (47) | 27(53) | 51 |
| Total | 60 (58) | 44 (42) | 104 |
Chi-square test of association was not significant (P > .05), however, the time-independent proportional hazards models reveal a significantly lower risk of rejection among recipients of initial high-dose steroid prophylaxis (P = .007).
DISCUSSION AND CONCLUSIONS
One aim was to discover whether lowering the dose of FK 506 increased the risk of rejection. Downward adjustments in FK 506 dose did not correlate significantly with the frequency of rejection. There were insufficient data points to correlate the results with FK 506 levels.
However, the initial 5 days of high-dose of prophylactic steroids had an effect on the 90-day incidence of rejection. This difference between high- or low-dose steroid inductions was not significant by Chi-square test of association, but it was significant with the time-independent proportional hazard model. Severity of rejection usually was mild and readily controlled with minor augmentation of maintenance steroids as reported before.2 Because of this, we currently do not give the 5-day course of prophylactic high-dose steroids to our OLT patients, except in those who have positive lymphocyte crossmatch or higher percentage of (>40%) panel-reactive antibodies, in whom incidence of rejection episodes are higher. 3
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Todo S, Fung JJ, Demetris AJ, et al. Transplant Proc. 1991;23:1397. [PubMed] [Google Scholar]
- 2.Jain AB, Fung JJ, Todo S, et al. Transplant Proc. 1991;23:928. [PMC free article] [PubMed] [Google Scholar]
- 3.Takaya S, Duquesnoy R, Iwaki Y, et al. Transplant Proc. 1991;23:396. [PMC free article] [PubMed] [Google Scholar]