Abstract
Mesenchymal stem cells (MSCs) have an extensive migratory capacity for gliomas, which is comparable to that of neural stem cells. Among the various types of MSCs, human adipose tissue-derived MSCs (hAT-MSC) emerge as one of the most attractive vehicles for gene therapy because of their high throughput, lack of ethical concerns, and availability and ease of isolation. We evaluated the therapeutic potential and safety of genetically engineered hAT-MSCs encoding the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) against brainstem gliomas. Human AT-MSCs were isolated from human fat tissue, characterized, and transfected with TRAIL using nucleofector. The therapeutic potential of TRAIL-producing hAT-MSCs (hAT-MSC.TRAIL) was confirmed using in vitro and in vivo studies. The final fate of injected hAT-MSCs was traced in long-survival animals. The characterization of hAT-MSCs revealed the expression of MSC-specific cell-type markers and their differentiation potential into mesenchymal lineage. Short-term outcomes included a 56.3% reduction of tumor volume (P < .001) with increased apoptosis (3.03-fold, P < .05) in animals treated with hAT-MSC.TRAIL compared with the control groups. Long-term outcomes included a significant survival benefit in the hAT-MSC.TRAIL-treated group (26 days of median survival in the control group vs 84 days in the hAT-MSC.TRAIL-treated group, P < .0001), without any evidence of mesenchymal differentiation in vivo. Our study demonstrated the therapeutic efficacy and safety of nonvirally engineered hAT-MSCs against brainstem gliomas and showed the possibility of stem-cell–based targeted gene therapy for clinical application.
Keywords: adipose tissue-derived mesenchymal stem cells, antitumor effect, brainstem glioma, tumor necrosis factor-related apoptosis-inducing ligand
Diffuse intrinsic pontine gliomas, which are the most common form of brainstem gliomas, cannot be removed by surgery and remain virtually inevitably lethal, with a mean survival period of less than a year, despite some progress in adjuvant radio-and chemotherapy. Currently, there is no effective treatment modality for brainstem gliomas.1,2 These disappointing results in the treatment of brainstem gliomas have encouraged numerous experimental trials in the search for a novel treatment, such as stem-cell–based gene therapy.
Recently, gene therapy using neural stem cells (NSCs) as the vehicle for therapeutic agents has emerged as a promising treatment modality for malignant brain tumors.3–8 Furthermore, studies have confirmed that NSCs and various mesenchymal stem cells (MSCs), such as bone marrow-derived MSCs (BM-MSCs), adipose tissue-derived MSCs (AT-MSCs), and umbilical cord blood-derived MSCs, can target brain tumors.9–18 Among the MSCs from various sources, AT-MSCs exhibit clear advantages, which include the easy and repeatable access to subcutaneous adipose tissue, the simple isolation procedure, and the higher yield of MSCs from adipose tissue compared with BM (approximately 500-fold).19 Our previous study confirmed that human AT-MSCs (hAT-MSCs) have an extensive migratory capacity for experimental brainstem gliomas, which is comparable to that of NSCs.20
To investigate the therapeutic potential of genetically modified hAT-MSCs for brainstem gliomas, we used the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. TRAIL, which is a member of the tumor necrosis factor protein superfamily, is an attractive genetic tool because it induces apoptosis selectively in a variety of neoplastic cells without causing toxicity to normal cells. TRAIL activates the p53-independent extrinsic pathway by binding to the death receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2/KILLER) at the cell surface.21–24
In the present study, we demonstrate, for the first time, that TRAIL-producing hAT-MSCs can induce potent apoptotic activity in vitro and in vivo, resulting in short- and long-term therapeutic efficacy. For safety reasons, we conducted gene transduction using a nonviral vector and monitored the long-term fate of injected hAT-MSCs. The purpose of this study is to provide the rationale for clinical application of the hAT-MSC–based gene therapy against human brainstem gliomas.
Materials and Methods
Isolation and Expansion of hAT-MSCs and Cell Culture
Human AT-MSCs were isolated from human adipose tissue. Adipose tissue samples were obtained from the abdominal fat prepared for sellar floor reconstruction in patients who had undergone transsphenoidal surgery at the Seoul National University Children's Hospital. All eligible patients or their parents provided written informed consent. Permission to isolate MSCs from the fat tissues was given by the institutional review board of the Seoul National University Hospital. Human AT-MSCs were suspended in MSC expansion medium (Chemicon) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic solution (Invitrogen). F98 cells that were originally obtained from American Type Culture Collection (ATCC) were cultured in Dulbecco's modified Eagle's medium (WelGene Biopharmaceuticals) supplemented with 10% FBS and 1% antibiotic–antimycotic solution. All cells were incubated at 37°C in an incubator in a 5% CO2/95% air atmosphere.
Fluorescence-activated Cell Sorting Analysis
Fluorescence-activated cell sorting (FACS) analyses were performed on hAT-MSCs. Human AT-MSCs were cultured in a control medium for 48 hours before analysis. Cells were harvested in 0.25% trypsin/ EDTA and fixed for 30 minutes in ice-cold 2% formaldehyde. The fixed cells were washed in flow cytometry buffer (FCB; 2% FBS, 0.2% Tween-20 in phosphate-buffered saline [PBS]) and incubated for 30 minutes in FCB containing fluorescein isothiocyanate-conjugated monoclonal antibodies directed against cluster of differentiation (CD) antigens (CDs 14, 34, and 90, BD Biosciences Pharmingen; CD 105, Chemicon) or phycoerythrin-conjugated monoclonal antibodies directed against CD antigens (CD 73, BD Biosciences Pharmingen; CD 45, Chemicon). Flow cytometry was performed using a FACscan argon laser cytometer (Becton Dickinson).
In vitro Adipogenic, Osteogenic, and Chondrogenic Differentiation of hAT-MSCs
The potential of hAT-MSCs to differentiate into adipogenic, osteogenic, and chondrogenic lineages was assayed. Cells were seeded in culture plates at 2.5 × 104 cells/cm2 in hMSC culture media until confluence. Cells were then stimulated under appropriate inducible conditions. Unstimulated cells and cells treated with identical amounts of diluents were used as controls.
For adipogenic differentiation, cells were induced with adipocyte induction media (Invitrogen) for 2 weeks with a medium change three times a week. Cells were then rinsed twice with PBS, fixed with 10% formalin for 10 minutes, washed with distilled water, rinsed in 60% isopropanol, and covered with a 0.3% oil red O solution (Sigma) for 10 minutes. Stained cells were briefly rinsed in 60% isopropanol and thoroughly washed in distilled water and left to dry at room temperature (RT).
For osteogenic differentiation, cells were stimulated every 2 days in osteogenic induction media (Invitrogen). After 3 weeks, cells were rinsed twice with PBS, fixed with formalin for 10 minutes, and washed with distilled water. To stain calcium deposits, cells were covered with a 2% aqueous solution of alizarin red S (Sigma), pH 4.2, for 3 minutes. Cultures were then washed thoroughly with distilled water and left to dry at RT.
For chondrogenic differentiation, cells were stimulated in chondrogenesis induction media (Invitrogen) for 2 weeks. Cells were then rinsed twice with PBS and fixed in 10% formalin for 10 minutes at RT. Alcian Blue (Sigma) was used to stain chondrogenic pellets. Excess dye was removed, and cells were visualized by light microscopy.
Engineering of TRAIL-producing hAT-MSCs
The pIRES2.GFP (5.3 kb) vector was purchased from BD Biosciences Clontech and the TRAIL cDNA was courtesy of Dr. Myong Hun Seong (Seoul National University Hospital). TRAIL (850 bp) was inserted into the XhoI/EcoRI (Invitrogen) cloning site of pIRES2.GFP. Nucleofection was performed using the Nucleofector machine (Amaxa Biosystems) according to the manufacturer's instructions with some modifications.25 For each nucleofection assay, 5 × 105 cells were resuspended in 100 μL of Nucleofector buffer (Amaxa Biosystems) and nucleofected with 3 μg of plasmid DNA. The U-23 protocol was tested on the Nucleofector II device. Immediately after nucleofection completion, cells were plated onto 6-well plates. Culture medium was changed 5 hours after nucleofection to remove dead cells. Transfection efficiency in all samples was verified using a fluorescent microscope and FACS analysis.
Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription (RT)–PCR analysis was conducted using the PrimeScript RT–PCR kit (Takara). Thirty PCR cycles were performed for all transcripts using the following primers: TRAIL, forward: 5′-CGGCTGAGATGGCTATGATGGAGGTCC-3′, reverse: 5′-GCCGAATTCTTAGCCAACTAAAAAGGC-3′ (850 bp amplicon) and GAPDH, forward: 5′-CGTGGAAGGACTCATGAC-3′, reverse: 5′-CAATTCGTTGTCATACCAG-3′ (513 bp amplicon). The PCR products were resolved on a 1% agarose gel stained with ethidium bromide and were visualized using a UV transilluminator.
In vitro Therapeutic Efficacy of hAT-MSC.TRAIL
To quantify TRAIL expression, hAT-MSC and hAT-MSC.TRAIL cells (1 × 105/250 μL/well) were seeded in 24-well plates and cultured for 24 hours. Culture supernatants were harvested, and secreted TRAIL was measured using the TRAIL Immunoassay Kit (Invitrogen) according to the manufacturer's protocol. Plates were read at 450 ηm using an absorbance plate reader (Molecular Devices), and data were analyzed.
The therapeutic efficacy of hAT-MSC.TRAIL cells was analyzed by coculture experiments using a cell viability assay. F98 cells (4 × 103) were grown in 96-well plates. Human AT-MSC or hAT-MSC.TRAIL cells (1.2 × 104) were added to the tissue culture transwell plate (0.4 μm pore size, Nunc International) on Day 1. Cells were incubated for 3 days and F98-cell viability was measured using a colorimetric assay (Cell Counting Kit-8, Dojindo Molecular Technologies). Experimental values were expressed as the mean percentage of control viability ± SEM. All experiments were conducted in triplicate.
In vivo Short-term Therapeutic Efficacy of hAT-MSC.TRAIL
All animal studies were carried out at the animal facility of the Seoul National University Hospital in accordance with national and institutional guidelines. Female Fischer 344 rats (Central Lab Animal) weighing 150–200 g were used in this experiment. Rats (n = 21) were anesthetized with an intramuscular injection of a solution of 20 mg/kg Zoletil (Virbac) and 10 mg/kg xylazine (Bayer Korea). The posterior cranial region was shaved and prepared in a sterile fashion. A midline scalp incision of approximately 2 cm was made, and a small burr hole was created using a 22-gauge needle. To establish brainstem gliomas, F98 tumor cells were stereotactically implanted into the right brainstem, as described previously.20,26 The stereotactic coordinates were 1.4 mm to the right of the sagittal suture and 1 mm anterior to the lambdoid suture. A 26-gauge Hamilton needle was inserted at an angle of 5° anteflexed from vertical, to a depth of 7 mm from the dura mater. F98 tumor cells (40 000 cells in 3 μL; n = 21) were then implanted via the needle at an injection rate of 1 μL/min. Three days after tumor cell implantation, the animals were randomized into 3 groups and treated with intratumoral inoculations of PBS (12 μL; n = 7), hAT-MSC cells (160 000 cells; n = 7), or hAT-MSC.TRAIL cells (160 000 cells; n = 7) in 12 μL of PBS at the established tumor site, using the same burr hole and stereotactic coordinates. Eighteen days after tumor implantation, the animals were perfused with 4% paraformaldehyde under deep anesthesia and sacrificed. After fixation, the entire brain was sequentially dehydrated in 10%, 20%, and 30% sucrose solution, embedded in an optimum cutting temperature compound (Tissue-Tek), and stored at −80°C. The brains were then sectioned using a cryotome and stained with hematoxylin and eosin (H&E).
The size of the tumor and the effect of therapeutic cells in brainstem structures were assessed. Tumor volumes were estimated using the formula for ellipsoid and expressed as the mean ± SEM, as described previously.20 The primary antibodies used for immunofluorescence staining included anti-Ki67 nuclear antigen (1:100; DAKO) for the detection of proliferating cells and anticleaved caspase-3 (1:100; Cell Signaling Technology) for the detection of apoptosis. The secondary antibody used was the Alexa Fluor 633-conjugated rabbit anti-mouse immunoglobulin-G (IgG) (1:200; Invitrogen). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and negative control slides were established by omitting the primary antibody. The apoptotic and proliferating indices were defined as the percentage of cells stained positively per 100 nuclei from 5 randomly selected high-power fields. The sections were observed using a confocal microscope (Zeiss).
In vivo Long-term Therapeutic Efficacy of hAT-MSC.TRAIL
F98 tumor cells were stereotactically implanted into the right brainstem (n = 27), as described previously. After 3 days, rats were randomized into 3 groups (n = 9 per group) and treated with PBS, hAT-MSC, or hAT-MSC.TRAIL. Discomfort or distress was assessed by animal care personnel with no knowledge of the protocol design. Survival was followed until the rats were dead or for a maximum of 100 days, at which time animals were sacrificed. All euthanized rats were verified as bearing tumors by necropsy.
Two rats that survived for 100 days after tumor injection were sacrificed, and their brains were collected for confirmation of differentiation of injected hAT-MSC.TRAIL. Neural and mesenchymal differentiation were investigated and evaluated using primary antibodies directed against Tuj1 (1:500; Chemicon) as a neuronal marker, GFAP (1:500; Covance) as an astrocyte marker, CNPase (1:200; Chemicon) as an oligodendrocyte marker, adiponectin (1:200; ProSci) as an adipose-tissue marker, bone sialoprotein (BSP, 1:500; Chemicon) as a bone marker, and aggrecan (1:100; Abcam) as a cartilage marker. The secondary antibody used was the Alexa Fluor 594-conjugated goat anti-mouse IgG (1:200; Invitrogen). Sections were mounted with antifading solution containing DAPI. Sections were observed using a confocal microscope.
Statistical Analysis
All values were calculated as means ± SEM or were expressed as a percentage of controls ± SEM. The GraphPad Prism software (GraphPad Software) was used for all the analyses. Significant differences in the assessment of cell viability, tumor volume, proliferation, and apoptosis index were determined using the Kruskal–Wallis test with post hoc analysis. The survival data are presented as Kaplan–Meier plots and were analyzed using a log-rank test. Significance was set at P < .05.
Results
hAT-MSCs Could Be Isolated and Expanded from Adipose Tissue
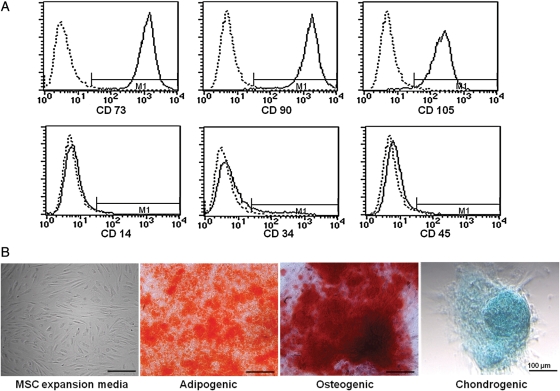
To characterize the hAT-MSC population, the isolated cells were analyzed using flow cytometry analysis. Cells were analyzed for the expression of cell membrane protein markers: positive for CD73, CD90, and CD105, which are generally considered as markers of MSCs, but negative for the expression of hematopoietic markers such as CD14, CD34, and CD45 (Fig. 1A).
Fig. 1.
Characterization and differentiation of hAT-MSCs analyzed by flow cytometry and microscopy. The MSC-specific markers CD73, CD90, and CD105 were expressed in hAT-MSCs, whereas the hematopoietic stem-cell markers CD14, CD34, and CD45 were not (A). hAT-MSCs showed a fibroblast-like morphology and had the ability for adipogenic, osteogenic, and chondrogenic differentiation in differentiation induction medium (B). Differentiated cells were stained with oil red O, alizarin red S, and alcian blue.
Human AT-MSCs showed a fibroblast-like morphology and were plastic adherent under standard culture conditions (Fig. 1B).
The differentiation potential for mesenchymal lineage was assessed by oil red O, alizarin red S, and alcian blue staining. Human AT-MSCs exhibited a characteristic of differentiation morphology in induction medium (+) but not control medium (−) (Fig. 1B). Two weeks after adipogenic induction, morphology of lipid droplets was observed and stained by oil red O. Under osteogenic culture conditions after 3 weeks, calcium deposits were detected by alizarin red S staining. Chondrogenic differentiation was revealed by alcian blue staining after 2 weeks of chondrocyte induction.
hAT-MSCs Were Engineered to Produce TRAIL Using Nucleofector
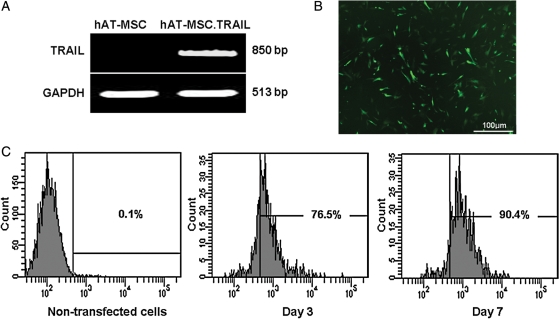
After transfection of hAT-MSCs with the TRAIL construct (or vector alone as a control) and subsequent antibiotic selection, G418-resistant cells were expanded in T75 flasks. RT–PCR produced the expected 850 bp band of the inserted TRAIL cDNA in hAT-MSC.TRAIL cells, but not in hAT-MSC cells (Fig. 2A). These cells were positive for green fluorescent protein (GFP) expression, as assessed by fluorescence microscopy (Fig. 2B). Of the hAT-MSC-TRAIL cells 62.6% showed GFP positivity on FACS analysis 3 and 7 days after transfection (Fig. 2C).
Fig. 2.
Confirmation of TRAIL expression in hAT-MSC after nucleofection. Expression of the TRAIL transcript was confirmed by RT–PCR. GAPDH controls confirmed equal protein loading. The TRAIL transcript was expressed in hAT-MSC.TRAIL cells but not in hAT-MSC cells (A). Expression of TRAIL protein (green) was detected 24 hours after nucleofection using fluorescence microscopy (B). FACS analysis of nucleofected hMSCs revealed that 76.5% of cells expressed GFP Day 3 and 90.4% Day 7 (C).
hAT-MSC.TRAIL Retained Therapeutic Efficacy In vitro
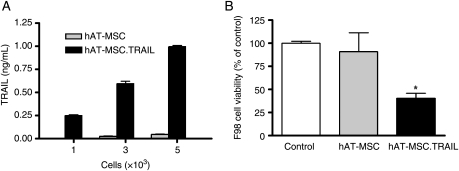
An enzyme-linked immunosorbent assay (ELISA) was performed to quantify the secreted TRAIL protein in hAT-MSC.TRAIL culture media. Human AT-MSC.TRAIL supernatant contained increased levels of TRAIL, in a cell number–dependent fashion. Approximately 5 × 103 hAT-MSC.TRAIL cells secreted 1 ηg/mL of TRAIL protein in 24 hours (Fig. 3A).
Fig. 3.
In vitro quantitative analysis of TRAIL expression in hAT-MSC.TRAIL cells and their therapeutic efficacy on F98 cells. TRAIL protein secreted into hAT-MSC.TRAIL medium was quantified using ELISA. hAT-MSC.TRAIL supernatant contained increased levels of TRAIL, in a cell number-dependent fashion. Approximately 5 × 103 hAT-MSC.TRAIL cells secreted 1 ηg/mL of TRAIL protein (A). The therapeutic efficacy of hAT-MSC-TRAIL cells was analyzed in coculture experiments. F98 cells cultured in the presence of hAT-MSC-TRAIL cells exhibited significant growth inhibition; however, there was no significant growth inhibition in coculture with hAT-MSC cells (B). Columns represent cell viability as a percentage of the control viability; bars, SE (P < .05, Kruskal–Wallis test with post hoc analysis).
To confirm the cytotoxic effect of hAT-MSC.TRAIL, cell viability was observed in a coculture system. The number of F98 cells cultured with hAT-MSC.TRAIL cells decreased by 59.5% compared with control F98 cells alone (P < .05; Fig. 3B).
hAT-MSC.TRAIL Led to Tumor Volume Reduction and Induced Apoptosis
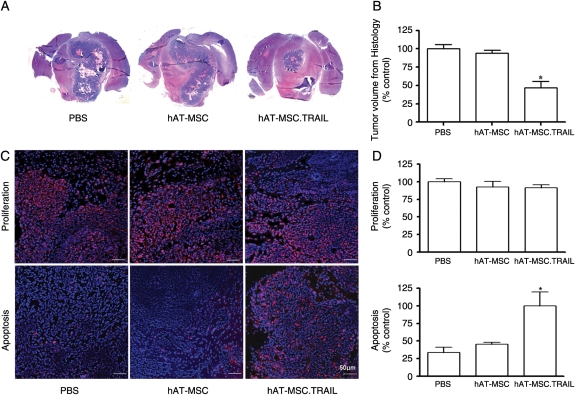
In vivo studies showed that the majority of intracranially injected hAT-MSC.TRAIL cells localized to the tumor bed and to the tumor–normal parenchyma interface. Histological analysis showed a 56.3% reduction of tumor volume in the hAT-MSC-TRAIL-treated rats compared with PBS-treated control animals and hAT-MSC-treated rats (PBS vs hAT-MSC vs hAT-MCS.TRAIL, 117.1 ± 21.9vs 105.9 ± 35.0 vs 51.1 ± 29.7 mm3; P < .001; Fig. 4A and B). We did not detect any abnormalities in the parenchyma surrounding the tumor in treated rats. We assessed the biological action of hAT-MSC.TRAIL on the brainstem gliomas. Immunofluorescence analysis revealed a significant increase (3.01-fold) in the number of apoptotic cells in animals treated with hAT-MSC.TRAIL compared with control groups (P < .05; Fig. 4C and D). In contrast, the proliferative indices revealed no significant differences among the groups (Fig. 4C and D).
Fig. 4.
Short-term therapeutic efficacy of hAT-MSC.TRAIL in vivo. (A) Representative histological images (H&E staining; magnification, ×1) (A). A 56.3% decrease in tumor volume was observed in hAT-MSC.TRAIL–treated animals compared with PBS-treated animals (P < .001; Kruskal–Wallis test) (B). Representative immunofluorescence images. Primary antibodies included anti-Ki67 nuclear antigen (for the detection of proliferating cells; red color) and anticleaved caspase-3 (for the detection of apoptosis; red color) (C). Sections were counterstained with DAPI (blue). Bars indicate 50 μm. Immunofluorescence analysis revealed that the proliferative indices were not different among the groups. In contrast, the number of apoptotic cells was increased 3.01-fold in the hAT-MSC.TRAIL-treated group compared with the control groups (P < .05; Kruskal–Wallis test) (D).
hAT-MSC.TRAIL Conferred a Survival Gain Without Mesenchymal Differentiation
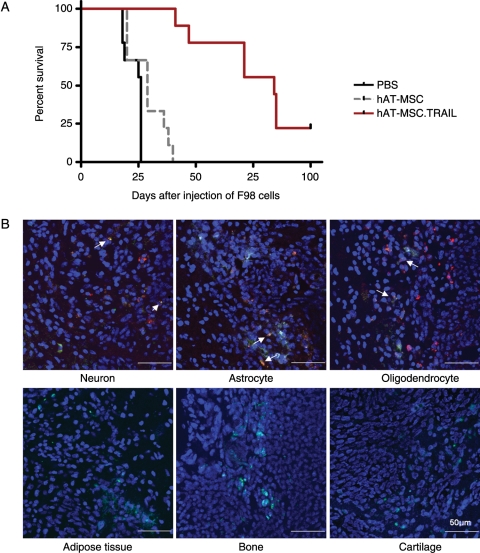
Our data showed that hAT-MSC.TRAIL–treated rats survived significantly longer (median survival, 84 days) compared with control animals treated with PBS (median survival, 26 days) or hAT-MSC (median survival, 29 days; P < .0001; Fig. 5A). Tissues from TRAIL-treated long-survival rats exhibited expression of the neural differentiation markers Tuj1, GFAP, and CNPase but did not express the mesenchymal differentiation markers adiponectin, BSP, and aggrecan (Fig. 5B).
Fig. 5.
Long-term therapeutic efficacy and safety of hAT-MSC.TRAIL in vivo. Kaplan–Meier plots revealed a significant survival gain in hAT-MSC.TRAIL-treated animals (median survival, 84 days) compared with animals treated with PBS (median survival, 26 days) or hAT-MSC (median survival, 29 days; P < .0001), within 100 days of survival endpoint (A). Two rats treated with hAT-MSC.TRAIL survived for 100 days after tumor cell injection. hAT-MSC.TRAIL cells (green color) injected into these long-term survival rats exhibited neuronal differentiation (red color, white arrow) but lacked mesenchymal differentiation (red color). Sections were counterstained with DAPI (blue) (B).
Discussion
Several essential premises should be satisfied for the clinical application of stem-cell–based gene therapy to brain malignancy. First, the supply of stem cells should be secure and stable. Second, the therapeutic gene transfection method should be safe and effective. Third, stem-cell-related complications should be absent or minimal.
The prototype cell used in stem-cell–based gene therapy for brain tumors is the NSC.3–8 However, the practical application of NSCs is limited by ethical and logistic problems related to their isolation and to their potential immunogenicity because of a requirement for allogenic transplantation.9,10,12,15 Increasing recognition is being given to the plasticity of stromal cells within BM (termed MSCs) as an alternative method. These cells can differentiate into multiple mesenchymal lineages such as osteocyte, adipocyte, and chondrocyte.18,27 Previous studies have confirmed that BM-MSCs have all the properties of NSCs, such as an extensive migratory capacity and tropism for gliomas.20 Therefore, they have been investigated as vehicles for gene therapy targeting malignant glioma.14,15 However, invasive isolation procedures, low yield, and potential malignant transformation for BM-MSCs represent an obstacle to their use in cellular therapy applications.28 Recently, adult adipose tissue has emerged an alternative method for autologous adult NSC therapy.29 Adipose tissue contains a specialized class (the stromal vascular fraction), which is thought to harbor stem cells that display an extensive proliferative capacity and multilineage potential, provide a rich source of pluripotent stromal stem cells, and have the capacity for autologous transplantation. Compared with BM-derived stem cells, adipose tissue-derived stem cells have an equal potential regarding morphology, immune phenotype, success rate in isolating MSCs, colony frequency, and differentiation into cells and tissues of mesodermal origin. The easy and repeatable access to subcutaneous adipose tissue and simple, uncomplicated enzyme-based isolation procedure represent a clear advantage.18,19,29,30 Therefore, we selected the adipose tissue as a cellular source of MSCs among the various possible sources. We successfully isolated and maintained hAT-MSCs from human adipose tissue. These hAT-MSCs showed a fibroblast-like morphology, expressed typical surface markers, and had differentiation potential to mesenchymal lineage.31–33 As the case of the adipose tissue-derived stem-cell therapy for cancer, this study is the first trial for brain malignancy after colon cancer.29
Viral-based techniques are the most efficient therapeutic gene transfection systems used to deliver DNA into stem cells, as they yield high gene transduction and transgenic expression in many cellular models. However, viral methods are practically complex, labor intensive, and involve safety risks complicated by immune response, intracellular trafficking, potential mutations, and genetic alterations caused by integration. Therefore, we chose a nonviral transfection method– the nucleofection technique. Nucleofection is a recent electroporation-based technique that combines electrical parameters and a cell-type solution to drive plasmid DNA, oligonucleotides, or siRNA directly into the cell nucleus. This technology has been successfully used to transfect several primary cell types, including mouse T cells, neurons, and keratinocytes, as well as human and mouse stem cells of diverse origins.34 This method does not alter the differentiation ability of these clones, as they underwent differentiation without altering the differentiation potential and loss of transgene expression.35,36 We successfully transfected the recombinant TRAIL gene using the nucleofection technique, with a transfection efficiency of 62.6% in living cells. Our data clearly demonstrated that nucleofection can be used to generate efficient and stable transgene expression in hAT-MSCs, without altering stem cell features and functions.
In this study, the in vivo hAT-MSC.TRAIL experimental design led to a significant short-term (56.3% reduction of tumor volume; P < .001) and long-term (>3-fold survival gain; P < .0001) therapeutic efficacy in the hAT-MSC.TRAIL–treated group. Although the therapeutic efficiency of stem cell-based gene therapy has been published, its safety has been addressed poorly.37,38 In the present study, the histological analysis of the short-term therapeutic efficacy of hAT-MSC.TRAIL showed no normal parenchymal injury. Furthermore, histological analysis of long-term survival animals showed no mesenchymal differentiation. These results provide evidence of the safety of this approach in clinical applications.
In spite of the short and long-term therapeutic efficacy observed, complete remission was not accomplished. Repeated injection of therapeutic MSCs or using the secretable TRAIL may improve the therapeutic efficiency. However, an omnipotent therapeutic gene is still lacking. Although such a gene remains unidentified, a combination therapy using traditional standard treatments remains valid.
In conclusion, we successfully isolated hAT-MSCs from human adipose tissue and modified cells with an effective recombinant gene using a nonviral method. Genetically modified hAT-MSCs exhibited short- and long-term therapeutic effects without any complications related to stem cell therapy. Our results bring stem-cell–based gene therapy for brain malignancy one step closer to clinical application.
Conflict of interest statement. None declared.
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0820310), and by grant from the Seoul National University Hospital Research Fund (03-2008-009).
References
- 1.Dunn IF, Black PM. The neurosurgeon as local oncologist: cellular and molecular neurosurgery in malignant glioma therapy. Neurosurgery. 2003;52:1411–1422. doi: 10.1227/01.neu.0000064808.27512.cf. doi:10.1227/01.NEU.0000064808.27512.CF. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms–an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. doi:10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 3.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. doi:10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. doi:10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 5.Kim SK, Cargioli TG, Machluf M, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. doi:10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 6.Kim SK, Kim SU, Park IH, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. doi:10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 7.Staflin K, Honeth G, Kalliomäki S, Kjellman C, Edvardsen K, Lindvall M. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res. 2004;64:5347–5354. doi: 10.1158/0008-5472.CAN-03-1246. doi:10.1158/0008-5472.CAN-03-1246. [DOI] [PubMed] [Google Scholar]
- 8.Yip S, Aboody KS, Burns M, et al. Neural stem cell biology may be well suited for improving brain tumor therapies. Cancer J. 2003;9:189–204. doi: 10.1097/00130404-200305000-00007. doi:10.1097/00130404-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol. 2002;178:288–293. doi: 10.1006/exnr.2002.8039. doi:10.1006/exnr.2002.8039. [DOI] [PubMed] [Google Scholar]
- 10.Kan I, Melamed E, Offen D. Integral therapeutic potential of bone marrow mesenchymal stem cells. Curr Drug Targets. 2005;6:31–41. doi: 10.2174/1389450053344902. doi:10.2174/1389450053344902. [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. doi:10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Elkahloun AG, Messina SA, et al. Cellular and genetic characterization of human adult bone marrow-derived neural stem-like cells: a potential antiglioma cellular vector. Cancer Res. 2003;63:8877–8889. [PubMed] [Google Scholar]
- 13.Menon LG, Kelly K, Yang HW, Kim SK, Black PM, Carroll RS. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27:2320–2330. doi: 10.1002/stem.136. doi:10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 14.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. doi:10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 16.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 17.Yuan X, Hu J, Belladonna ML, Black KL, Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. doi:10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 18.Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. doi:10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 19.Black LL, Gaynor J, Gahring D, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272–284. [PubMed] [Google Scholar]
- 20.Lee DH, Ahn Y, Kim SU, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. doi:10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 21.Hao C, Beguinot F, Condorelli G, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res. 2001;61:1162–1170. [PubMed] [Google Scholar]
- 22.Kim CY, Jeong M, Mushiake H, et al. Cancer gene therapy using a novel secretable trimeric TRAIL. Gene Ther. 2006;13:330–338. doi: 10.1038/sj.gt.3302658. doi:10.1038/sj.gt.3302658. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Hampl M, Albert P, Fine HA. Antitumor activity and prolonged expression from a TRAIL-expressing adenoviral vector. Neoplasia. 2002;4:312–323. doi: 10.1038/sj.neo.7900245. doi:10.1038/sj.neo.7900245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. doi:10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 25.Zaragosi LE, Billon N, Ailhaud G, Dani C. Nucleofection is a valuable transfection method for transient and stable transgene expression in adipose tissue-derived stem cells. Stem Cells. 2007;25:790–797. doi: 10.1634/stemcells.2006-0235. doi:10.1634/stemcells.2006-0235. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Jallo GI, Guarnieri M, Carson BS, Sr., Penno MB. A novel brainstem tumor model: guide screw technology with functional, radiological, and histopathological characterization. Neurosurg Focus. 2005;18:E11. [PubMed] [Google Scholar]
- 27.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. doi:10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 28.Rosland GV, Svendsen A, Torsvik A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. doi:10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 29.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. doi:10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 30.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. doi:10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 31.Kassis I, Zangi L, Rivkin R, et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–976. doi: 10.1038/sj.bmt.1705358. doi:10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 32.Funes JM, Quintero M, Henderson S, et al. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc Natl Acad Sci USA. 2007;104:6223–6228. doi: 10.1073/pnas.0700690104. doi:10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai A, Kim WK, Lee HJ, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. doi: 10.1371/journal.pone.0001272. doi:10.1371/journal.pone.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Ex vivo expansion of adipose tissue-derived stem cells in spinner flasks. Biotechnol J. 2009;4:1198–1209. doi: 10.1002/biot.200800130. doi:10.1002/biot.200800130. [DOI] [PubMed] [Google Scholar]
- 35.Aluigi M, Fogli M, Curti A, et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:454–461. doi: 10.1634/stemcells.2005-0198. doi:10.1634/stemcells.2005-0198. [DOI] [PubMed] [Google Scholar]
- 36.Gresch O, Engel FB, Nesic D, et al. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–163. doi: 10.1016/j.ymeth.2003.11.009. doi:10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Muehlberg FL, Song YH, Krohn A, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. doi:10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 38.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463–473. doi: 10.1089/scd.2007.0181. doi:10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]