Abstract
Metastatic renal cell carcinoma has harboured a poor prognosis for decades with immunotherapy being the only available therapy with high toxicity and modest effect. Dependance of renal cell carcinoma oncogenesis on the mTOR pathway has led to clinical development of temsirolimus in this setting. This sirolimus derivative has shown clinical efficacy in monotherapy for poor-risk renal cell carcinoma leading to an overall survival of 10.8 months in the pivotal phase III trial of this agent. Its specific adverse events consist of metabolic dysregulation (hyperlipemia, hyperglycemia), mucositis, rash and pneumonitis which can be severe and need careful monitoring and management. In this review, we will discuss of the clinical development of this molecule, its efficacy, its safety profile and future perspectives.
Keywords: clear cell carcinoma, temsirolimus, mTOR, rapamycin, safety, efficacy
Introduction
Renal cell carcinoma (RCC) is the most common malignancy of the kidney and accounts for 2%–3% of all adult cancers.1 Although surgical resection can be curative in localized disease, prognosis of advanced renal cell carcinoma is very poor with a 5-year survival rate of 5%–10%. Immunotherapy with interferon-α has produced modest survival benefice in clinical trials2–7 while high dose interleukin- 2, although active in highly selected patients, is associated with severe toxicity.8,9 Phase III studies since 2007 have emphasized the importance of targeting angiogenesis through vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibition with sunitinib10 and sorafenib11 or direct VEGF inhibition with bevacizumab in combination with IFN.12,13 These anti angiogenic agents have demonstrated improved overall survival (sunitinib)14 or progression free survival (sorafenib15 and bevacizumab/IFN)16,17 for patients with advanced RCC. The mammalian target of rapamycin (mTOR), a member of the phosphatidyl inositol 3′ kinase family, is a multifunctional serine-threonine kinase that acts as central regulator of cell growth, proliferation, and apoptosis.18,19 It modulates the expression and stability of hypoxia- inducible factor (HIF)-1α, which regulates expression of VEGF. Temsirolimus, although known as CCI-779, is a potent and selective inhibitor of mTOR. It has demonstrated its efficacy as first line monotherapy in poor-prognosis metastatic RCC in comparison with IFN.20 This review will focus on data supporting temsirolimus efficacy in RCC and will address its safety profile with emphasis on specific side effects.
Efficacy of Temsirolimus in Renal Cell Cancer
Pharmacokinetics and pharmacodynamics
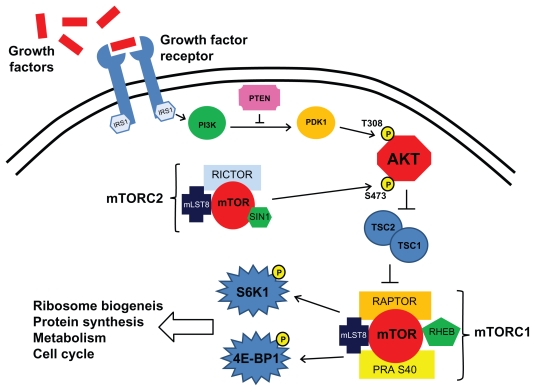
mTOR is a highly conserved serine/threonine kinase that forms multimolecular complexes and has a key function in apoptosis, cell growth, and tumor proliferation by controlling cellular catabolism and anabolism.21 mTOR complexes with raptor (regulatory-associated protein of mTOR) to form mTORC1 and with rictor (rapamycin-insensitive companion of mTOR) to form another multimolecular complex named mTORC2. mTOR is activated through the phosphatidylinositol 3-kinase (PI3K) pathway by growth factors receptors such as epidermal growth factor receptor (EGFR) or insulin growth factor receptor (IGFR), for example (Fig. 1). Once activated, mTORC1 phosphorylates its downstream effectors, for example, eukaryotic translation initiation factor 4E-binding protein (4EBP1) and S6 kinase (S6K). mTOR activation downstream of growth factor receptors promotes protein translation, cell growth, ribosome biogenesis, metabolism increase, proliferation and decreased autophagy.
Figure 1.
Cell signalling involving mTORC1 and mTORC2 in cancer cells.
Temsirolimus is a functional analog of rapamycin (also named sirolimus) like everolimus and deforolimus.22 Temsirolimus binds with high affinity to the immunophilin FKBP 12 (FK506, binding protein 12) and selectively inhibits mTORC1 but have no direct effect on mTORC2.23 Inhibition of mTORC1 activity is reversible only slowly (5 days).23 Inhibition of mTORC1 kinase activity results in decreased phosphorylation of S6K and 4EBP1.24 Ultimately, temsirolimus inhibits the synthesis of various proteins that have important roles in the cell cycle and tumorigenesis, such as cyclin D1, p27, and apoptosis regulators such as BAD, Bcl2 and p53.25 Inhibition of mTORC1 by temsirolimus has also been shown to reduce expression of HIF-1α and HIF-2α under both normoxic and hypoxic conditions in mouse xeno-graft models.26 This reduced expression will lead to decreased VEGF and PDGF expression. The observed clinical efficacy of temsirolimus in patients with renal cell carcinoma may be mediated in one hand by inhibition of efficient HIF-1α translation resulting in interception of the VEGF/VEGFR and/or PDGF/PDGFR signaling cascades and in another way by inhibition of protein synthesis that are involved in cell cycle and tumorigenesis.
Temsirolimus is a macrocyclic lactone and a water-soluble ester derivative of sirolimus with better chemical stability and solubility which make it suitable for intravenous (IV) administration. Following IV administration, exposure, measured as total area under the concentration time curve (AUC), is less than proportional to dose.29 The mean steady-state volume of distribution of temsirolimus is high, indicating extensive tissue distribution; clearance of the drug increases in a less than proportional manner with increased dosing, suggesting saturation of drug metabolism, modification of protein binding, and/or erythrocyte sequestration.27 After IV injection, temsirolimus is rapidly cleared from the plasma and converted in the liver by cytochrome CYP 4503A4/5 into sirolimus that becomes the most prevalent drug. Terminal half life decreases with increasing dose. Excretion is predominantly via the feces, with renal elimination of drug and metabolites accounting for 4.6% of the administered dose.22 The higher relative half-life of the sirolimus metabolite is considered to contribute to sustained and clinically important exposures for the duration of the weekly dosage interval. The mean ratio of sirolimus AUC/temsirolimus AUC is 2.7.22
Controlled trials have not been performed in special populations such as renal impaired, elderly, obese or hepatic insufficiency patients. Renal impairment, in particular, is not expected to influence drug exposure, and no dose adjustments of temsirolimus are recommended in this population because of predominant feces elimination of the drug. In two patients on hemodialysis who were treated with temsirolimus 25 mg once weekly for metastatic RCC, pharmacokinetic profiles were similar with those observed in patients with normal renal function.31
In phase I studies, temsirolimus was initially administered at doses that were corrected for body surface area (BSA) but it was then showed that the degrees of variability between BSA-normalized and flat dosing were comparable; therefore, flat doses were selected for further evaluation in phase II and III trials.30 In the phase II study from Atkins et al, 3 doses were evaluated: 25,75 and 250 mg: no differences were seen in terms of median survival, tumor response rates and toxicities between the three doses.32 Thus, temsirolimus, administered as a 30- to 60-minute IV infusion once weekly at a flat dose of 25 mg alone or in combination was the therapeutic regimen chosen for subsequent evaluation in phase III study.
Temsirolimus in monotherapy
Phase I studies
Temsirolimus efficacy in patient with heavily pretreated malignancies was initially observed in two phase 1 studies. In the study by Raymond et al, 24 patients were included with 6 RCC patients30 (Table 1). Temsirolimus was administered once weekly as a 30-minute IV infusion after pretreatment with IV antihistamine, with escalated doses ranging from 7.5 to 220 mg/m2. Maximum tolerated Dose (MTD) was not reached. Confirmed partial responses were observed in two patients: in a patient with breast cancer and in a patient with renal cell carcinoma who received 15 mg/m2 temsirolimus after documented tumor progression of lung and pleural metastasis under treatment with interferon-α and interleukin 2. The partial response was observed after 8 weeks of treatment and lasted for 6.5 months under therapy. Minor responses lasting for 3 and 4.9 months were reported in two additional patients with renal cell carcinoma treated at the dose of 15 mg/m2 and 45 mg/m2, respectively. Flat dose was adopted because of the lack of variability in comparison with BSA-normalized doses. Toxicity analysis of this study will be reviewed in another paragraph.
Table 1.
Efficacy of temsirolimus.
| Trial | ORR | PFS (months) | 95% CI | OS (months) | 95% CI | |
|---|---|---|---|---|---|---|
| Phase I (monotherapy)25 | 8.3% | ND | ND | ND | ND | |
| Phase I/II (+IFNα)35 | 11% | 9.1 | [6.2–13] | 18.8 | [15–25] | |
| Phase II (monotherapy 25 mg)27 | 7% | 6.3 | [3.6–7.8] | 13.8 | [9–18.7] | |
| Phase III34 | Monotherapy | 8.6% | 5.5 | [3.9–7] | 10.9 | [8.6–12.7] |
| +IFN α | 8.1% | 4.7 | [3.9–5.8] | 8.4 | [6.6–10.3] | |
Abbreviations: ORR, overall response rate = complete response + partial response; PFS, progression free survival; OS, overall survival; 95% CI= 95% confidence interval.
In the study by Hidalgo et al, 63 patients were included with 16 having RCC.33 Temsirolimus was administered as a 30-minute IV infusion once daily on days 1 to 5 of each treatment cycle of 2 weeks with doses ranging from 0.75 to 24 mg/m2/d with MTD in heavily pretreated patients being 15 mg/m2 (grade 3 aspartate and alanine aminotransaminase elevations, vomiting, diarrhea, and asthenia). One patient had a partial response, 3 had an unconfirmed partial response, 3 patients had stable disease. The responses in RCC patients in these two phase I studies provided the rationale for further evaluation of temsirolimus in patient with advanced RCC in phase II studies.
Phase II study
In phase II study evaluating temsirolimus as a monotherapy, 111 patients with advanced refractory RCC were randomly assigned to receive 25 mg, 75 mg, or 225 mg of temsirolimus every week as a 30-minute infusion32 (Table 1). Median age was 57, 69% of the patients were male, ECOG performance status (PS) was 0 or 1, patients had extensive disease with half of them having three or more metastatic sites and were heavily pretreated: 28% had had 3 or more previous immunotherapy or chemotherapy regimen. The objective response rate was 7%, which included 1 complete response and 7 partial responses, with 26% minor response also reported. The median time to tumor progression was 5.8 months for the total patient population and 6.3 (95% confidence interval (CI) [3.6–7.8], 6.7 (95% CI [3.5–8.5]) and 5.2 (95% CI [3.7–7.4]) months in the 25-, 75- and 250-mg dose group, respectively. Median survival was 15.0 months for the total patient population and 13.8 (95% CI [9–18.7]), 11 (95% CI [8.6–18.6]) and 17.5 (95% CI [12–24.6]) months in the 25-, 75- and 250-mg dose group, respectively. Additional analysis on the basis of previously described prognostic factors was undertaken. Five poor prognosis factors, adapted from Motzer et al,34 were taken into account: performance status less than ECOG PS 0 or 1, lactate dehydrogenase levels more than 1.5 × upper limit of normal, corrected serum calcium levels more than 10 mg/dl, serum hemoglobin level less than lower limit of normal, and time from initial RCC diagnosis to start of first immunotherapy or chemotherapy of less than 1 year. Patients were separated into a good-risk group that had none of these poor-prognosis factors, an intermediate-risk group that had one or two factors and a poor-risk group that had three or more factors. The median survivals of the heavily pretreated patients in the different risk groups in this study were compared with the median survivals of first-line RCC patients in the different risk groups treated with IFN-α from a previous study.35 For patients in the intermediate- and poor-prognosis populations, median survivals of temsirolimus–treated patients appeared to be 1.6 to 1.7-fold longer than those of IFN-α–treated patients. No such advantage was seen in the good-prognosis patients; however, this may have been due to the small number of patients with good prognosis who received temsirolimus in this study, resulting in a lack of statistical power. Temsirolimus was generally well tolerated at all dose levels, hyperglycemia (17%) and hypophosphatemia (13%) were the most frequently grade 3 or 4 adverse events. Results of this phase II study led to design a phase III trial comparing temsirolimus alone or in combination with IFN-α to IFN-α alone in first line treatment of poor-prognosis RCC.
Phase III trial
Global Advanced Renal Cell Carcinoma (ARCC) Trial, a phase III open label multicenter trial, included 626 patients with previously untreated, poor-prognosis, metastatic RCC who were randomly assigned to receive 25 mg of weekly IV temsirolimus, 3 million units (U) of IFN-α (with an increase to 18 million U) subcutaneously three times weekly, or combination therapy with 15 mg of temsirolimus weekly plus 6 million U of IFN- α three times weekly36 (Table 1). At least three of the following six poor-prognosis factors were required for inclusion: a serum lactate dehydrogenase level of more than 1.5 times the upper limit of the normal range, a hemoglobin level below the lower limit of the normal range; a corrected serum calcium level of more than 10 mg per deciliter (2.5 mmol per liter), a time from initial diagnosis of renal-cell carcinoma to randomization of less than 1 year, a Karnofsky performance score of 60 or 70, or metastases in multiple organs; these factors included the 5 adverse prognosis factors described by Motzer et al plus the presence of multiple metastatic sites. Characteristics of randomized patients were as following: median age 59, 69% male, 82% patients with Karnofsky performance status of 70 or 60, 82% renal clear cell histology, 94% of patients having 3 or less poor-prognosis factors. Seventy four percent of the patients were classified as poor-risk RCC and 26% were classified as intermediate-risk RCC. The primary endpoint was overall survival of the temsirolimus group and the combination-therapy group compared with the interferon group. Patients who received temsirolimus alone had superior overall survival (hazard ratio (HR) for death: 0.73; 95% CI [0.58–0.92]; P = 0.008) and PFS (P < 0.001) compared with patients who received interferon alone. Overall survival in the combination therapy group did not differ significantly from that in the interferon group (HR: 0.96; 95% CI [0.76–1.20]; P = 0.70). Median overall survival was 7.3 months (95% CI [6.1–8.8]), 10.9 months (95% CI [8.6–12.7]), and 8.4 months (95% CI [6.6–10.3]); the objective response rates were 4.8%, 8.6% and 8.1% in the interferon, temsirolimus, and combination therapy groups, respectively.
In exploratory subgroup analyses with Cox proportional-hazards model, the effect of temsirolimus on overall survival was found to be greater among patients under 65 years of age than among older patients (P = 0.02) and among patients with a serum lactate dehydrogenase level of more than 1.5 times the upper limit of the normal range than among those with lower levels (P = 0.008). The most frequently occurring grade 3 or 4 adverse events were asthenia, nausea, hyperglycemia, rash and neutropenia. Mean dose intensity was 30.2 million U/week in the IFN- α alone group, 23.1 mg/week in the temsirolimus alone group and 10.9 mg/wk of temsirolimus with 13.1 million U IFN-α in the combination group. The data from this pivotal trial were the basis for May 2007 FDA approval of temsirolimus for advanced RCC.
Temsirolimus in combination
Combination with IFN-α
Temsirolimus was first investigated as combination therapy with IFN-α in phase I/II study37 (Table 1). Patients were enrolled onto a multicenter, ascending-dose study of temsirolimus (5, 10, 15, 20, or 25 mg) administered intravenously once a week combined with IFN-α (6 or 9 million U) administered subcutaneously three times per week. An expanded cohort was treated at the recommended dose to obtain additional safety and efficacy information. Seventy-one patients received one of six dose levels. Median age was 59 year, 76% of patients were male, 55% had had 1 or 2 prior chemotherapy regimen, 82% had clear cell histology, 55% of the patients had an intermediate-risk RCC and 24% had a poor-prognosis RCC according to MSKCC model. The recommended dose was temsirolimus 15 mg/IFN 6 millions U based on dose-limiting toxicities of stomatitis, fatigue, and nausea/vomiting, which were observed at higher doses of temsirolimus and IFN-α. The most frequent grade 3 or 4 toxicities occurring in any cycle included leukopenia, hypophosphatemia, asthenia, anemia, and hypertriglyceridemia. Among patients who received the recommended dose (n = 39), 8% achieved partial response and 36% had stable disease for at least 24 weeks. For the entire population, median progression-free survival was 9.1 months (95% CI [6.2–13]) and median overall survival was 18.8 months (95% CI [15–25]). For patients in the recommended-dose cohort median progression-free survival was 7.6 months (95% CI [5.5–11.0]) and median overall survival was 22.1 months (95% CI [11.0–26.0]).
This results contrast with those seen in the combination arm of the ARCC phase III trial. Although the chosen dose for the combination arm in the phase III trial was the same as the recommended dose of the phase I/II study, no differences in overall survival nor in progression free survival were seen in comparison to the IFN-α alone group. The overall survival of the combination group was 8.4 months (95% CI [6.6–10.3]) in the ARCC trial in contrast with overall survival of the combination arm in the phase I/II study of 22.1 months (95% CI [11.0–26.0]). In the ARCC trial, 30% of the combination group discontinued treatment for adverse event or symptomatic deterioration in comparison with 14% of patient in the temsirolimus alone group. Therefore, investigators attributed the reduced overall survival in the combination group to the high rate of serious adverse events. However, grade 3–4 adverse events were not more important in the phase III trial: asthenia 13.4% versus (vs.) 23% in the phase II study, Anemia 18.2% vs. 23%, neutropenia 7.2% vs. 33%. Patients in the phase III trial had poorer prognosis with 74% of them having a poor-risk RCC in comparison with 23% of the patients in the phase II study. This could explain differences seen in terms of efficacy and toxicity. To conclude, owing to the results of the phase III trial, temsirolimus has been approved as a monotherapy.
Combination with antiangiogenic agents
Phase I studies have been conducted with both sunitinib and sorafenib. One phase I study evaluated temsirolimus in combination with sunitinib in patients with advanced RCC with at most two previous regimen.38 At the starting dose, temsirolimus 15 mg was administered by IV infusion once weekly, and sunitinib 25 mg was administered orally once daily for 4 weeks, followed by a 2-week rest period. In the first cohort, dose-limiting toxicities (grade 3 treatment- related toxicities that lasted more or equal to 7 days) were observed in 2 of 3 patients. One patient experienced grade 3 rash during week 3, which led to treatment discontinuation. A second patient had grade 3 thrombocytopenia, cellulitis, and gout during week 3 and was hospitalized. A third patient experienced rash, asthenia, diarrhea, stomatitis, constipation, fever, and rectal hemorrhage, all of which were mild in severity. The study was terminated because of dose-limiting toxicity observed at low starting doses of both agents. Another phase I study evaluated temsirolimus in combination with sorafenib: eligible patients were treated with escalating continuous oral doses of sorafenib (200 and 400 mg twice daily) and weekly Temsirolimus IV (15 mg, 25 mg).39 Twenty four evaluable patients received 85 courses [median 3; range 1–12] of combination therapy. Dose limiting toxicities (DLT) were grade 3 typhlitis (n = 1), mucositis (n = 1), hand foot syndrome (n = 2), thrombycytopenia/rash (n = 1) and creatinine elevation (n = 1). The combination of sorafenib and temsirolimus demonstrated significant mucocutaneous toxicity at full doses of sorafenib, although preliminary PK analyses show no evidence of drug-drug interactions. To conclude, phase I studies of antiangiogenic tyrosine kinase inhibitors have not shown sufficient safety so far.
Bevacizumab has shown its efficacy with improved progression free survival and overall survival ranging from 18.3 to 23.3 months in the AVOREN trial16 and CALGB90206,17 in combination with IFN-α. TORAVA is an open label, multicenter, non comparative phase II trial that evaluated temsirolimus plus bevacizumab combination versus bevacizumab plus IFN-α versus sunitinib.40 Preliminary results have been recently presented at the 2010 American Society of Clinical Oncology (ASCO) annual meeting. One hundred and seventy one untreated metastatic RCC patients with ECOG PS ≤2 and measurable disease were randomized to Temsirolimus-Bevacizumab combination (Tem/Bev) n = 88, sunitinib (S) n = 42 or bevacizumab and IFN-α (Bev/IFN) n = 41. Patients were treated until disease progression or toxicity. The primary objective was to estimate the non-progression rate at 48 weeks (NPR-48) for combination arm. Major secondary end-points were toxicity, response rate and survival. Treatments were prematurely stopped for other reasons than progression in 43% (Tem/Bev), 12% (S) and 23% (Bev/IFN) patients. Grade 3/4 events were observed in 36%, 14% and 27% pts in Tem/Bev, S and Bev/IFN arms respectively; two toxic deaths occurred in Tem/Bev arm. In an intent-to-treat analysis with a median follow-up of 43 weeks, NPRs-48 were 43.2% (95% CI, 32.7–54.2), 47.6% (95% CI, 32.0–63.6) and 65.9% (95% CI, 49.4–79.9) in Tem/Bev, S and Bev/IFN arms respectively. Best response rates (RECIST) were 25%, 24% and 34% respectively. The toxicity profile of the Temsirolimus/Bevacizumab combination was higher than expected, leading to a high drop-out rate. The results do not suggest any evidence of a synergistic/additive efficacy of this combination. Phase III study of combined temsirolimus and bevacizumab (INTORACT) is ongoing.
To conclude, temsirolimus combination with antiangiogenic therapies (monoclonal antibody or tyrosine kinase inhibitors) has been disappointing so far, with high toxicity, phase III studies are still ongoing and will probably help to define the best sequence in therapy.
Biomarkers
In an effort to identify potential predictors of response to temsirolimus, tumor samples from a subset of patients within a randomized phase II trial of temsirolimus in advanced renal cell carcinoma were studied.41 Paraffin- embedded tissue sections from patients who had received temsirolimus were immunostained with antibodies to phosphorylatd S6 ribosomal protein (phospho- S6), phosphorylated Akt (pAkt), carbonic anhydrase IX and PTEN. In addition, von Hippel-Lindau (VHL) mutational analysis was performed. Immunohistochemistry expression levels and mutational analysis were correlated with objective response to temsirolimus. Tissue specimens were obtained from 20 patients who were evaluable for both tumor response and staining for phospho-S6. In addition, 19 specimens were evaluable for pAkt. VHL mutational analysis was performed on 16 samples. Five patients achieved an objective response (1 partial response/4 minimal responses) to temsirolimus. There was a positive association of phospho-S6 expression (P = 0.02) and a trend toward positive expression of pAkt (P = 0.07) with response to temsirolimus. No patient without high expression of either phospho-S6 or pAkt experienced an objective tumor response. There was no correlation of carbonic anhydrase IX and PTEN expression or VHL status with response to temsirolimus. These results suggest that phospho-S6 and pAkt expression could be predictive biomarkers for response to temsirolimus. However, they were obtained on limited samples and need further evaluation in larger populations of patients.
A pharmacodynamic evaluation was performed using p70S6 kinase activity measurement in peripheral blood mononuclear cells from nine patients with renal cell cancer treated with a single dose of 25, 75, or 250 mg of temsirolimus IV (three patients each) in the phase II study by Atkins et al.42 PBMCs were collected on days 2,4, and 8 after temsirolimus treatment. Eight of the nine patients had evidence of p70S6 kinase activity inhibition after treatment that was independent of the administered dose. There was a significant linear association between time to disease progression and inhibition of p70s6 kinase activity 24 h after treatment (P = 0.004). These results indicated that the pharmacodynamic effects of temsirolimus could be determined using a p70s6 kinase assay in PBMCs. However, the limited sample size in this study does not permit to conclude for the value of this assay to predict the outcome of patients treated with the drug, validation would necessitate larger studies.
Exploratory subgroup analyses from the ARCC trial were conducted to determine if baseline levels of the tumor molecular markers PTEN and HIF-1α correlated with efficacy in patients treated with temsirolimus versus IFN.43 Of the 416 patients in the intent-to-treat population for the temsirolimus and IFN single study arms, tumor PTEN levels were available for 51% of patients and HIF-1α levels were available for 60% of patients. Of patients with PTEN data, 71% had tumors that stained positively (scoring intensity >0). Of patients with HIF-1α data, 62% had tumors that stained negatively (scoring intensity = 0) for HIF-1α. The baseline status of the molecular markers PTEN and HIF-1α did not correlate with efficacy in renal cell carcinoma patients treated with temsirolimus versus IFN. Patients demonstrated OS and progression-free survival benefit when treated with temsirolimus regardless of PTEN and HIF-1α a status. Thus, baseline PTEN and HIF-1α levels may not predict response to temsirolimus.
Safety Profile of Temsirolimus
Temsirolimus shares common specific side effects with other sirolimus derivatives such as everolimus owing to a classs effect. These specific side effects are summarized in Table 2.
Table 2.
Safety profile of temsirolimus.
| Trial | Metabolic adverse events |
Cutaneomucous |
Hematologic adverse events |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertryglyceridemia/hypercholesterolemia |
Hyperglycemia |
Rash |
Mucositis |
Anémia |
Thrombopenia |
Leucopenia |
Pneumonitis |
|||||||||
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Raymond et al25 | 42% | 34% | ND | ND | 75% | 4% | 75% | 4% | ND | ND | 29% | 8% | ND | ND | ND | ND |
| Motzer et al35 | 32% | 15% | ND | ND | 48% | 6% | ND | ND | ND | ND | 35% | 7% | 37% | 32% | ND | ND |
| Atkins et al27 | 28% | 6% | 20% | 17% | 76% | <5% | 70% | <5% | 29% | 9% | 25% | <5% | ND | ND | 5.4% | 1.8% |
| Hudes et al34 | 27% | 3% | 26% | 11% | 47% | 4% | 20% | 1% | 45% | 20% | 14% | 1% | 6% | 1% | 4% | 1% |
| Trial | Pneumonitis | |
|---|---|---|
| Any grade | Grade 3–4 | |
| Raymond et al25 | ND | ND |
| Motzer et al35 | ND | ND |
| Atkins et al27 | 5.4% | 1.8% |
| Hudes et al34 | 4% | 1% |
Abbreviation: ND, not determined.
Pharmacokinetics considerations
Overall exposure is represented as the composite AUC of temsirolimus and sirolimus moieties (AUCsum). The severity and duration of some adverse events appear to be related to pharmacokinetic exposure, particularly AUCsum.22 Assessment of safety from a phase II study in RCC indicated that AUCsum was correlated with the severity of thrombocytopenia (P = 0.007), pruritus (P = 0.011), and hyperlipidemia (P = 0.04), and with duration of thrombocytopenia (P = 0.015) and dry mouth (P = 0.036).28 The peak concentration (Cmax) of temsirolimus following single doses is also associated with toxicity, occurrence and severity of acne and mucositis. Acne frequency was 22%, and the odds increased approximately 4% with each 10 ng/mL increase in Cmax. For mucositis, the probability of occurrence was approximately 84%, and the odds increased by approximately 13% with each 10 ng/mL increase in temsirolimus Cmax.22
Metabolic side effects
In the phase I study, 21% grade 3–4 hypercholesterolemia and 13% grade 3–4 hypertriglyceridemia were reported. All grade hypertriglyceridemia ranged from 28% to 33% in phase II studies with grade 3–4 ranging from 6 to 13%. Hyperglycemia occurred in 20% of patients with 17% grade 3–4, hypophosphatemia was also reported in 17 to 28% of the patient (grade 3–4).30 These adverse events were also reported in the ARCC phase III trial with 25% hypertriglyceridemia, 21% hypercholesterolemia, 18% hyperglycemia, 6% hypophosphatemia and 11% creatinine increase (all grade)44 (Table 2). Among patients with such toxicity, preexisting laboratory abnormalities were present in many patients in this study. At baseline, 42% had grades 1–2 elevated serum glucose and one patient (0.5%) had grades 3–4. Additionally, 35% of patients had grade 1–2 high total cholesterol/lipid levels at baseline. According to temsirolimus safety analysis in the phase III study by Bellmunt et al,44 guidelines have been published regarding management of these toxicities. When considering renal adverse events, patients in the ARCC study were predisposed to nephrotoxicity because 67% had under-gone prior nephrectomy. Drug-related renal events in the temsirolimus arm (25%) were approximately two times greater than the IFN arm (12%). Drug-related creatinine increase in the temsirolimus arm (11%) was approximately three times greater than the IFN arm (4%). Mechanism of renal insufficiency following temsirolimus administration has not been precisely described. A case report of Temsirolimus-induced Glomerulopathy with proteinuria related to ischemic glomerulopathy and/or focal segmental glomerulosclerosis has been published by Izzedine et al.45
Pneumonitis
The association between sirolimus and pulmonary toxicity was first described in kidney transplant recipients.46 Since then, 41 additional cases have been reported in the literature. Lung toxicity with temsirolimus has also been described. In the phase II study by Atkins and colleagues, out of 111 patients, six patients were reported to have had possible nonspecific pneumonitis, including five at the 75-mg dose level and one at the 25-mg dose level. Of these, two were withdrawn from additional treatment and four were re-treated, with two patients experiencing recurrent pneumonitis.
In the phase III trial,44 regardless of causality, 26% of patients on temsirolimus had increased cough versus 15% on IFN (P = 0.006). Beginning on study weeks 9–41, four patients in the temsirolimus group had drug-related pneumonitis of differing severity and consequences: grade 1 (asymptomatic radiographic finding) with no dose interruption (n = 1); grade 2 with dose delay and reduction from 25 to 20 mg (n = 1); grade 2 progressing to grade 3 with discontinuation of treatment (n = 1); and grade 3 progressing to grade 4 to 5 with dose delay, then reduction from 15 to 10 mg, and finally treatment discontinuation (n = 1) (Table 2). One patient whose pneumonitis progressed was treated with antibiotics. In the temsirolimus group, cough was associated with pneumonitis grade 2 or higher; dyspnea was associated with pneumonitis that progressed in severity. One fatal pneumonitis occured in a patient with a pleural-based mass after 40 weeks on temsirolimus. Death was reported to be due to disease progression although the causality of the pneumonitis could not be excluded. Patients experiencing pneumonitis in this study were managed with antibiotics and/or steroids and/or temsirolimus dose reduction.
In a report of 10 patients who had developped pulmonary abnormalities during telmsirolimus treatment for neuroendocrine tumor or endometrial cancer (45% of all patients in the trial, n = 22), Duran and colleagues described clinical and radiological course of these temsirolmius induced pneumonitis.47 Two of the ten patients had infectious pneumonitis successfully treated with antibiotics. Eight patients were classified as experiencing possible drug-induced pneumonitis, 6 were non-smokers, 1 was an ex-smoker and 1 was an active smoker. Fifty percent of the patients were asymptomatic, the other experienced dry cough or dyspnea on exertion. Pulmonary abnormalities were categorized into two different radiological patterns: ground glass opacities with or without diffuse interstitial disease and lung parenchymal consolidation. Five patients had pulmonary function tests that demonstrated decreased diffusing lung capacity for carbon monoxide. Asymptomatic patients were maintained under temsirolimus until disease progression, dose discontinuation led to resolution of radiological abnormalities in all the patients.
To conclude, patients under temsirolimus should be cautiously monitored for pulmonary toxicity with radiographic or scannographic evaluation during treatment.
Dermatologic toxicity
In early phase I study, dermatologic side effects were reported in 71% of the 24 patients enrolled, consisting of grade 1 to 2 herpes simplex lesions (5 patients), acne-like rash (n = 9), maculopapular rash (n = 12), dry skin (n = 9), pruritus (n = 7), and nail disorders (n = 11). Maculopapular rashes, generally consisting of 5 to 10 cm reactions on face and neck, mainly occurred during the first few weeks of treatment and were spontaneously reversible. Grade 1 to 2 acne-like rash on erythematous base occurred on the face and the upper part of the trunk and was reversible with and without topical steroid cream. Stomatitis occured in 71% of patients; it consisted mainly of 1 to 3 round grade 1 to 2 aphtous lesions in the mouth and tongue.
All grade dermatologic toxicity and mucosistis induced by temsirolimus monotherapy affected 76 and 70% of patients in the phase II study respectively and 47 and 20% of patients respectively in the phase III study with grade 3–4 toxicities affecting less than 5% of the patients in both studies (Table 2).
Drug interactions through CYP3A4
Because temsirolimus and sirolimus are substrates of the cytochrome P450 3A4 (CYP3A4) coadministration of drugs that inhibit, induce, or compete for CYP3A4/5 activity may alter their disposition. In a pharmacokinetic assesment including healthy volunteers, coadministration of 5 mg temsirolimus and 400 mg oral ketoconazole, a potent cytochrome P450 3A4 blocker, increased sirolimus mean Cmax by 2.2- fold and AUC by 3.2-fold compared with temsirolimus alone.48 Therefore, if a concomitant strong CYP3A4 inhibitor is necessary, a temsirolimus dose reduction to 12.5 mg weekly should be considered. If the strong CYP3A4 inhibitor is discontinued, a washout period of approximately 1 week should be allowed before the temsirolimus dose is adjusted back to the dose used before initiation of the strong CYP3A4 inhibitor.44
Other toxicities
Nausea and vomiting occurred respectively in about 40% and 30% of the patients, diarrhea in 20% to 30% patients, grade 3–4 adverse events were inferior to 5%.
Hematologic toxicity (Table 2) occured in 41% (grade 3–4, 19%) of the patients enrolled in the phase III studies with most frequent hematologic side effect being anemia in 33% of the patient (grade 3–4, 13%). Thrombocytopenia occurred in 13% of the patients and leukopenia in 5% (all grades). As far as temsirolimus is a sirolimus derivative which is used as an immunosuppressive drug, infection-related adverse events have been reported to affect 27% of patients (all grades, 5% grade 3–4). No abnormal clinical pattern of infectious disease has been reported with this drug suggesting that with its schedule of weekly administration, temsirolimus does not display immunosuppressive property. Allergic reactions, mostly low severity, occurred in 10 (5%) patients receiving temsirolimus, despite premedication with an antihistamine.
Conclusion
Temsirolimus has been approved for first line treatment of poor-prognosis RCC after having demonstrated its efficacy on overall and progression free survival in the ARCC phase III study. Toxicity was acceptable with most frequent side effects being mucositis, rash, hyperglycemia, hyperlipemia and anemia. Although rare, drug induced pneumonitis have raised concern and need to be monitored in patients receiving mTOR inhibitors. Next step to develop this drug is to determine its position in the sequential therapy of RCC and to investigate the potential impact of combination therapies. Predictive factors for efficacy ant toxicity are obviously needed for any further development and biological studies should be included in future trials.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Aass N, De Mulder PH, Mickisch GH, et al. Randomized phase II/III trial of interferon Alfa-2a with and without 13-cis-retinoic acid in patients with progressive metastatic renal cell Carcinoma: the European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group (EORTC 30951) J Clin Oncol. 2005;20;23(18):4172–8. doi: 10.1200/JCO.2005.07.114. [DOI] [PubMed] [Google Scholar]
- 3.Fossa SD, Martinelli G, Otto U, et al. Recombinant interferon alfa-2a with or without vinblastine in metastatic renal cell carcinoma: results of a European multi-center phase III study. Ann Oncol. 1992;3(4):301–5. doi: 10.1093/oxfordjournals.annonc.a058185. [DOI] [PubMed] [Google Scholar]
- 4.Minasian LM, Motzer RJ, Gluck L, Mazumdar M, Vlamis V, Krown SE. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol. 1993;11(7):1368–75. doi: 10.1200/JCO.1993.11.7.1368. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Costanzi JJ, Leavitt R, et al. Recombinant alfa interferon in renal cell carcinoma: a randomized trial of two routes of administration. J Clin Oncol. 1987;5(2):286–91. doi: 10.1200/JCO.1987.5.2.286. [DOI] [PubMed] [Google Scholar]
- 6.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med. 1998;338(18):1272–8. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 7.Otto U, Schneider AW, Conrad S, Klosterhalfen H. Recombinant alpha-2 or gamma interferon in the treatment of metastatic renal cell carcinoma: results of two phase II/III trials. Prog Clin Biol Res. 1990;350:275–82. [PubMed] [Google Scholar]
- 8.Atkins MB, Sparano J, Fisher RI, et al. Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. J Clin Oncol. 1993;11(4):661–70. doi: 10.1200/JCO.1993.11.4.661. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–41. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;20;26(33):5422–8. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–8. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–43. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8(8):647–65. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 19.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16(4):525–37. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 20.Hudes GR, Berkenblit A, Feingold J, Atkins MB, Rini BI, Dutcher J. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol. 2009;36 (Suppl 3):S26–36. doi: 10.1053/j.seminoncol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5(8):671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 22.Boni JP, Hug B, Leister C, Sonnichsen D. Intravenous temsirolimus in cancer patients: clinical pharmacology and dosing considerations. Semin Oncol. 2009;36 (Suppl 3):S18–25. doi: 10.1053/j.seminoncol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Le TC, Faivre S, Serova M, Raymond E. mTORC1 inhibitors: is temsirolimus in renal cancer telling us how they really work? Br J Cancer. 2008;99(8):1197–203. doi: 10.1038/sj.bjc.6604636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park IH, Bachmann R, Shirazi H, Chen J. Regulation of ribosomal S6 kinase 2 by mammalian target of rapamycin. J Biol Chem. 2002;277(35):31423–9. doi: 10.1074/jbc.M204080200. [DOI] [PubMed] [Google Scholar]
- 25.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12(1):122–7. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 27.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005;77(1):76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Chang SM, Kuhn J, Wen P, et al. Phase I/pharmacokinetic study of CCI- 779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs. 2004;22(4):427–35. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 29.Punt CJ, Boni J, Bruntsch U, Peters M, Thielert C. Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol. 2003;14(6):931–7. doi: 10.1093/annonc/mdg248. [DOI] [PubMed] [Google Scholar]
- 30.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22(12):2336–47. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 31.Lunardi G, Armirotti A, Nicodemo M, et al. Comparison of temsirolimus pharmacokinetics in patients with renal cell carcinoma not receiving dialysis and those receiving hemodialysis: a case series. Clin Ther. 2009;31(8):1812–9. doi: 10.1016/j.clinthera.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22(5):909–18. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12(19):5755–63. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 34.Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: memorial sloan-kettering cancer center experience. Clin Cancer Res. 2004;10(18 Pt 2):6302S–3. doi: 10.1158/1078-0432.CCR-040031. [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–96. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 36.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, Hudes GR, Curti BD, et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007;25(25):3958–64. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 38.Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7(1):24–7. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patnaik A, Ricart A, Cooper J, et al. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies. J Clin Oncol (Meeting Abstracts) 2007;25(18_Suppl):3512. [Google Scholar]
- 40.Escudier BJ, Negrier S, Gravis G, et al. Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma (mRCC)? Results of the randomized TORAVA phase II trial. J Clin Oncol (Meeting Abstracts) 2010;28(15_Suppl):4516. [Google Scholar]
- 41.Cho D, Signoretti S, Dabora S, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2007;5(6):379–85. doi: 10.3816/CGC.2007.n.020. [DOI] [PubMed] [Google Scholar]
- 42.Peralba JM, DeGraffenried L, Friedrichs W, et al. Pharmacodynamic Evaluation of CCI-779, an Inhibitor of mTOR, in Cancer Patients. Clin Cancer Res. 2003;9(8):2887–92. [PubMed] [Google Scholar]
- 43.Figlin RA, de SP, McDermott D, et al. Analysis of PTEN and HIF-1alpha and correlation with efficacy in patients with advanced renal cell carcinoma treated with temsirolimus versus interferon-alpha. Cancer. 2009;115(16):3651–60. doi: 10.1002/cncr.24438. [DOI] [PubMed] [Google Scholar]
- 44.Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann Oncol. 2008;19(8):1387–92. doi: 10.1093/annonc/mdn066. [DOI] [PubMed] [Google Scholar]
- 45.Izzedine H, Boostandoot E, Spano JP, Bardier A, Khayat D. Temsirolimus-induced glomerulopathy. Oncology. 2009;76(3):170–2. doi: 10.1159/000201930. [DOI] [PubMed] [Google Scholar]
- 46.Morelon E, Stern M, Israel-Biet D, et al. Characteristics of sirolimus- associated interstitial pneumonitis in renal transplant patients. Transplantation. 2001;72(5):787–90. doi: 10.1097/00007890-200109150-00008. [DOI] [PubMed] [Google Scholar]
- 47.Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer. 2006;42(12):1875–80. doi: 10.1016/j.ejca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Boni JP, Leister C, Burns J, Hug B. Differential effects of ketoconazole on exposure to temsirolimus following intravenous infusion of temsirolimus. Br J Cancer. 2008;98(11):1797–802. doi: 10.1038/sj.bjc.6604376. [DOI] [PMC free article] [PubMed] [Google Scholar]