Abstract
Recent years have seen many exciting developments in the treatment of rheumatoid arthritis. Tocilizumab (TCZ) is a monoclonal antibody which inhibits the interleukin-6 receptor. After initial studies in Japan, it has been extensively studied in five multicentre clinical trials. This report summarises the key efficacy and toxicity findings from the major clinical trials. TCZ works quickly and effectively in rheumatoid arthritis either as monotherapy or in combination with other agents in early disease, DMARD inadequate responders, seronegative disease and after anti-TNF failure. The toxicity profile is manageable but includes infections (most notably skin and soft tissue), increases in serum cholesterol, transient decreases in neutrophil count and abnormal liver function tests (especially in combination with methotrexate). In summary, there is sufficient evidence to make TCZ a first line biologic therapy for rheumatoid arthritis especially for those who are unable to take methotrexate or who fail anti-TNF therapy.
Keywords: clinical trials, rheumatoid, therapy, interleukin-6
Introduction
Rheumatoid arthritis is the most common inflammatory arthritis. There have been a number of non biologic treatments that slow down radiographic progression1 but generally, the effect of these agents was relatively modest and often limited by toxicity. Therapies have improved in recent years to the point where clinical remission can be achieved in up to 50% of patients with a combination of a biologic agent and methotrexate (MTX) or triple therapy. While this is very encouraging compared to ten years ago, it still means there are 50% of patients who get an incomplete response and 10%–20% who get a very limited response. Interleukin-6, a glycoprotein composed of 212 amino acids in human, has pleiotropic effects on various cells such as B cells, T cells, hematopoietic stem cells, hepatocytes, megakaryocytes, osteoclasts, synoviocytes, keratinocytes and myeloma cells; thus, it has a wide range of biological activity, including regulation of immune responses, support of hematopoiesis, generation of acute-phase reactions and induction of inflammation and oncogenesis.2 Therapies involving blockade of interleukin-6 functions have constituted a new therapeutic strategy for some inflammatory and autoimmune diseases, including rheumatoid arthritis (RA), systemic-onset juvenile idiopathic arthritis, Castleman’s disease, multiple myeloma and systemic lupus erythematosus (SLE).2 This review will cover its efficacy and safety in rheumatoid arthritis.
Mechanism of action, metabolism and pharmacokinetic profile
Initial attempts at IL-6 blockade used a monoclonal antibody (mAb) to human IL-6. However, administration of an early specific mAb against IL-6 resulted in the formation of immune complexes that prolonged the half-life of IL-6. This was, therefore, unsuccessful in blocking IL-6 activity but others have been more successful at developing these antibodies.3 The current approach utilises a humanized anti-IL-6 receptor antibody, Tocilizumab (TCZ) (also known as MRA or Actemra). TCZ can bind to both soluble IL-6R and transmembrane IL-6R and inhibit IL-6 binding to its receptors, leading to the blockade of IL-6 signaling through both receptors, while not blocking the signaling of other IL-6 family cytokines.4 It may also have an effect on autoimmunity given the the effect of IL6 blockade on TH17 function.5 It blocks the action of IL-6 without increasing the IL-6 half-life.6 Single-dose pharmacokinetic studies in both rats and monkeys showed that clearance of TCZ (5 and 50 mg/kg) was slow, with a half-life (t1/2) of between 6 and 9 days.2 TCZ has a long t1/2 in human serum (~240 h) after the third dose of 8 mg/kg,7 so it can be administered biweekly but is usually given monthly.
Efficacy
The ACR20 is a clinical response parameter established by the American College of Rheumatology (ACR) indicating a ≥20% decrease in the count of tender joints and swollen joints and a 20% improvement in at least five of the following measures: patient’s global assessment of disease, patient’s global assessment of physical function, physician’s global assessment of disease and CRP or ESR. ACR50 and ACR70 responses indicate 50% and 70% improvements, respectively.
An early open clinical study suggested that TCZ was quite effective in the treatment of RA with >80% of patients achieving ACR20 responses and >30% achieving ACR50 responses.7 The major clinical trials are outlined in Tables 1 and 2.8–16 It can be seen that most subjects were highly active in terms of their DAS28 scores at study entry. There was marked variation in prior therapy and disease duration between the trials reflecting differing entry criteria. However, it is clear that the patients included reflect the more severe end of the spectrum of patients seen in everyday rheumatology practice.
Table 1.
Earlier tocilizumab monotherapy studies.
Table 2.
Phase 3 international program.
| OPTION12 | TOWARD13 | RADIATE14 | AMBITION15 | LITHE16 | |
|---|---|---|---|---|---|
| Study description | Reducing signs and symptoms | Reducing signs and symptoms | Reducing signs and symptoms | Reducing signs and symptoms | Structural damage |
| Population | MTX-IR | DMARD-IR | Anti-TNF-IR | 6 mo MTX-free | MTX-IR |
| Treatment | TCZ + MTX | TCZ + DMARDs | TCZ + MTX | TCZ vs. MTX | TCZ + MTX |
| Duration | 6 mo study | 6 mo study | 6 mo study | 6 mo study | 2 yr study |
Monotherapy
In a randomized, double-blind, controlled Phase II trial (the Chugai Humanized Anti-Human Recombinant Interleukin-6 Monoclonal Antibody or CHARISMA study),10 patients with active RA and an inadequate response to methotrexate (MTX) were entered into four treatment arms: TCZ 2, 4 and 8 mg/kg (n = 53, 54 and 52, respectively; four infusions every 4 weeks plus MTX placebo once weekly at week 12) or MTX 10–25 mg/week plus placebo infusions (n = 49). A dose response was observed and tocilizumab at dose of 8 mg/kg was most effective. TCZ 8 mg/kg monotherapy was significantly superior to MTX alone for reducing the Disease Activity Score using a 28-joint count (DAS28) as well as for normalizing CRP and ESR. The ACR20 response in the MTX group was lower than in the TCZ monotherapy groups.10
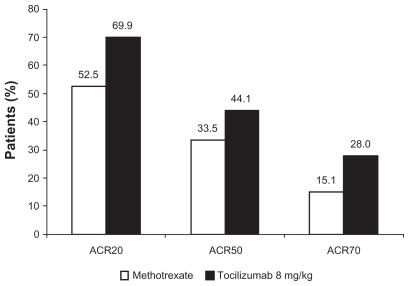
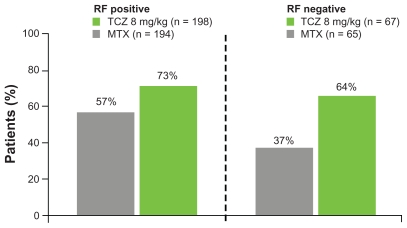
In an international multicenter, randomized, double-blind trial The Actemra versus Methotrexate double-Blind Investigative Trial In mONotherapy, AMBITION study,15 RA patients (n = 572) with moderate-to-severe active RA and who had not failed previous MTX or biologic therapy were randomized to receive either TCZ (8 mg/kg every 4 weeks, n = 286) or MTX (7.5 mg/week titrated to ≤20 mg/week within 8 weeks, n = 284) for 24 weeks. In the intention to treat analyses, TCZ was superior to MTX, with a significantly higher proportion in tocilizumab group achieving ACR responses (Fig. 1). TCZ treatment for 24 weeks achieved DAS28 remission (>5 times odds), normalized CRP (high sensitivity CRP < 0.3 mg/dL; 89% with TCZ versus 31% with MTX) and improved hemoglobin levels (mean change from baseline: +1.2 g/dl versus +0.1 g/dl with MTX) and these effects were greater than those with MTX treatment. 13 In addition, TCZ seemed to work regardless of whether the subject was rheumatoid factor positive or negative (Fig. 2). Prior disease modifying anti-rheumatic drugs (DMARDs) or methotrexate did not influence efficacy, however, both TCZ and methotrexate were more effective in those with a disease duration under 2 years.17 DAS28 remission rates are outlined in Figure 3 for all the key international trials. These show consistent remission rates with the highest in LITHE16 where the data is recorded at 12 months rather than 24 weeks suggesting increasing efficacy over time.
Figure 1.
Efficacy of tocilizumab compared to methotrexate in the AMBITION trial. Tocilizumab was statistically superior to methotrexate for all endpoints in this figure.
Note: Reproduced from15 with permission. All P < 0.05.
Figure 2.
Effect of rheumatoid factor on ACR20 response in the AMBITION trial. Tocilizumab was superior to methotrexate in both groups but the magnitude of benefit appeared greater in the seronegative arm primarily due to methotrexate being much less effective.
Figure 3.
DAS28 remission rates for tocilizumab 8 mg/kg versus comparator in the international phase 3 program. Tocilizumab was statistically superior to all comparators for DAS28 remission.
Other clinical trials all showed that targeting IL-6 by TCZ monotherapy was effective in reducing clinical symptoms, improving general health status and normalizing inflammatory markers.8–11 Overall, there have been >1,300 patients participating in TCZ monotherapy trials (including other doses) with ACR20 response of 63%–80% and ACR50 response of 40%–64% in the 8 mg/kg group, which were higher than with MTX therapy.
Combination therapy
In a double-blind, randomized, placebo-controlled, parallel group Phase III study (tOcilizumab Pivotal Trial in methotrexate Inadequate resONders, OPTION study),12 623 patients with moderate-to-severe active RA were randomly allocated to receive TCZ 8 or 4 mg/kg (n = 205 and 214, respectively), or placebo (n = 204) i.v. every 4 weeks, with MTX at stable pre-study doses (10–25 mg/week) for 24 weeks. Over 24 weeks, more patients in the placebo group (34%) than in the TCZ groups (15% and 9%) switched to rescue therapy. By week 24, the ACR20 response in the placebo group was lower than in the 4 and 8 mg/kg groups, respectively. The ACR50 and ACR70 (12 and 22% in 4 and 8 mg/kg groups, respectively, versus 2% in placebo group, all P < 0.001) responses in tocilizumab groups were also greater than in the placebo group. Similar to other trials, TCZ 8 mg/kg normalized CRP and ESR by week 2 of treatment and these remained normal until the end of the study but the effects of 4 mg/kg were less striking suggesting 8 mg/kg is the ideal dose. Rheumatoid factor concentrations had decreased from baseline more in both TCZ groups than in the placebo group by week 24.
The Tocilizumab in cOmbination With traditional disease modifying anti-rheumatic drug therapy (TOWARD) study was a double-blind, placebo-controlled, multicenter Phase III study examining the efficacy of TCZ in combination with a range of DMARDs for treatment of moderate-to-severe RA patients with inadequate response to these agents.13 A total of 1,220 patients, who had received stable doses of permitted DMARDs for ≥8 weeks before study entry, were randomized (2:1 ratio) to receive TCZ 8 mg/kg or placebo (control group) every 4 weeks for 24 weeks. Overall, 751 patients (93% including 2% receiving rescue therapy) in the tocilizumab group and 370 patients (89% including 11% receiving rescue therapy) in the control group completed 24 weeks’ treatment. At week 24, the TCZ group had a greater ACR20 response than placebo group irrespective of the types and number of concurrent DMARDs used. The TCZ group also had greater ACR50 and ACR70 (21 versus 3%, P < 0.0001) responses than the placebo group.
A Phase III, randomized, double-blind, placebo-controlled, parallel group study (The Research on Actemra Determining effIcacy after Anti-TNF failures, RADIATE study) examined the efficacy and safety of TCZ with MTX in patients with active RA who had failed at least one tumour necrosis factor (TNF) antagonist.14 A total of 499 patients who discontinued one or more TNF antagonists due to inadequate response before the study were randomly assigned to receive TCZ 4 or 8 mg/kg (n = 163 and 175, respectively) or placebo (control, n = 160) i.v. every 4 weeks for 24 weeks. Over 24 weeks, 84% patients (87% in 8 mg/kg group, 85% in 4 mg/kg group and 79% in placebo group) completed the study, and more patients in the control (41%) received rescue therapy after week 16 compared with 19% of patients in 4 mg/kg group and 11% in the 8 mg/kg group. Despite this refractory patient selection, ACR responses were greater in the TCZ groups than in the control group with a significant dose response between 4 and 8 mg/kg. The efficacy of TCZ was similar irrespective of the number of previous TNF agents.
Overall, there were >3,600 patients refractory to MTX or anti-TNF antagonists participating in TCZ in combination with either MTX or other DMARDs trials with ACR20 responses of 50%–74% and ACR50 responses of 29%–53% in the 8 mg/kg group.
Structure Modifying Effect
The disease-modifying effect of TCZ in combination with methotrexate was determined in the LITHE and OPTION trials by examining its effects on joint erosion, joint space narrowing and Total Sharp Score (TSS) from X-ray films9,16 and on biochemical markers. 18 Over 1 year, TCZ treatment (4 or 8 mg/kg i.v. every 4 weeks) significantly suppressed mean joint erosion, joint space narrowing and TSS score, which was superior to that of methotrexate either in combination16 or as monotherapy.9 Compared to placebo plus MTX, TCZ 8 mg/kg plus MTX induced a significant decrease of a bone resorption marker ( carboxy-terminal telopeptide of type-I collagen, ICTP) at week 16–24, and a significant decrease of the cartilage turnover markers (type IIA collagen N-propeptide, PIIANP and type II collagen helical peptide, Helix-II) at week 4–24.18
Safety and Tolerability
There are similarities for safety in most of the phase 3 program. In AMBITION,15 the overall incidence of AEs was similar in both groups (79.9% TCZ vs. 77.5% methotrexate; P = 0.48), as was the incidence of serious AEs. Most AEs were mild or moderate, with fewer than 7% of patients experiencing severe AEs.
The most common AEs during the study were infections (TCZ, 34.4% vs. methotrexate, 37.3%). Infection rates per patient year were similar (TCZ, 1.06% vs. methotrexate, 1.09%). Skin and subcutaneous infections were reported with a higher frequency in the TCZ (4.1%) vs. methotrexate group (0.7%). The incidence of herpes infection was similar in the two treatment groups (1.7% vs. 1.4%). No fungal infections were reported in the TCZ group, but five patients (1.8%) in the methotrexate group experienced fungal infection. The second most common AEs were gastrointestinal disorders, occurring with similar frequency in both groups with no difference in perforations. Five (1.8%) patients in the methotrexate group and 1 (0.3%) patient in the TCZ group were withdrawn from the study due to gastrointestinal AEs.
Infusion reactions (any AE occurring during, or within 24 hours following infusion) occurred in 5.6% of patients in the TCZ group and 1.8% in the methotrexate group (P = 0.0158). The majority occurred during the first 2 infusions (TCZ: 10/16; methotrexate: 3/6); no serious infusion reactions or anaphylaxis were reported.
Four patients died during the study; 1 in the methotrexate group (lung cancer), and 3 in the TCZ group (upper gastrointestinal hemorrhage/perforation, myocardial ischemia, and cardio-respiratory arrest in a patient with history of asthma and arrhythmia). The death due to gastrointestinal hemorrhage was considered by the investigator to be remotely related to treatment due to short exposure to TCZ and the patient’s medical history (peptic ulcer), although relationship to treatment could not be excluded. The other 3 deaths were deemed unrelated to trial treatment.
Fewer elevations in alanine aminotransferase (ALT) to >3 > the upper limit of normal (ULN) were observed with TCZ vs. methotrexate. One percent of TCZ-treated patients vs. 2.5% methotrexate-treated patients developed elevations between 3 > and 5 > ULN; 0.3 vs. 0.7%, respectively, developed elevations between >5 > and 8 > ULN, and one patient in each group developed elevations to >8 > ULN. Three patients in the TCZ group vs. 2 in the methotrexate group discontinued study therapy due to aminotransferase elevations. Total bilirubin elevations >ULN and <3 > ULN were seen in 0.7 and 7.6% of patients in the TCZ and methotrexate groups, respectively, with no elevations observed concurrently with aminotransferase elevations. There were no clinical signs or symptoms of hepatitis or hepatic dysfunction.
Increases in total cholesterol from <200 mg/dL at baseline to ≥240 mg/dL occurred with TCZ in 13.2% of patients compared to 0.4% with methotrexate. Increases in LDL from <100 mg/dL at baseline to ≥160 mg/dL occurred in 3.1 and 0% of patients, respectively. No patients in either treatment group had changes in triglycerides from <150 mg/dL to ≥500 mg/dL. There were no clinical symptoms or cardiovascular events (apart from the death mentioned above) reported in these patients.
More patients had transient grade 3 neutropenia (<1000–>500 cells/mm3) with TCZ, 3.1% vs. methotrexate, 0.4%. One patient in the placebo/TCZ sub-study arm discontinued due to grade 4 neutropenia (<500 cells/mm3) and one patient due to grade 3 neutropenia; both events resolved without sequelae. There was no correlation between neutropenia and infection risk. In fact, the correlation had a negative trend suggesting infections may be more likely in those who don’t drop their neutrophil counts.
The overall infection rates from four Phase III trials (OPTION, TOWARD, LITHE and RADIATE) were pooled for analysis.19 Patients received combination treatment with DMARDs plus tocilizumab 8 mg/kg i.v. (n = 1,582) every 4 weeks, or placebo (placebo + DMARD; n = 1,170). Over 24 weeks, infections were the most frequent type of adverse events observed in all groups. Serious infection rates were low in all groups with slightly higher rates in the tocilizumab group. Tuberculosis was not observed in all these studies unlike anti-TNF agents. The rate of serious infections was 5.2%, or 3.9/100 patient-years, with no evidence of increased risk with continued tocilizumab exposure over time. This is similar to infection rates on those on antiTNF therapy. Diabetes, age ≥65 years, a history of infection and corticosteroid use were associated with an increased probability of developing a serious infection. No association was observed between low neutrophil counts and serious infections,20 even though neutrophil counts decreased in a dose-dependent fashion after TCZ treatment.
Hepatic aminotransferases and bilirubin levels during TCZ combined therapy of patients with RA in four Phase III trials (OPTION, TOWARD, RADIATE and LITHE) were pooled for analysis.21 The incidence of ALT and AST abnormalities during treatment was higher in the TCZ combined therapy group than in the DMARDs group. Most of these patients did not require dose modification to achieve values equal to or less than the upper limit of normal. Hepatic aminotransferase elevations were not associated with increases in total bilirubin. In the TCZ combination therapy group, 25 patients discontinued treatment due to significant increases in ALT or AST levels (more than fivefold the upper limit of normal). After a median of 1.5 years of treatment, ALT and AST were elevated more than threefold the upper limit of normal in 7.6 and 2.4% of 2,562 patients, respectively. Over 5 years of treatment, increases in AST and ALT (more than threefold the upper limit of normal) occurred in 9 (6.3%) and 14 (9.8%) of 143 patients, respectively, but most were transient and resolved without any particular treatment.11 Greater than 3 times elevation of ALT was more common with methotrexate therapy than with TCZ monotherapy in AMBITION15 suggesting that the TCZ methotrexate combination may not be ideal with regard to liver toxicity.
Lipid profiles in patients receiving TCZ for treatment of RA in four Phase III trials were also pooled.22 Increases from baseline in total cholesterol (TC), low density lipoprotein (LDL), High density lipoprotein (HDL) and triglycerides were observed within 6 weeks of initiating treatment, and these persisted to week 24 in tocilizumab-treated patients. Over 1.5 years, the levels of TC, HDL, LDL and triglycerides were elevated from the first measurement at week 6, and remained elevated but without further increases with continued treatment. Initiation of statin treatment after receiving TCZ was effective in reducing LDL levels to below baseline values.23 The increase in lipid levels may be secondary to the control of inflammation and explainable by the apparent effect of IL-6 on lipid metabolism.24 Triglyceride levels also increased but this was not associated with clinical symptoms, particularly pancreatitis.13 In addition, the increase in total TC was not associated with an excess in major adverse cardiovascular events in the short term. More patients in the TCZ groups had a greater increase in atherogenic index (ratio LDL/HDL) than in the control group.15 This can be balanced against the marked decrease in CRP, which is an independent cardiovascular risk factor.
The potential development of anti-tocilizumab human anti-human antibodies (HAHAs) and their effect on both efficacy and incidence of allergic-type adverse event was evaluated in four Phase III trials (OPTION, TOWARD, RADIATE and AMBITION).25 Over 24 weeks, 24 out of a total of 1,747 TCZ-treated patients developed neutralizing (n = 18), Ig class (n = 9) or uncharacterized (n = 4) HAHAs, with some patients appearing in several categories. The incidence of neutralizing HAHAs was highest in patients treated with TCZ (8 mg/kg) + DMARD (n = 16), compared with TCZ (4 mg/kg + DMARD, n = 1; or tocilizumab 8 mg/kg, n = 1). However, the efficacy remained in patients with neutralizing HAHAs with no withdrawals due to lack of efficacy. A total of 6 patients had 12 allergic adverse events but 4 of these developed HAHAs before these allergic events. Of these 4 patients, 3 withdrew from the treatment due to severe anaphylactic reaction (1 patient) or moderate infusion-related reactions (2 patients). Since then there has been one death from anaphylaxis worldwide suggesting careful monitoring is necessary for this rare side effect during the first 5–6 infusions.
The suggested management for TCZ side effects is outlined in Table 4.
Table 4.
Side effects with Tocilizumab and suggested management.
| Side effects | Action |
|---|---|
| Skin and soft tissue infections | Standard antibiotic therapy. Omit infusion for one month then restart if infection has cleared |
| Abnormal liver function tests | Monitor monthly for first 6 months Cease if >5 times ULN Omit for one month if 3–5 times ULN No action if <3 times ULN Consider modifying methotrexate dose |
| Hypercholesterolemia | Monitor 2–3 times in first 6 months and annually thereafter Treat with statins if elevated according to local guidelines |
| Neutropenia | Monitor monthly for first 6 months Cease if <0.5 Omit for one month if 0.5–1 No action if greater than one |
| Anaphylaxis | Monitor BP more carefully during first 6 infusions Do not rechallenge if BP drops substantially or patient can’t complete a prior infusion |
Place in Therapy
Currently, there are many effective treatments for RA so it is a good time to be either a sufferer of rheumatoid arthritis or a rheumatologist. There is substantial experience with anti-TNF agents suggesting most rheumatologists will continue to use them first line in difficult rheumatoid arthritis. However, TCZ now has sufficient evidence of efficacy in all the stages of rheumatoid arthritis to justify it being a first-line biologic agent. Unlike, the anti-TNF agents, this agent works well either as monotherapy or in combination whereas the anti-TNF agents do best when combined with methotrexate. Thus, in the not uncommon methotrexate intolerant patient, TCZ monotherapy now appears the treatment of choice. There are no data directly comparing this agent with other biologic agents so the only comparisons are indirect and cautiously suggest similar efficacy. The long term safety remains to be determined. Despite the increase in lipids, the effect on cardiovascular disease might actually be protective given the normalisation of C reactive protein. We would like to see data on factors predicting infection risk as the neutrophil data suggest this is not mediated by lower neutrophil counts. At present, we would be reluctant to use TCZ in those on corticosteroids or with diabetes mellitus given the increased risk of gram positive skin infections. We would treat cholesterol according to local guidelines and similarly would monitor liver function regularly and suspend treatment if transaminases are greater than 3 times ULN and reinstate if they normalised as these changes are often transient. Lastly, TCZ is already being widely used in Australia but this will increase once there are simpler modes of administration.
Conclusions
Tocilizumab is the first agent (biologic or otherwise) that has been shown to be superior to MTX as monotherapy for the signs and symptoms of rheumatoid arthritis. It is also effective in combination with methotrexate or other DMARDs. The effect is consistent across all outcome measures and is rapid in onset at 2–4 weeks. This is especially notable for hemoglobin as methotrexate did not improve this in this trial. The side effect profile is as expected given its mode of action and is largely consistent with the other trials. There are some points of difference. Liver toxicity was less common with tocilizumab compared to methotrexate whereas the combination seems to be a bit more toxic in other trials compared to DMARDs or methotrexate alone (2,3). In other trials both LDL and HDL cholesterol increased but that was not seen AMBITION where only LDL increased.15 The cholesterol effect may be consistent with the marked decrease in inflammation which has also been observed with anti-TNF agents.24 Reassuringly, there is no cardiovascular signal at this stage either in the trials or the long term open label studies.
Table 3.
Baseline characteristics of the tocilizumab phase three program.
Acknowledgements
G Jones is partially funded by an NHMRC Practitioner Fellowship and C Ding is supported by an NHMRC Clinical Career Development Award.
Footnotes
Disclosures
G Jones has received travel grants, research funding, speaker fees and advisory board fees from Roche (the maker of tocilizumab). He is also the principal investigator on the AMBITION trial. No writing assistance or input from Roche was sought or provided for this manuscript.
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no other conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Jones G, Halbert J, Crotty M, et al. The effect of treatment on radiological progression in rheumatoid arthritis: a systematic review of randomized placebo-controlled trials. Rheumatology (Oxford) 2003;42:6–13. doi: 10.1093/rheumatology/keg036. [DOI] [PubMed] [Google Scholar]
- 2.Ding C, Jones G. Anti-interleukin-6 receptor antibody treatment in inflammatory autoimmune diseases. Rev Recent Clin Trials. 2006;1:193–200. doi: 10.2174/157488706778250168. [DOI] [PubMed] [Google Scholar]
- 3.Imagawa T, Mori M, Takei S, et al. Efficacy and safety of tocilizumab, an anti- IL-6 receptor monoclonal antibody; in patients with polyarticular or oligoarticular onset juvenile idiopathic arthritis. Arthritis Rheum. 2006;54:S168. doi: 10.1007/s10165-011-0481-0. [DOI] [PubMed] [Google Scholar]
- 4.Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–40. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Ohsugi Y. Recent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti- human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol Pharm Bull. 2007;30:2001–6. doi: 10.1248/bpb.30.2001. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8:S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimoto N, Yoshizaki K, Maeda K, et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti- interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol. 2003;30:1426–35. [PubMed] [Google Scholar]
- 8.Nishimoto N, Miyasaka N, Yamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–9. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of Active Controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 inhibitor (SAMURAI):—Evidence of clinical and radiographic benefit from an X-Ray Reader-Blinded Randomized Controlled Trial of TCZ. Ann Rheum Dis. 2007;66:1162–7. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 11.Nishimoto N, Miyasaka N, Yamamoto K, et al. Long-term safety and efficacy of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580–4. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with TCZ in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 13.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with TCZ reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The TCZ in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 14.Emery P, Keystone E, Tony HP, et al. IL-6 Receptor inhibition with TCZ improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-TNF biologics: results from a 24-week multicentre Randomised Placebo Controlled Trial. Ann Rheum Dis. 2008;67:1516–22. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones G, Sebba A, Gu J, et al. Comparison of TCZ monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer JM, Fleischmann RM, Halland AM, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with an inadequate response to methotrexate: the LITHE study. Arthritis Rheum. 2008;58:S4031. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- 17.Jones G. The AMBITION trial: Tocilizumab monotherapy for rheumatoid arthritis. Exp Rev Clin Immunol. 2010;6:189–95. doi: 10.1586/eci.10.2. [DOI] [PubMed] [Google Scholar]
- 18.Garnero P, Thompson E, Woodworth T, Smolen JS. Rapid and sustained improvement in bone and cartilage turnover markers with the anti- interleukin- 6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 2010;62:33–43. doi: 10.1002/art.25053. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Beaulieu AD, Dikranian A, et al. Safety of tocilizumab in patients with rheumatoid arthritis: pooled analysis of five phase 3 clinical trials. Arthritis Rheum. 2008;58:S784. [Google Scholar]
- 20.Kremer JM, van Vollenhoven RF, Ridley DJ, et al. Relationship between patient characteristics and the development of serious infections in patients receiving tocilizumab: results from long-term extension studies with a follow-up duration of 1.5 years. Arthritis Rheum. 2008;58:S783–4. [Google Scholar]
- 21.Kremer JM, Joh AK, Malamet R, Keystone EC. Hepatic aminotransferases and bilirubin levels during tocilizumab treatment of patients with rheumatoid arthritis: pooled analysis of five phase 3 clinical trials. Arthritis Rheum. 2008;58:S783. [Google Scholar]
- 22.Genovese MC, Smolen JS, Emery P, et al. Lipid and inflammatory biomarker profiles in patients receiving tocilizumab for rheumatoid arthritis: analysis of five phase 3 clinical trials. Arthritis Rheum. 2008;58:S531. [Google Scholar]
- 23.Genovese MC, Smolen JS, Emery P, et al. Concomitant use of statins in tocilizumab-treated patients with rheumatoid arthritis with elevated low density lipoprotein cholesterol: analysis of five phase 3 clinical trials. Arthritis Rheum. 2008;58:S785. [Google Scholar]
- 24.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–9. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Remus C, Genovese MC, Harre RA, et al. Low immunogenic potential of tocilizumab in patients with rheumatoid arthritis: analysis of four phase 3 clinical trials. Arthritis Rheum. 2008;58:S534. [Google Scholar]