Abstract
Introduction
Both indole-3-carbinol and dietary lignans have beneficial effects on estrogen metabolism and breast cancer risk. There is no published literature on the effects of a combination product. This study was designed to investigate the impact of a combination product on estrogen metabolism. The major trial objective was to determine whether a breast health supplement containing indole-3-carbinol and hydroxymatairesinol lignan would alter estrogen metabolism to favour C-2 hydroxylation and reduce C-16 hydroxylation. Higher concentrations of C-2 metabolites and lower concentrations of C-16 metabolites may reduce breast cancer risk and risk for other hormonally-related cancers.
Methods
Forty-seven pre-menopausal and forty-nine post-menopausal women were recruited for this study, and were divided by random allocation into treatment and placebo group. The treatment supplement contained HMR lignan, indole-3-carbinol, calcium glucarate, milk thistle, Schisandra chinesis and stinging nettle, and each woman consumed either treatment or placebo for 28 days. At day 0 and day 28, blood samples were analysed for serum enterolactone concentrations, and first morning random urine samples were assessed for estrogen metabolites. Repeated measures ANOVA statistical testing was performed.
Results
In pre-menopausal women, treatment supplementation resulted in a significant increase (P < 0.05) in urinary 2-OHE concentrations and in the 2:16α-OHE ratio. In post-menopausal women, treatment supplementation resulted in a significant increase in urinary 2-OHE concentrations. In pre- and post-menopausal women combined, treatment supplementation produced a significant increase in urinary 2-OHE concentration and a trend (P = 0.074) toward an increased 2:16α-OHE ratio. There were no significant increases in serum enterolactone concentrations in the treatment or placebo groups.
Conclusions
Supplementation with a mixture of indole-3-carbinol and HMR lignan in women significantly increased estrogen C-2 hydroxylation. This may constitute a mechanism for the reduction of breast cancer risk as well as risk for other estrogen-related cancers. Further studies with higher numbers of subjects are indicated.
Trial registration
ClinicalTrials.gov registration #NCT01089049.
Keywords: herbal supplement, breast health, estrogen metabolites
Introduction
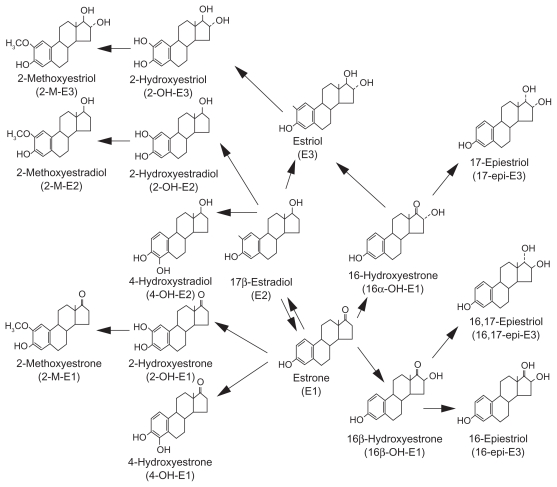
The relationship between breast cancer risk and concentrations of specific urinary estrogen metabolites is well-established.1–8 Figure 1 illustrates estrogen Phase 1 metabolic pathways.9 Estradiol and estrone are interconvertible. Both are metabolized by cytochrome P450 isoenzymes to hydroxylated metabolites and excreted in the urine or bile.3 Lord et al.3 and others4–7 have suggested that the relative amounts of these metabolites influence risk for breast cancer and other estrogen-related cancers. C-2 hydroxylation metabolites 2-hydroxyestrone (2-OHE1) and 2- hydroxyestradiol (2-OHE2) tend to be either weakly estrogenic or possibly anti-estrogenic.
Figure 1.
Metabolism of estrogen. Reprinted from Cancer letters, 211, Takahashi M, Shimomoto T, Miyajima K, et al., Effects of estrogens and metabolites on endometrial carcinogenesis in young adult mice initiated with N-ethyl-N1-nitro-N-nitroguanidine, 1–9, Copyright (2004) with permission from Elsevier.
The metabolite 16α-hydroxyestrone (16α-OHE1) binds covalently and irreversibly to the estrogen receptor and is an endogenous carcinogen. The metabolites 4-hydroxyestrone (4-OHE1) and 4-hydroxyestradiol (4-OHE2) bind to DNA, forming semi-quinones that cause mutations which result in carcinogenesis, although they are present in much smaller concentrations than 16α-OHE1.3,4,8
The roles of dietary lignans, normally found in the diet from whole grain and fruits and vegetable sources, and of indole-3 carbinol (I3C), most often sourced dietarily from cruciferous vegetables, have received increasing attention in the modulation of breast cancer risk. Evidence for their metabolic effects on estrogen elimination pathways is emerging, in both epidemiological studies, and in clinical trials using either source foods or derived supplements (10–22). The primary acid condensation product of I3C, diindolylmethane (DIM) induces the cytochrome P450 enzyme CYP1A1, which increases the 2- hydroxylation of estrogens.13,20
The effects of lignan consumption are influenced by enzyme genotypes, specifically catechol-O-methyltransfrase (COMT), required for the conversion of 4-OHE1 to its proliferative form (catechol estrogen-3,4-quinones) and cytochrome P451 enzyme CYP1B1, needed in the conversion of estrogens to 16α-OHE1.8 The ratio of 2-OHE to 16α-OHE1 increased with lignan consumption, and correlated directly with the number of variant alleles for COMT and for CYP1B1.22 A lignan source derived from the Norwegian spruce tree, 7-hydroxymatairesinol (HMR lignan) had antitumor effects in rat mammary cancers, and positively influenced the concentration of 2-OHE and the ratio of 2-OHE : 16α-OHE1 in human clinical trials.23,24
The purpose of this study was to determine the effects of supplementation the diets of women (not taking hormonal supplements or oral contraceptives) with an encapsulated combination of I3C and HMR lignan on urinary concentrations of 2-OHE and 16α-OHE, on the ratio of 2-OHE to 16α-OHE, and on concentrations of serum enterolactone.
Subjects and Methods
Subjects
Pre- and post-menopausal women were recruited for the trial through local newspaper advertising. Prior to their first lab visit, subjects underwent a telephone interview to assess their eligibility for the study, and to determine the current stage of their menstrual cycle. For pre-menopausal women, lab appointments were booked based on timing each subject’s first lab visit at the mid-point of the follicular phase of her menstrual cycle. After pre-screening and exclusion for use of any type of sex hormone supplementation (estrogen, progesterone, androgens, hormonal oral contraceptives), and for a family history of breast cancer, 48 healthy pre-menopausal women aged 19 to 54, and 50 healthy post-menopausal women aged 46 to 70 were recruited for the study. Besides the use of hormones, exclusion criteria included being less than 18 years of age, pre-existence of any serious illness including fibrocystic breast disease or family history of ovarian cancer, and pregnancy or lactation.
Supplements
Supplements were encapsulated in vegetarian softgel white capsules, and each treatment capsule contained 200 mg I3C and 10 mg HMR lignans, in accordance with previous research.14,24 Minor constituents included 100 mg Milk Thistle [Silybum marianum], 75 mg calcium-D-glucarate, 75 mg Schisandra chinesis, 50 mg Stinging Nettle Root [Urtica dioica] and 10 mcg vitamin D. The placebo consisted of microcrystalline cellulose, and the daily dosage was 2 capsules, one to be taken with breakfast and one with dinner. The supplements, including encapsulation, were provided by Fem Med (Fem Med Formulas Limited Partnership, Toronto, Canada). Both the clinical coordinator and trial participants were blinded to the type of supplement.
Study design
The trial was a double-blind, placebo-controlled, parallel study in which 98 subjects were recruited to one of two arms of the study. One arm consisted of pre-menopausal women not using hormonal contraceptives, while the other consisted of post-menopausal women not receiving HRT. Each arm of the study was carried out concurrently and in one phase, with no washout period. Subjects in each arm were randomly allocated to consume either the treatment or a placebo for a period of 28 days. The randomization list was generated using an internet program from Tufts University and the procedure was verified using SAS™ software (http://www.tufts.edu/~gdallal/random_block_size.htm).
Duplicate measures of sitting blood pressure were measured using an automated digital blood pressure device. Height and weight were measured in duplicate, and Body Mass Index (BMI) calculated using the equation BMI = weight in kg/height in metres squared. Blood samples and first morning urine samples were collected from each group at day 0 and day 28, and adverse events diaries, for the recording of possible side-effects, were distributed to all subjects.
This study has been reviewed and approved by the Canadian Shield Ethics Review Board [CSERB], with tracking #08-10-001. All of the procedures followed throughout this study were in accordance with the Declaration of Helsinki, and followed the International Conference on Harmonisation (ICH)/World Health Organisation (WHO) Good Clinical Practice standards for clinical trials. The study was also reviewed and approved by Health Canada’s Natural Health Products Directorate (File #137987). All subjects signed an Informed Consent document that followed ICH guidelines and had been approved by the CSERB and by Health Canada’s Natural Health Products Directorate.
Laboratory analyses
First morning urine samples were collected, aliquoted into cryovials and stored at −80 °C until analyzed. They were analysed for creatinine concentration using a colourimetric detection ELISA methodology (Assay Designs, Ann Arbor, MI), and for estrogen metabolites, including 2-OHE1, 2OHE2 and 16α-OHE1 concentrations using stable isotope dilution gas chromatography-mass spectrometry.25 Estrogen metabolites were calculated as ng per mg of creatinine, in order to standardise the results and facilitate comparisons between participants. Blood samples were collected by venipuncture into evacuated tubes with no anti-coagulant. After allowing time for the blood to clot, tubes were centrifuged at 3,200 rpm for 10 minutes at 25 °C. Serum supernatant was portioned into cryovials and stored at −80 °C until analysed for concentrations of serum enterolactone using gas chromatography-mass spectrometry.26
Statistical analyses
Statistical analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC). ANOVA for repeated sampling was used to compare the treatment group with the placebo group, after pooling of the pre- and post-menopausal treatment and placebo group results. Additionally, each treatment subgroup, ie, post-menopausal women without HRT, and pre-menopausal women not using hormonal contraceptives, was compared with its corresponding placebo group, again using ANOVA for repeated sampling. A power analysis to determine the appropriate number of subjects was carried out, using known urinary estrogen metabolite variability. For a two-tailed α level of 0.05 (P-value for statistical significance), and a β level of 80% (desired power), it was estimated that a minimum of 44 subjects would be required in order to detect a minimum change of 0.3 ng/mg creatinine in estrogen metabolite concentration. To allow for drop-outs/attrition, a number of 48 was deemed appropriate. All of the statistical analyses were performed by an independent Ph.D. in biostatistics and Senior Analyst at the University of Guelph, Ontario, Canada.
Results
Although a total of 98 pre- and post-menopausal women were initially recruited, the number of subjects whose data was statistically analysed was 68. Table 1 illustrates the reasons for this reduction in numbers. A creatinine level of ≤0.20 mg is very low for a first morning sample, and indicates the possibility of a missed first morning sample and/or middle-of-the-night uncollected bladder evacuation. Nonetheless, it results in very dilute and probably inaccurate measurements of the estrogen biomarkers of interest; hence samples with creatinine levels of ≤0.20 mg were omitted from the data base. If the capsule count indicated an intake of <85% over the previous 28 days, subjects were assumed to be non-compliant, and their data was omitted.
Table 1.
Subject numbers and reasons for data removal.
| Pre-menopausal women | # | Post-menopausal women | # |
|---|---|---|---|
| Initial total | 48 | Initial total | 50 |
| 1 blood sampling inability | 47 | 1 side-effects drop-out | 49 |
| 1 urine sample result missing | 46 | 12 very low creatinine levels1 | 37 |
| 1 very low creatinine level | 45 | 1 nd2 urine sample for 2-OHE | 36 |
| 2 nd urine samples for 2-OHE | 43 | 5 nd urine samples for 16α-OHE | 31 |
| 1 nd urine sample for 16α-OHE | 42 | 1 non-compliant3 subject | 30 |
| 4 non-compliant subject | 38 | TOTAL | 30 |
| TOTAL | 38 | ||
| Total for both pre- and post-menopausal women: 68 | |||
Notes: ≤0.20 mg creatinine;
nd = non-detected;
Capsule count <85%.
The baseline characteristics of the subjects are presented in Table 2, and include data for all subjects enrolled in the trial, excluding the subject who was unable to give blood, and the early drop-out. The range in age for the pre-menopausal women was 19 to 54 years, and for post-menopausal women it was 46–70 years. Not unexpectedly, blood pressure measurements for post-menopausal (older) women tended to be somewhat higher than those for pre-menopausal (younger) women, given that blood pressure tends to increase with age.
Table 2.
Demographic and anthropometric data for participants at baseline (mean ± SD).
| Group > | Pre-menopausal women |
Post-menopausal women |
||
|---|---|---|---|---|
| Placebo | Treatment | Placebo | Treatment | |
| N | 17 | 21 | 13 | 17 |
| Age (years) | 38 ± 10 | 36 ± 9 | 56 ± 6 | 57 ± 6 |
| Weight (kg) | 77.8 ± 24.3 | 75.5 ± 17.5 | 78.7 ± 19.0 | 67.2 ± 13.0 |
| BMI (kg/m2) | 27.82 ± 8.87 | 26.83 ± 6.04 | 30.03 ± 7.18 | 25.66 ± 4.30 |
| Blood pressure (mmHg) | 112/74 ± 12/11 | 110/75 ± 14/11 | 118/81 ± 12/11 | 121/79 ± 20/13 |
Results for estrogen metabolite concentrations for pre- and post-menopausal women combined are presented in Table 3. For the treatment group, the mean change in concentration of 2-OHE was highly significant, and there was a trend towards an increase in the mean ratio of 2-OHE to 16α-OHE. Statistically, 16α-OHE concentrations were unchanged in both groups. None of the concentrations of the biomarkers of interest were significantly changed in the placebo group.
Table 3.
Comparison of day 0 vs. day 28 concentrations of urinary biomarkers of interest for pre- and post-menopausal women consuming the treatment or a placebo.
| Biomarker |
Pre- and post-menopausal women n = 68 |
||||||
|---|---|---|---|---|---|---|---|
| ng/mg creatinine | Treatment n = 38 |
Placebo n = 30 |
|||||
| Day 0 | Day 28 | P-value | Day 0 | Day 28 | P-value | ||
| 2-OHE 1 + 2 | Mean | 7.63 | 15.11 | 0.001 | 9.77 | 11.13 | 0.524 |
| SD1 | 8.31 | 17.50 | 11.5 | 9.64 | |||
| 16α-OHE | Mean | 5.46 | 6.50 | 0.082 | 6.05 | 5.66 | 0.620 |
| SD | 4.50 | 5.07 | 4.76 | 3.93 | |||
| 2:16α-OHE ratio | Mean | 2.52 | 3.33 | 0.074 | 2.17 | 2.70 | 0.451 |
| SD | 3.70 | 3.78 | 2.95 | 3.46 | |||
Abbreviation: SD, standard deviation.
Table 4 lists estrogen metabolite concentrations for the pre-menopausal group. In pre-menopausal women, both the mean 2-OHE concentration and the mean ratio of 2-OHE to 16α-OHE1 were significantly increased in the treatment group. None of these biomarker concentrations significantly changed in the placebo group, nor was the mean concentration of 16α-OHE.
Table 4.
Comparison of day 0 vs. day 28 concentrations of urinary biomarkers of interest for pre-menopausal women consuming the treatment or a placebo.
| Biomarker |
Pre-menopausal women n = 38 |
||||||
|---|---|---|---|---|---|---|---|
| ng/mg creatinine | Treatment n = 21 |
Placebo n = 17 |
|||||
| Day 0 | Day 28 | P-value | Day 0 | Day 28 | P-value | ||
| 2-OHE | Mean | 6.34 | 13.29 | 0.003 | 9.37 | 10.5 | 0.667 |
| SD1 | 7.01 | 14.05 | 11.4 | 8.42 | |||
| 16α-OHE | Mean | 7.69 | 8.58 | 0.380 | 7.38 | 7.01 | 0.817 |
| SD | 4.66 | 5.64 | 5.28 | 4.52 | |||
| 2:16α-OHE ratio | Mean | 0.88 | 1.66 | 0.016 | 1.39 | 1.62 | 0.444 |
| SD | 0.74 | 1.27 | 1.17 | 1.01 | |||
Abbreviation: SD, standard deviation.
For post-menopausal women, the results are presented in Table 5. Only the mean 2-OHE concentration was significantly increased in the post-menopausal treatment group. In this group, the mean concentration of 16α-OHE was very close to significance (P = 0.057), and the mean ratio change was not significant. None of the placebo biomarkers exhibited any significant change in mean concentration.
Table 5.
Comparison of day 0 vs. day 28 concentrations of urinary biomarkers of interest, for post-menopausal women consuming the treatment or a placebo.
| Biomarker |
Post-menopausal women n = 30 |
||||||
|---|---|---|---|---|---|---|---|
| ng/mg creatinine | Treatment n = 17 |
Placebo n = 13 |
|||||
| Day 0 | Day 28 | P-value | Day 0 | Day 28 | P-value | ||
| 2-OHE | Mean | 9.22 | 17.37 | 0.035 | 10.29 | 11.9 | 0.656 |
| SD1 | 9.67 | 21.27 | 11.99 | 11.4 | |||
| 16α-OHE | Mean | 2.70 | 3.92 | 0.057 | 4.30 | 3.79 | 0.556 |
| SD | 2.23 | 2.63 | 3.43 | 1.81 | |||
| 2:16α-OHE ratio | Mean | 4.54 | 5.40 | 0.387 | 3.20 | 4.13 | 0.570 |
| SD | 4.81 | 4.77 | 4.14 | 4.87 | |||
Abbreviation: SD, standard deviation.
Although 4-OHE was not identified as a biomarker of specific interest in this trial, it has been recognised as a cell proliferator similar to 16α-OHE,3,4,8 and results for 4-OHE were available from the estrogen metabolite analyses. There were fewer 4-OHE values for statistical analysis, because several of the subjects’ 4-OHE concentrations were non-detectable. The results that are available are presented in Table 6. In the pre-menopausal group, both the treatment and the placebo group exhibited a reduction in mean 4-OHE concentration, though neither reduction was statistically significant. In post-menopausal women, the placebo group increased the mean 4-OHE concentration, from 2.46 to 4.75 ng/mg creatinine, while the treatment group decreased 4-OHE concentration from 7.49 to 4.70 ng/mg creatinine. Neither result was statistically significantly; however, only 10 and 12 4-OHE results were available for post-menopausal placebo and treatment groups, respectively.
Table 6.
Comparison of day 0 vs. day 28 concentrations of urinary 4-OHE, for pre- and post-menopausal women consuming the treatment or a placebo (mean and SD).
| Treatment |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | P-value | N | Day 0 | Day 28 | P-value | N | |
| All women | 4.10 | 2.91 | 0.158 | 32 | 3.42 | 2.95 | 0.750 | 25 |
| SD | 6.94 | 3.73 | 5.35 | 4.72 | ||||
| Pre-menopausal women | 2.05 | 1.37 | 0.307 | 20 | 3.95 | 1.60 | 0.146 | 15 |
| SD | 3.08 | 1.10 | 6.61 | 1.41 | ||||
| Post-menopausal women | 7.49 | 4.70 | 0.321 | 12 | 2.46 | 4.75 | 0.393 | 10 |
| SD | 9.57 | 4.78 | 2.53 | 6.67 | ||||
In order to further elucidate this data, a ratio of “pro-carcinogenic” and “anti-carcinogenic” metabolites was devised. The numerator consisted of 4-OHE (1 and 2), 16α-OHE1 and estriol (E3), and the denominator was 2-OHE (1 and 2). E3 was included in the numerator because it is the product of 16α-OHE hydroxylation, and has itself, along with its other metabolites, been implicated in possible carcinogenic activity, in animal models.25 Non-detectable levels of E3, added to non-detectable levels of 2-OHE and/or 4-OHE and/or 16α-OHE, resulted in the number of subjects in this analysis being quite small. Table 7 illustrates these ratios. Because of the small number of subjects, it is difficult to draw conclusions from this table. Nonetheless, in the treatment group, there was a highly significant reduction the carcinogenic ratio for all women, a significant reduction in this ratio for the pre-menopausal women, and a trend towards a significant reduction in this ratio for the post-menopausal group (P = 0.067 for the latter). However, the reduction in this ratio for the pre-menopausal placebo group was almost identical to that of the pre-menopausal treatment group, and the lack of significance in the former was undoubtedly due to the smaller number of subjects (10 vs. 19, respectively).
Table 7.
Comparison of day 0 vs. day 28 concentrations of calculated carcinogenic ratios, for pre- and post-menopausal women consuming the treatment or a placebo (mean and SD).
| Treatment |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | P-value | N | Day 0 | Day 28 | P-value | N | |
| All women | 4.91 | 2.12 | 0.005 | 26 | 3.58 | 3.40 | 0.881 | 17 |
| SD | 5.02 | 1.76 | 4.22 | 6.26 | ||||
| Pre-menopausal women | 3.90 | 2.08 | 0.031 | 19 | 3.59 | 1.64 | 0.121 | 10 |
| SD | 3.38 | 1.82 | 3.85 | 1.00 | ||||
| Post-menopausal women | 7.64 | 2.22 | 0.067 | 7 | 3.55 | 5.93 | 0.231 | 7 |
| SD | 7.66 | 1.69 | 5.03 | 9.51 | ||||
Serum enterolactone concentrations for the whole group and the two subgroups are presented in Table 8. There were no differences among any of the groups or subgroups in mean serum enterolactone concentrations.
Table 8.
Mean serum enterolactone concentrations for day 0 and day 28 in the treatment and placebo groups, for pre- and post-menopausal women together and separately.
| Group | Day 0 |
Day 28 |
|||
|---|---|---|---|---|---|
| Average | SD1 | Average | SD | ||
| All women | Treatment N = 38 | 5.0 | 5.9 | 4.9 | 7.1 |
| Placebo N = 30 | 3.6 | 3.0 | 4.0 | 5.7 | |
| Pre-menopausal women | Treatment N = 21 | 4.2 | 5.0 | 4.2 | 7.8 |
| Placebo N = 17 | 4.2 | 3.4 | 5.3 | 7.4 | |
| Post-menopausal women | Treatment N = 17 | 6.1 | 6.8 | 5.8 | 6.2 |
| Placebo N = 13 | 2.9 | 2.4 | 2.4 | 1.1 | |
Abbreviation: SD, standard deviation.
Adverse events were collated from diaries that study subjects completed as needed. There were more adverse events reported in the treatment group than in the placebo group. Of 47 adverse events diaries received from pre-menopausal women, 21 noted adverse events, 14 in the treatment group and 7 in the placebo group. By far, the most common adverse event reported was headache (20 incidences in the treatment group, 8 in the placebo group) with stomach pain as the second most common event (14 in the treatment group), and single occurrences of other minor adverse events such as nausea, irritability, rash. Of 50 adverse events diaries received from post-menopausal women, 13 noted adverse events, 10 in the treatment group and 3 in the placebo group. Again, the most common adverse event reported was headache (11 incidences in the treatment group, 6 in the placebo group), as well as 2 incidences of heartburn reported in the treatment group and 4 incidences of jaw pain reported in the placebo group.
Discussion
For pre-menopausal women, these results suggest that consumption of a daily dosage of 2 capsules of the treatment offers beneficial outcomes, in that the mean 2-OHE concentration and the mean 2-OHE:16α-OHE ratio significantly increased during a 28-day supplementation. In contrast, there were no significant differences in the placebo group. The estrogen metabolite 2-OHE2 undergoes conjugation to 2-methoxyestradiol (2-MeO-E2), which has been shown to inhibit cell proliferation.27 This 2-OHE2 conjugation product has also been identified as an inhibitor of angiogenesis and tumour growth; thus, an increase in 2-OHE concentration, and the subsequent increase in 2-MeO-E2, is considered a beneficial outcome independent of the 2-OHE:16α-OHE ratio.28–80 The increased ratio, having been clearly identified as a biomarker of a reduction in breast cancer risk in pre- menopausal women,2,5,31 is a very positive outcome, and an outcome that has previously been observed in pre-menopausal women taking either I3C or dietary lignans.10,12,14
For post-menopausal women, the results are more complex. There was a significant increase in the concentration of 2-OHE from day 0 to day 28 in the treatment group, but not in the placebo group. As with pre-menopausal women, this represents a significant beneficial effect, because previous research has indicated that an increase in the concentration of 2-OHE in the urine is correlated independently with a reduction in breast cancer risk.28–30 Furthermore, this metabolite is regarded as a weak estrogen, with minimal negative effects, and possibly anti-estrogenic effects, compared to the much more potent negative effects of 16α-OHE.3
Given the expectation that there is a limited pool of estrogen substrates for the various metabolic pathways, one would expect that an increase in the activity of the pathway producing 2-OHE would concomitantly result in a reduction in the activity of the 16α-OHE pathway, and this was the hypothesis confirmed in an IC3 supplementation trial.32 In another I3C supplementation trial, the authors noted that although a significant increase in the 2:16 ratio was observed, 3 of 20 subjects experienced no change in this ratio at any time-point.10 They noted that “ … some individuals may be resistant to change”. In our study, 4 of 21 pre-menopausal and 5 of 17 post-menopausal women experienced a decrease in this ratio. In a study on soy consumption in post-menopausal women, only women who were equol producers increased the 2:16 ratio.33 It may be that future nutrigenomic studies may elucidate genetic differences in the way some women metabolize estrogen, and/or in how they digest, absorb and metabolize dietary components that influence estrogen metabolism.
For the combined results in both pre- and postmenopausal women, the increase in 2-OHE in the treatment group was highly significant, and there was a trend towards significance in the 2:16a-OHE ratio. The unexpected trend towards an increase in the concentration of 16α-OHE in both the pre- and post-menopausal groups undoubtedly attenuated what might have been a significant increase in the 2-OHE:16α-OHE ratio when the results for both preand post-menopausal women were combined. The P-value for the 2:16 ratio, in the combined groups, was 0.074, and this is most likely a combination of the significant pre-menopausal P-value of 0.016, and the non-significant post-menopausal P-value of 0.387. However, the small increase in average levels of 16α-OHE in both pre- and post-menopausal women needs to be investigated further, to assess whether this is a spurious finding or the result of metabolic alterations in estrogen metabolism. This estrogen metabolite is associated with an increase in breast cancer risk;1–7 thus, any supplement that appears to increase the level of this metabolite requires further investigation.
The analyses of 4-OHE and the so-called carcinogenic ratio provided somewhat interesting results. Although there were no significant differences in 4-OHE1 concentrations among any of the groups or sub-groups, the apparent reduction in 4-OHE levels in the treatment group, with only 12 subjects, in comparison with an increase in 4-OHE levels in the placebo group of 10 subjects, warrants further research, although a larger trial may show these results to be extraneous. The carcinogenic ratio, which combines E3, 4-OHE (1 and 2), 16α-OHE1 and 2-OHE (1 and 2) provided more interesting results, given the significant or close-to-significant results for the treatment groups and lack thereof in the placebo groups.
Although there may be dispute as to whether E3 belongs among the carcinogenic metabolites, Takahashi et al.9 reported on the carcinogenic effects of estriol and its metabolites in young adult mice. After initiation of endometrial carcinogenesis with N-ethyl-N1-nitro-N-nitroguanidine, the researchers measured the incidences of endometrial hyperplasia/ proliferative lesions and adenocarcinomas following implantation with estrogen metabolite pellets. The metabolites that produced significant incidences of proliferative lesions were 2-hydroxyestriol (2-OH-E3), 2-methoxyestriol (2-MeO-E3), 2-methoxyestradiol (2-MeO-E2) and 16-epiestriol, and those that resulted in adenocarcinomas were estrone (E1), estradiol (E2), E3, 16α-OHE1, 16β-OHE1 and 17-epiestriol (a metabolite of 16α-OHE1). In effect, two estriol metabolites produced lesions, and two produced adenocarcinomas. Human studies are less definitive, but there is some evidence that estriol metabolites may be carcinogenic in some instances.34
In this trial, it was expected that serum enterolactone concentrations would rise as dietary HMR lignan intake rose. The fact that there were no significant increases in the average serum enterolactone concentrations in any of the groups or sub-groups is puzzling. Originally, the manufacturer of this specific lignan (Linnea, Inc., Switzerland) stated, in a brochure for public consumption, that a significant increase in serum enterolactone concentration may only be expected if dietary intake of HMR lignans is 25 to 50 mg/day.35 However, a later brochure indicated that 10 to 50 mg/day would be sufficient to increase serum enterolactone concentrations,36 and this was based on a study by Cosentino.37 The daily intake of HMR lignan in this trial was 20 mg, and this may or may not have been sufficient to see a significant increase in serum enterolactone concentrations. Further research on the most efficacious dosage of HMR lignans is warranted. Serum quantification of the HMR lignin metabolite 7-hydroxy-HMR lignin and the enterolactone precursor enterodiol would also be advisable, in order to verify adequate HMR lignan absorption and metabolism.
Although it was determined, by power analysis, that 44 subjects per group would be needed in order to obtain statistically robust results, the unexpected reduction in group numbers to 38 women consuming the treatment and 30 women consuming the placebo attenuated the number and power of statistical findings. These findings were further attenuated when the treatment and placebo groups were subdivided into pre- and post-menopausal women, and when non-detectable concentrations of other metabolites of interest, such as 4-OHE, were analysed statistically. Thus, the fact that several statistically-significant results were obtained with only 21 pre-menopausal women in the treatment group, and 17 post-menopausal women in the treatment group, for the major metabolites, and even fewer for other metabolites, indicates that these findings are important, and that future research with a greater number of participants, ie, sufficient to subdivide into pre- and post-menopausal groups while retaining statistical robustness, may well elicit more favourable results.
Conclusions
In pre-menopausal women, consumption of a specific mixture of I3C and HMR lignan increased the mean urinary concentration of the estrogen metabolite 2-OHE and the mean urinary ratio of 2-OHE:16α-OHE, and this may reduce breast cancer risk. In postmenopausal women, consumption of a specific mixture of I3C and HMR lignan increased the mean urinary concentration of the estrogen metabolite 2-OHE, and this may reduce breast cancer risk. However, the evidence for this is weaker than for premenopausal women because the 2-OHE:16α-OHE ratio did not increase significantly in this group.
In their 2002 review article, Lord et al. noted that the maximum 2:16 OHE ratio one could safely strive towards without encountering undesirable adverse effects (such as an increase in osteoporosis) would be 8.0. In our trial, where the daily supplement dose was 400 mg I3C, the average maximum ratio we achieved was 5.4, and this was in post-menopausal women. For the pre-menopausal women, there was a 110% increase in the average urinary 2-OHE concentration, and a 100% increase in the 2:16 OHE ratio, while these numbers for post-menopausal women were 88% and 19%, respectively. Except for the postmenopausal 2:16 ratio reduction, these results compare favourably with other trial results, where 2-OHE levels increased by 50%,38 and 2:16 ratio increased by 66%.14 It also resulted in a Relative Risk reduction of breast cancer risk from 1.00 to 0.763 in premenopausal women.
Acknowledgements
NDI was contracted to design and conduct this clinical trial by Fem Med Formulas Limited Partnership, 64 Bakersfield Street, Toronto, ON M3J 2W7 Canada. All costs associated with the design and conduct of this trial were fully funded by Fem Med, and Fem Med also provided encapsulated treatment and placebo supplements. Fem Med has funded the writing of this manuscript, and will fund any article-processing charges associated with its publication. The salaries of Dr. M. Laidlaw and C. Cockerline are the responsibility of NDI, but the time contributions of these two individuals for the trial were included in the cost of the trial, and were paid by Fem Med. Thus, NDI was fully reimbursed for their staff time for this trial. All analyses costs performed by Dr. Sepkovic, including his time, were paid by Fem Med. NDI suggested submission of the manuscript to Breast Cancer Research, and Fem Med accepted this suggestion.
Dr. M. Edwards was contracted to perform all of the statistical analyses associated with this clinical trial. Her invoices for these analyses were paid by Fem Med.
William Rowe is the CEO of NDI and provided the facility and general support for the clinical trial.
List of Abbreviations (In Order of Usage)
- 2-OHE1
2-hydroxyestrone
- 2-OHE2
2-hydroxyestradiol
- 16α-OHE1
16α-hydroxyestrone
- 4-OHE1
4-hydroxyestrone
- 4-OHE2
4-hydroxyestradiol
- I3C
indole-3 carbinol
- DIM
diindolylmethane
- COMT
catechol-O-methyltransfrase
- HMR lignin
7-hydroxymatairesinol
- BMI
body mass index
- CSERB
canadian shield ethics review board
- ICH
international conference on harmonisation
- WHO
world health Laidlaw et al organisation
- ELISA
enzyme-linked immunosorbent assay
- E3
estriol; 2-MeO-E2-2-methoxyestradiol
- 2-OH-E3
2-hydroxyestriol
- 2-MeO-E3
2-methoxyestriol
- E1
estrone
- E2
estradiol
Footnotes
Authors’ Contributions
Dr. M. Laidlaw is the primary author of this manuscript. She designed the trial, aided in its implementation, collated the data for statistical analyses, and wrote the bulk of the manuscript.
C. Cockerline had significant input into the design of this trial and was the person primarily responsible for the implementation of the trial. She also contributed significantly to the manuscript, reviewed the final draft, and offered comments on the final draft.
Dr. D. Sepkovic performed all of the estrogen metabolite analyses. He also reviewed the manuscript and offered comments.
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors confirm that they have permission to reproduce any copyrighted material. Nutrasource Diagnostics Inc. (NDI) was contracted by Fem Med Formulas Limited Partnership to design and conduct this clinical trial, and was fully reimbursed for this work. The first author of this manuscript, Maggie Laidlaw, Ph.D., is the Director of the Clinical Trials Division of NDI, and Carla Cockerline, M.Sc., is the Associate Director of the Clinical Trials Division of NDI.
References
- 1.Kabat GC, Chang CJ, Sparano JA, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiology, Biomarkers and Prevention. 1997;6:505–9. [PubMed] [Google Scholar]
- 2.Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16α-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11(6):635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the dietcancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Alternative Medicine Review. 2002;7(2):112–29. [PubMed] [Google Scholar]
- 4.Okobia MN, Bunker CH. Estrogen metabolism and breast cancer risk—a review. Afr J Reprod Health. 2006;10(1):13–25. [PubMed] [Google Scholar]
- 5.Kabat GC, O’Leary ES, Gammon MD, et al. Estrogen metabolism and breast cancer. Epidemiology. 2006;17(1):80–8. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- 6.Sepkovic DW, Bradlow HL. Estrogen hydroxylation—the good and the bad. Ann N Y Acad Sci. 2009;1155:57–67. doi: 10.1111/j.1749-6632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- 7.Im A, Vogel VG, Ahrendt G, et al. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009;30(9):1532–5. doi: 10.1093/carcin/bgp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalieri EL, Rogan EG. A unifying mechanism in the initiation of cancer and other diseases by catechol quinines. Ann N Y Acad Sci. 2004;1028:247–57. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi M, Shimomoto T, Miyajima K, et al. Effects of estrogens and metabolites on endometrial carcinogenesis in young adult mice initiated with N-ethyl-N1-nitro-N-nitroguanidine. Cancer Letters. 2004;211:1–9. doi: 10.1016/j.canlet.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Bradlow HL, Michnovicz JJ, Halper M, Miller DG, Wong GY, Osborne MP. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidem Biomarkers Prev. 1994;3(7):591–5. [PubMed] [Google Scholar]
- 11.Bradlow HL, Sepkovic DW, Telang NT, Osborne MP. Multifunctional aspects of the action of indole-3 carbinol as an antitumor agent. Ann N Y Acad Sci. 1999;889:204–13. doi: 10.1111/j.1749-6632.1999.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 12.McCann SE, Muti P, Vito D, Edge SB, Trevisan M, Freudenheim JL. Dietary lignan intakes and risk of pre- and post-menopausal breast cancer. Int J Cancer. 2004;111(3):440–3. doi: 10.1002/ijc.20262. [DOI] [PubMed] [Google Scholar]
- 13.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiological evidence and mechanistic base. Pharmacol Res. 2007;55(3):224–36. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed GA, Peterson KS, Smith HJ, et al. A phase 1study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1953–60. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- 15.Brignall M. Prevention and treatment of cancer with indole-3-carbinol. Altern Med Rev. 2001;6(6):580–9. [PubMed] [Google Scholar]
- 16.Touillaud MS, Thiébaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99(6):475–86. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks JD, Ward WE, Lewis JE, Hilditch J, Nickell L, Wong E, Thompson LU. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr. 2004;79:318–25. doi: 10.1093/ajcn/79.2.318. [DOI] [PubMed] [Google Scholar]
- 18.Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chand-Claude J. Meta- analysis of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr. 2010;92(1):141–53. doi: 10.3945/ajcn.2009.28573. [DOI] [PubMed] [Google Scholar]
- 19.Telang N, Katdare M, Bradlow HL. Inhibition of proliferation and modulation of estradiol metabolism: novel mechanisms for breast cancer prevention by the phytochemical indole-3-carbinol. Proc Soc Biol Med. 1997;216:246–52. doi: 10.3181/00379727-216-44174. [DOI] [PubMed] [Google Scholar]
- 20.Saarinen NM, Wärri A, Airio M, Smeds A, Makela S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Res. 2007;51:857–66. doi: 10.1002/mnfr.200600240. [DOI] [PubMed] [Google Scholar]
- 21.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006;17:1253–61. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 22.McCann Se, Wactawaski-Wende J, Kufel K, et al. Changes in 2-hydroxyestrone and 16alpha-hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiol Biomarkers Prev. 2007;16(2):256–62. doi: 10.1158/1055-9965.EPI-06-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saarinen N, Huovinen R, Wärri A, et al. Uptake and metabolism of hydroxymatairesinol in relation to its anticarcinogenicity in DMBA-induced rat mammary carcinoma model. Nutr Cancer. 2001;41(1–2):82–90. doi: 10.1080/01635581.2001.9680616. [DOI] [PubMed] [Google Scholar]
- 24.Kangas L, Saarinen N, Mutanen M, et al. Antioxidant and antitumor effects of hydroxymatairesinol (HM-3000, HMR), a lignan isolated from the knots of spruce. Eur J Cancer Prev. 2002;11(Suppl 2):S48–57. [PubMed] [Google Scholar]
- 25.Sepkovic DW, Bradlow HL, Michnovicz JJ, Murtezani S, Levy I, Osborne MP. Catechol Estrogen Production in Rat Microsomes After Treatment with Indole-3-Carbinol, Ascorbigen, or B-napthaflavone: A Comparison of Stable Isotope Dilution Gas Chromatography-Mass Spectrometry and Radiometric Methods. Steroids. 1994;59:318–23. doi: 10.1016/0039-128x(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Jung BH, Kim SY, Chung BC. Determination of phytoestrogens in traditional medicinal herbs using gas chromatography-mass spectrometry. J of Nutr Biochem. 2004;15:452–60. doi: 10.1016/j.jnutbio.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–77. [PubMed] [Google Scholar]
- 28.Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368(6468):237–9. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher G, Neuhaus P. The physiological estrogen metabolite 2-methoxyestradiol reduces tumor growth and induces apoptosis in human solid tumors. J Cancer Res Clin Oncol. 2001;127:405–10. doi: 10.1007/s004320000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowke JH, Qi D, Bradlow HL, et al. Urinary estrogen metabolites and breast cancer: differential pattern of risk found with pre-versus post-treatment collection. Steroids. 2003;68:65–72. doi: 10.1016/s0039-128x(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 32.Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89(10):718–23. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- 33.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16 hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;135(3):603–8. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 34.IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer; 1972-PRESENT. (Multivolume work). Available at: http:/monographs.iarc.fr/ENG/Monographs/vol91/mono91-7D.pdf. [Google Scholar]
- 35.HMRlignan™. http://www.linnea-worldwide.com/downloadsarticles/hmrlignan.pdf.
- 36.HMRlignan™. http://www.hmrlignan.com/HMRwoBroch.pdf.
- 37.Cosentino M. In New clinical research—the HMRlignan™ strategy for sustainable women’s health support; 2007 May 9; Vitafoods, Geneva, Switzerland. [Google Scholar]
- 38.Michnovicz JJ, Bradlow HL. Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer. 1991;16(1):59–66. doi: 10.1080/01635589109514141. [DOI] [PubMed] [Google Scholar]