Abstract
This randomized, open-label, active-controlled, dose-finding phase IIb study evaluated the efficacy and safety of trabedersen (AP 12009) administered intratumorally by convection-enhanced delivery compared with standard chemotherapy in patients with recurrent/refractory high-grade glioma. One hundred and forty-five patients with central reference histopathology of recurrent/refractory glioblastoma multiforme (GBM) or anaplastic astrocytoma (AA) were randomly assigned to receive trabedersen at doses of 10 or 80 µM or standard chemotherapy (temozolomide or procarbazine/lomustine/vincristine). Primary endpoint was 6-month tumor control rate, and secondary endpoints included response at further timepoints, survival, and safety. Six-month tumor control rates were not significantly different in the entire study population (AA and GBM). Prespecified AA subgroup analysis showed a significant benefit regarding the 14-month tumor control rate for 10 µM trabedersen vs chemotherapy (p= .0032). The 2-year survival rate had a trend for superiority for 10 µM trabedersen vs chemotherapy (p = .10). Median survival for 10 µM trabedersen was 39.1 months compared with 35.2 months for 80 µM trabedersen and 21.7 months for chemotherapy (not significant). In GBM patients, response and survival results were comparable among the 3 arms. Exploratory analysis on GBM patients aged ≤55 years with Karnofsky performance status >80% at baseline indicated a 3-fold survival at 2 and 3 years for 10 µM trabedersen vs chemotherapy. The frequency of patients with related or possibly drug-related adverse events was higher with standard chemotherapy (64%) than with 80 µM trabedersen (43%) and 10 µM trabedersen (27%). Superior efficacy and safety for 10 µM trabedersen over 80 µM trabedersen and chemotherapy and positive risk–benefit assessment suggest it as the optimal dose for further clinical development in high-grade glioma.
Keywords: anaplastic astrocytoma, antisense oligonucleotide, convection-enhanced delivery, glioblastoma multiforme, recurrent or refractory high-grade glioma, targeted therapy, temozolomide, trabedersen, transforming growth factor beta 2
The high-grade gliomas, mainly anaplastic astrocytoma (AA; WHO grade III) and glioblastoma multiforme (GBM; WHO grade IV), represent about 60% of all primary malignant brain tumors. Prognosis in these cases has only marginally improved despite technical advances in neurosurgery and radiotherapy and the approval of novel anticancer agents. Chemotherapy with the alkylating agent temozolomide (TMZ) has shown a clinically meaningful and statistically significant survival benefit in patients with newly diagnosed GBM with a median survival of 14.6 months with a combination of radiotherapy and TMZ vs 12.1 months with radiotherapy alone.1 The respective 5-year survival rates were 9.8% vs 1.9%.2 However, overall survival (OS) is still poor, especially in patients with recurrent or refractory tumors with a median survival of 11.3 months for patients with AA and 7.4 months for patients with GBM.3
The transforming growth factor 2 (TGF-β2) is overexpressed in more than 90% of high-grade gliomas, and its levels are closely related to tumor progression.4,5,6 Inhibition of TGF-β2 in tumor tissue leads to reversal of tumor-induced immune suppression as well as inhibition of tumor growth, invasion, and metastasis.7,8,9
Trabedersen (AP 12009) is a synthetic antisense phosphorothioate oligodeoxynucleotide complementary to the mRNA of the human TGF-β2 gene, developed as a novel, targeted treatment for patients with high-grade glioma.10
The safety and efficacy of trabedersen has been established through various pharmacokinetic and toxicology studies, both in vitro and in vivo.11 In 3 phase I/II dose escalation studies, adult patients with recurrent or refractory AA or GBM and evidence of tumor progression were treated with trabedersen.12 The maximum tolerated dose was not reached despite up to a 64-fold increase in the administered concentration. Long-lasting complete remissions were observed at doses of 10 and 80 µM trabedersen.
The current randomized and controlled phase IIb study evaluated the efficacy and safety of 2 doses (10 and 80 µM) of trabedersen in comparison with standard chemotherapy in patients with recurrent high-grade glioma of either AA or GBM.
Methods
This study was conducted in accordance with the International Conference on Harmonisation (ICH) Consolidated Good Clinical Practice (GCP) Guideline E6 (Note for Guidance CPMP/135/95), the ethical principles of the Declaration of Helsinki, and the rules of the EU Directive 2001/20/EC. The study protocol was fully approved by regulatory authorities and Independent Ethics Committees/Institutional Review Boards. Written informed consent was obtained from all patients. An independent Data and Safety Monitoring Board periodically reviewed safety and efficacy data.
Study design
The study was multinational, open-label, randomized, and active controlled. Patients were randomized in a 1:1:1 ratio using a centralized randomization procedure to treatment with either 10 µM trabedersen, 80 µM trabedersen, or standard chemotherapy.
Main inclusion criteria were: male or female patients between 18 and 75 years of age with a reference neuropathology-confirmed diagnosis of recurrent/refractory AA or GBM, Karnofsky performance status (KPS) ≥70% at baseline, with tumor lesions ≤50 cm3, and a measurable enhancing tumor lesion in MRI with a diameter ≤4.5 cm. Main exclusion criteria were: patients with tumor surgery within the last 2 weeks, radiotherapy within 8 weeks, chemotherapy within 4 weeks, or a baseline MRI showing a significant mass effect.
The diagnosis of AA or GBM was confirmed by central neuropathology assessment of tissue specimens (Institute for Neuropathology, German Brain Tumor Reference Center, University Hospital Bonn, Germany).
Treatment
The planned duration of treatment in the study was approximately 6 months in all 3 treatment arms. The survival status of all patients was followed for at least 48 months.
The standard chemotherapy control was either TMZ (150–200 mg/m2 on days 1–5 in 28-day cycles) or procarbazine/CCNU (N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea; lomustine)/vincristine (PCV; lomustine 110 mg/m2 on day 1, procarbazine 60 mg/m2 on days 8–21, and vincristine 1.4 mg/m2 on days 8 and 29 in 56-day cycles) depending on patient's previous therapy: TMZ if the patient had not been treated with TMZ before (35 patients) and PCV if prior TMZ treatment had failed (10 patients). Study treatment for patients of the standard chemotherapy group included up to 6 treatment cycles with TMZ or 3 treatment cycles with PCV. Patients showing a response to therapy might have continued treatment at the discretion of the investigator.
Patients in the trabedersen groups received up to 11 treatment cycles (14 days per cycle). One cycle consisted of trabedersen for 7 days with a flow rate of 4 µL/min, followed by isotonic saline for 7 days with a flow rate of 1 µL/min. The trabedersen dose per cycle was 2.48 mg (10 µM group) or 19.81 mg (80 µM group). Trabedersen was infused intratumorally using convection-enhanced delivery (CED) via a subcutaneous port access system connected to an external pump allowing outpatient treatment. The CED technique allows a highly efficient, homogeneous, and targeted administration of the drug, bypassing the blood–brain barrier.13,14 In contrast to other CED studies,15,16 a single catheter was placed into the solid and contrast-enhancing area of the largest tumor lesion. The drug delivery system was implanted 2 days before the start of the infusion.
Statistical endpoints and analysis
This randomized and controlled phase II study was designed to be hypothesis generating. The primary endpoint was the comparison of the 2 trabedersen dose groups for the tumor control rate after 6 months using Fisher's exact test at a 2-sided significance level of 5%. In addition, as the internal control, the trabedersen groups were compared descriptively with the standard chemotherapy group. Tumor assessments were done via central, blinded MRI readings of 2 independent radiologists and 1 adjudicator using the classical Macdonald criteria.17 The tumor control rate was defined as the percentage of patients with complete response (CR) + partial response (PR) + stable disease (SD).
Assuming a tumor control rate of 50% in the 80 µM trabedersen group and of 20% in the 10 µM trabedersen group, 32 evaluable patients per arm were needed to give 80% power to reject the null hypothesis of no difference between the 2 trabedersen groups at an α-level of 10%. Assuming a dropout rate of 18%, 39 GBM patients per arm were planned to be randomized. About 8 AA patients were expected to be included in each treatment arm, resulting in a total of 141 patients to be randomized.
Secondary endpoints included response at time points other than 6 months, with 14 months as the last time point with sufficient MRI data available for interpretable analysis. Further secondary endpoints included survival, progression-free survival, and safety. The OS was calculated from the date of randomization to date of death and analyzed using the Kaplan–Meier methodology. Patients who had not died at the time of analysis or had been lost to follow-up were censored at the time of their last survival assessment.
Exploratory analyses were planned to be done separately for the prespecified subpopulations of AA and GBM patients. An additional exploratory analysis was done on GBM patients with the prognostic factors age ≤55 years and KPS >80%.
Adverse events (AEs) were graded according to the National Cancer Institute–Common Toxicity Criteria (NCI-CTC), version 2.0.
Results
Altogether 145 patients were randomized, 103 with recurrent GBM and 42 with recurrent AA. Eight trabedersen patients discontinued before catheter-port implantation and 2 standard chemotherapy patients discontinued before start of treatment, thus the safety population included 135 patients. One trabedersen patient discontinued after catheter-port surgery but before start of treatment. Therefore, a total of 134 patients were treated with study medication (40 with 10 µM trabedersen, 49 with 80 µM trabedersen, and 45 with standard chemotherapy: 35 with TMZ and 10 with PCV) and represented the primary efficacy population. During the treatment period, 83 patients discontinued the study due to disease progression (57), AEs (8), patient request (6), death (5), study drug toxicity (2), loss to follow-up (1), and other reasons (4).
All patients had previous first-line tumor surgery, and 91% had previous first-line radiotherapy (Table 1). The 10 µM trabedersen group included more patients >55 years (43%) than the 80 µM trabedersen (18%) or the standard chemotherapy (29%) group. Patients of the 10 µM trabedersen group also had the largest total tumor volume at baseline (Table 1). Almost all patients had brain edema at baseline.
Table 1.
Patient baseline characteristics (primary efficacy population, n= 134): all patients, GBM patients, and AA patients (primary efficacy population)
| Patient characteristics | 10 µM trabedersen | 80 µM trabedersen | Standard chemotherapy (TMZ/PCV) |
|---|---|---|---|
| All patients | n= 40 | n= 49 | n= 45 |
| Median age (years) | 46.5 | 44.0 | 45.0 |
| Patients >55 y (%) | 17 (43%) | 9 (18%) | 13 (29%) |
| Female (%) | 13 (33%) | 12 (25%) | 22 (49%) |
| Median duration of disease (months) | 7.0 | 6.0 | 7.0 |
| Previous radiation therapy (%) | 35 (88%) | 46 (94%) | 41 (91%) |
| Previous surgery (%) | 40 (100%) | 49 (100%) | 45 (100%) |
| Previous chemotherapy (%) | 22 (55%) | 26 (53%) | 28 (62%) |
| Karnofsky performance status | |||
| 90–100 (%) | 30 (75%) | 34 (69%) | 30 (67%) |
| 70–80 (%) | 10 (25%) | 15 (31%) | 14 (31%) |
| Median tumor volume (mm3) | 24,159 | 21,510 | 15,834 |
| GBM patients | n= 28 | n= 34 | n= 33 |
| Median age (years) | 56.5 | 45.5 | 52.0 |
| Patients >55 y (%) | 15 (54%) | 7 (21%) | 12 (36%) |
| Female (%) | 7 (25%) | 10 (29%) | 16 (49%) |
| Median duration of disease (months) | 7.0 | 6.0 | 7.0 |
| Previous radiation therapy (%) | 23 (82%) | 32 (94%) | 29 (88%) |
| Previous surgery (%) | 28 (100%) | 34 (100%) | 33 (100%) |
| Previous chemotherapy (%) | 14 (50%) | 21 (62%) | 21 (64%) |
| Karnofsky performance status | |||
| 90 or 100 (%) | 22 (79%) | 23 (68%) | 20 (61%) |
| 70 or 80 (%) | 6 (21%) | 11 (32%) | 12 (36%) |
| Median tumor volume (mm3) | 27,072 | 26,885 | 17,664 |
| AA patients | n= 12 | n= 15 | n= 12 |
| Median age (years) | 39.0 | 40.0 | 36.5 |
| Patients >55 y (%) | 2 (17%) | 2 (13%) | 1 (8%) |
| Female (%) | 6 (50%) | 2 (13%) | 6 (50%) |
| Median duration of disease (months) | 5.5 | 7.0 | 8.0 |
| Previous radiation therapy (%) | 12 (100%) | 14 (93%) | 12 (100%) |
| Previous surgery (%) | 12 (100%) | 15 (100%) | 12 (100%) |
| Previous chemotherapy (%) | 8 (67%) | 5 (33%) | 7 (58%) |
| Karnofsky performance status | |||
| 90–100 (%) | 8 (67%) | 11 (73%) | 10 (83%) |
| 70–80 (%) | 4 (33%) | 4 (27%) | 2 (17%) |
| Median tumor volume (mm3) | 19,976 | 14,346 | 15,701 |
AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; PCV, procarbazine/CCNU (lomustine)/vincristine; TMZ, temozolomide.
Patients received a median of 7 and 6 treatment cycles in the 10 and 80 µM trabedersen groups, respectively. TMZ-treated patients (n= 35) received a median of 6 treatment cycles and PCV-treated patients (n= 10) a median of 1 treatment cycle. The median overall treatment duration was 91 days in the 10 µM and 78 days in the 80 µM trabedersen groups, and 145 days for TMZ vs 29 days for PCV. The short treatment duration in PCV-treated patients could be explained by their higher median age at baseline (54 years) compared with TMZ-treated patients (43 years) and the associated inferior bone marrow capacity and prognosis.
Efficacy in the entire study population
Tumor control rate at 6 months (Table 2) in the entire study population was nonsignificantly higher in the 10 µM trabedersen group (33%) compared with the 80 µM trabedersen group (20%, p= .1298) and the standard chemotherapy group (27%, p= .6219). After 14 months, the 10 µM trabedersen group had the highest tumor control rate (23%) compared with the 80 µM trabedersen group (8%, p= .1197) and the standard chemotherapy group (7%, p= .0529) (Table 2). Median survival was 12.0 months (95% CI: 7.0–26.6) with the 10 µM trabedersen group compared with 13.1 months with the 80 µM trabedersen group (95% CI: 9.3–14.8, p= .5153), and 11.0 months (95% CI: 8.7–13.7, p= .5119) with the standard chemotherapy group. The OS rate was similar in all 3 groups at 12 months (Supplementary Material, Table S1), but starting from 24 months, survival rates were nonsignificantly higher in the 10 µM trabedersen group (39%, 95% CI: 24–54) compared with the 80 µM trabedersen group (29%, 95% CI: 17–42, p= .2186) and the standard chemotherapy group (22%, 95% CI: 12–35, p= .0592).
Table 2.
Tumor response: all patients, GBM patients, and AA patients (primary efficacy population)
| Tumor response | Number of patients (%) |
||
|---|---|---|---|
| 10 µM trabedersen | 80 µM trabedersen | Standard chemotherapy (TMZ/PCV) | |
| All patients | n = 40 | n = 49 | n = 45 |
| 6 months | |||
| Tumor control rate (CR + PR + SD) | 13 (33%) | 10 (20%) | 12 (27%) |
| Overall response rate (CR + PR) | 2 (5%) | 3 (6%) | 3 (7%) |
| Progressive disease | 19 (48%) | 34 (69%) | 23 (51%) |
| Missing MRI data | 8 (20%) | 5 (10%) | 9 (20%) |
| 14 months | |||
| Tumor control rate (CR + PR + SD) | 9 (23%) | 4 (8%) | 3 (7%) |
| Overall response rate (CR + PR) | 6 (15%) | 3 (6%) | 2 (4%) |
| Progressive disease | 22 (55%) | 31 (63%) | 32 (71%) |
| Missing MRI data | 9 (23%) | 14 (29%) | 10 (22%) |
| GBM patients | n = 28 | n = 34 | n = 33 |
| 6 months | |||
| Tumor control rate (CR + PR + SD) | 4 (14%) | 4 (12%) | 5 (15%) |
| Overall response rate (CR + PR) | 0 | 1 (3%) | 0 |
| Progressive disease | 16 (57%) | 26 (77%) | 19 (58%) |
| Missing MRI data | 8 (29%) | 4 (12%) | 8 (24%) |
| 14 months | |||
| Tumor control rate (CR + PR + SD) | 2 (7%) | 1 (3%) | 3 (9%) |
| Overall response rate (CR + PR) | 1 (4%) | 0 | 2 (6%) |
| Progressive disease | 20 (71%) | 25 (74%) | 25 (76%) |
| Missing MRI data | 6 (21%) | 8 (24%) | 5 (15%) |
| AA patients | n = 12 | n = 15 | n = 12 |
| 6 months | |||
| Tumor control rate (CR + PR + SD) | 9 (75%) | 6 (40%) | 7 (58%) |
| Overall response rate (CR + PR) | 2 (17%) | 2 (13%) | 3 (25%) |
| Progressive disease | 3 (25%) | 8 (53%) | 4 (33%) |
| Missing MRI data | 0 | 1 (7%) | 1 (8%) |
| 14 months | |||
| Tumor control rate (CR + PR + SD) | 7 (58%) | 3 (20%) | 0 |
| Overall response rate (CR + PR) | 5 (42%) | 3 (20%) | 0 |
| Progressive disease | 2 (17%) | 6 (40%) | 7 (58%) |
| Missing MRI data | 3 (25%) | 6 (40%) | 5 (42%) |
AA, anaplastic astrocytoma; GBM, glioblastoma; PCV, procarbazine/CCNU (lomustine)/vincristine; TMZ, temozolomide; CR, complete response; PR, partial response; SD, stable disease.
Case reports of 1 GBM patient and 1 AA patient treated with 10 µM trabedersen are presented in Supplementary Material, Figures S1 and S2.
Efficacy in GBM and AA subgroups
As AA and GBM patients differ in their prognoses,18,19 efficacy analyses were repeated on an exploratory basis separately for the 2 subpopulations of AA and GBM patients.
GBM patients
The primary efficacy population included 95 patients with recurrent/refractory GBM: 28 were treated with 10 µM trabedersen, 34 with 80 µM trabedersen, and 33 with standard chemotherapy. Baseline characteristics are shown in Table 1, and safety results are given in Supplementary Material, Table S2.
In GBM patients, the tumor control rates at 6 months were comparable in all 3 groups (Table 2): 14% (10 µM trabedersen), 12% (80 µM trabedersen), and 15% (standard chemotherapy). Tumor control rates subsequently decreased and were 7% (10 µM trabedersen), 3% (80 µM trabedersen), and 9% (standard chemotherapy) after 14 months.
Median survival was 7.3 months (95% CI: 5.0–12.0) with 10 µM trabedersen compared with 10.9 months with 80 µM trabedersen (95% CI: 5.6–13.9, p= .9370), and 10.0 months (95% CI: 7.0–13.0, p= .7310) with standard chemotherapy. At 24 months, OS was nonsignificantly higher with 10 µM trabedersen (20%, 95% CI: 7–36) compared with 80 µM trabedersen (18%, 95% CI: 7–32, p= .6783) and standard chemotherapy (15%, 95% CI: 6–29, p= .4861) (Supplementary Material, Table S1). The median of the prognostic factor age for GBM patients of the 10 µM trabedersen group was higher than in the other 2 treatment groups (56.5 vs 45.5 and 52.0 years) (Table 1) as well as the number of patients of age >55 years (54% vs 21% and 36%), which may have biased the survival results for this group.
Furthermore, as it is well known that high-grade glioma patients with lower age and higher KPS have a better prognosis,18,20 an additional exploratory analysis was performed for GBM patients with the prespecified prognostic factors age ≤55 years and KPS >80%. This subgroup included 45 patients (primary efficacy population): 9 were treated with 10 µM trabedersen, 21 with 80 µM trabedersen, and 15 with standard chemotherapy. Both trabedersen groups showed favorable survival results, especially the 10 µM trabedersen group. Median survival for this group was 12.0 months (95% CI: 5.9 to not defined [ND]) compared with 10.1 months (95% CI: 7.8–13.7, p= .2972) for standard chemotherapy. At 12 months, OS was 40% (95% CI: 6–74) with 10 µM trabedersen and 40% (95% CI: 15–65) with standard chemotherapy. Of note, at 2 and 3 years, the OS rate was 3-fold higher for 10 µM trabedersen (40%, 95% CI: 6–74) compared with standard chemotherapy (13%, 95% CI: 0–31).
AA patients
The primary efficacy population included 39 patients with recurrent AA: 12 were treated with 10 µM trabedersen, 15 with 80 µM trabedersen, and 12 with standard chemotherapy. Baseline characteristics are shown in Table 1, and safety results are given in Supplementary Material, Table S2.
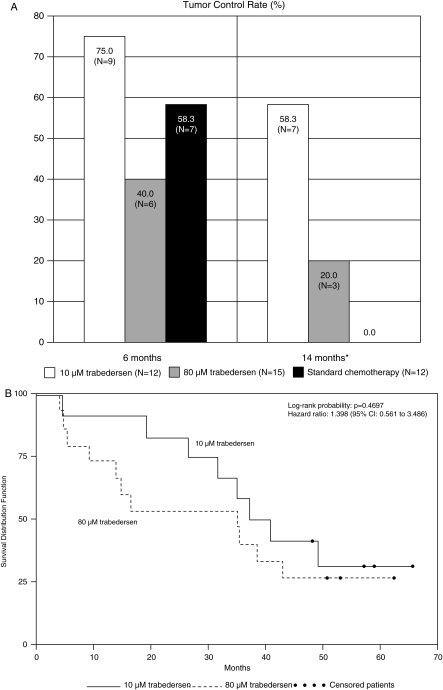
In AA patients, the tumor control rate was nonsignificantly higher with 10 µM trabedersen at 6 months (75%) compared with 80 µM trabedersen (40%, p= .1302) and standard chemotherapy (58%, p= .6668) and significantly higher vs standard chemotherapy at 14 months (58% vs 20% with 80 µM trabedersen, p= .1534, and 0% with standard chemotherapy, p= .0032) (Fig. 1A, Table 2). The median duration of response was 29.1 months (95% CI: 4.1–49.8) with 10 µM trabedersen, 24.0 months (95% CI: 2.3–44.9) with 80 µM trabedersen, and 8.0 months (95% CI: ND) with standard chemotherapy. The median time to progression was 22.4 months (95% CI: 1.2–42.0) with 10 µM trabedersen, 3.4 months (95% CI: 1.2–7.8) with 80 µM trabedersen, and 13.0 months (95% CI: 3.7–14.3) with standard chemotherapy.
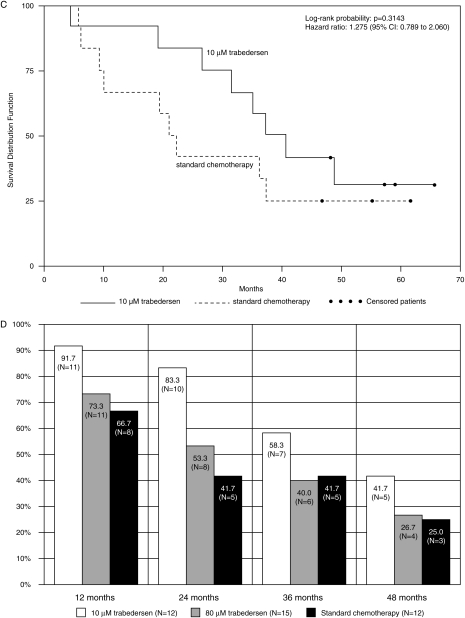
Fig. 1.
Efficacy results in patients with AA (primary efficacy population, n= 39). (A) Tumor control rates (complete response + partial response + stable disease) at months 6 and 14. *Comparison between 10 µM trabedersen and standard chemotherapy: p= .0032. (B) Kaplan–Meier plot for patients treated with 10 µM trabedersen (n= 12) vs 80 µM trabedersen (n= 15). (C) Kaplan–Meier plot for patients treated with 10 µM trabedersen (n= 12) vs standard chemotherapy (n= 12). (D) Overall survival rates.
Median survival in AA patients was considerably longer in the trabedersen groups: 39.1 months (95% CI 31.6 to ND) for 10 µM trabedersen and 35.2 months (95% CI: 13.9–43.1) for 80 µM trabedersen compared with 21.7 months (95% CI: 10.1–37.4) for standard chemotherapy (Fig. 1B and C), resulting in a median survival benefit of 17.4 months for 10 µM trabedersen compared with standard chemotherapy (not significant). After 12 months, the 10 µM trabedersen group had the highest survival rate (92% vs 73% and 67%) (Fig. 1D, Supplementary Material, Table S1). After 2 years, the 10 µM trabedersen group had a higher survival rate (83%, 95% CI: 48–96) compared with the 80 µM trabedersen group (53%, 95% CI: 26–74, p= .2071) and the standard chemotherapy group (42%, 95% CI: 15–67, p= .1038). At 3 and 4 years, the survival rate remained highest for 10 µM trabedersen (Fig. 1D, Supplementary Material, Table S1).
Safety
A total of 98% of patients had at least one AE (Table 3). AEs of NCI-CTC grade 3 or 4 were reported for 76% of patients with 10 and 80 µM trabedersen, and 60% with standard chemotherapy (Table 4). Most of these events were nervous system disorders in all 3 groups (66% for 10 µM trabedersen, 59% for 80 µM trabedersen, and 31% for standard chemotherapy), with convulsion, brain compression, hemiparesis, and brain edema as the most common. Most AEs were related to the underlying disease.
Table 3.
AE overview (safety population, n= 135)
| AE | Number of patients (%) |
||
|---|---|---|---|
| 10 µM trabedersen (n = 41) | 80 µM trabedersen (n = 49) | Standard chemotherapy (TMZ/PCV) (n = 45) | |
| At least 1 AE | 40 (98%) | 48 (98%) | 44 (98%) |
| AEs leading to discontinuation of study drug | 19 (46%) | 24 (49%) | 10 (22%) |
| AEs drug related or possibly drug related | 11 (27%) | 21 (43%) | 29 (64%) |
| SAEs | 32 (78%) | 37 (76%) | 18 (40%) |
| SAEs drug related or possibly drug related | 0 | 3 (6%) | 1 (2%) |
| SAEs procedure related | 14 (34%) | 14 (29%) | n.a.* |
| SAEs with outcome death | 11 (27%) | 17 (35%) | 10 (22%) |
AE, adverse event; PCV, procarbazine/CCNU (lomustine)/vincristine; SAE, serious adverse event; TMZ, temozolomide.
*n.a., not applicable, no medical device was used.
Table 4.
Patients with NCI-CTC toxicity grade 3/4 AE in ≥10% of patients in any group (safety population, n= 135)
| AEs | Number of patients (%) |
||
|---|---|---|---|
| 10 µM trabedersen (n = 41) | 80 µM trabedersen (n = 49) | Standard chemotherapy (TMZ/PCV) (n = 45) | |
| Patients with NCI-CTC toxicity grade 3 or 4 AEs | 31 (76%) | 37 (76%) | 27 (60%) |
| Blood and lymphatic system disorders | 2 (5%) | 4 (8%) | 5 (11%) |
| General disorders and administration site conditions | 10 (24%) | 3 (6%) | 7 (16%) |
| Infections and infestations brain abscess | 3 (7%) | 6 (12%) | 0 |
| Injury, poisoning, and procedural complications | 7 (17%) | 8 (16%) | 0 |
| Investigations | 6 (15%) | 4 (8%) | 5 (11%) |
| Karnofsky scale worsened | 4 (10%) | 4 (8%) | 4 (9%) |
| Nervous system disorders | 27 (66%) | 29 (59%) | 14 (31%) |
| Aphasia | 6 (15%) | 5 (10%) | 2 (4%) |
| Brain compression | 3 (7%) | 5 (10%) | 2 (4%) |
| Brain edema | 11 (27%) | 10 (20%) | 2 (4%) |
| Convulsion | 5 (12%) | 4 (8%) | 3 (7%) |
| Depressed level of consciousness | 5 (12%) | 2 (4%) | 0 |
| Headache | 4 (10%) | 5 (10%) | 3 (7%) |
| Hemiparesis | 11 (27%) | 11 (22%) | 1 (2%) |
| Intracranial pressure increased | 8 (20%) | 7 (14%) | 2 (4%) |
| Neurological symptom | 7 (17%) | 4 (8%) | 2 (4%) |
| Psychiatric disorders | 5 (12%) | 3 (6%) | 1 (2%) |
AE, adverse event; NCI-CTC, National Cancer Institute–Common Toxicity Criteria; PCV, procarbazine/CCNU (lomustine)/vincristine; TMZ, temozolomide.
AEs leading to permanent discontinuation of treatment were more common in the trabedersen groups: 46% (10 µM) and 49% (80 µM) compared with 22% for standard chemotherapy (Table 3), but AEs considered possibly drug related were more common with standard chemotherapy: 64% compared with 27% (10 µM trabedersen) and 43% (80 µM trabedersen). These events were most commonly blood and lymphatic disorders in the standard chemotherapy group and nervous system disorders as defined above in the trabedersen groups. Only in 4 patients were drug-related or possibly drug-related serious adverse events (SAEs) reported: meningitis, hyponatremia and brain edema, and thrombocytopenia for 3 patients in the 80 µM trabedersen group and cerebral disorder for 1 standard chemotherapy patient. Procedure-related SAEs were reported in 34% of patients receiving 10 µM trabedersen and 29% of patients receiving 80 µM trabedersen; for those respective groups, application site infection occurred in 12% and 10% of patients and complications associated with catheter placement occurred in 7% and 2%.
Discussion
This randomized and active-controlled phase IIb study was designed to be hypothesis generating. It had the main goals to determine the optimal dose of trabedersen for use in further clinical development in patients with high-grade glioma and to compare the efficacy and safety of trabedersen to standard chemotherapy.
The primary endpoint tumor control rate at 6 months did not show statistically significant differences among the treatment groups. Endpoints based on tumor assessments early after treatment start are recognized surrogate endpoints for OS, the “gold standard” endpoint for oncology trials.21,22 However, it has turned out in recent years that this may not be appropriate for therapies that have a completely different mode of action from fast-acting cytotoxic or cytoreductive agents.22,23 This particularly applies to immuno-based therapies, such as trabedersen, which rely on building an immune response over time.23,24 Indeed, the results of this study show that the clinically relevant beneficial effect of trabedersen increases over time, which was visible especially in the 10 µM trabedersen group and was most evident at 14 months regarding tumor response (Table 2). An increase in tumor response over time for trabedersen had already been observed in phase I/II studies,12 where 2 patients had long-lasting complete tumor remissions appearing several months after initiation of treatment. One patient from these studies is still alive and in complete remission more than 8 years after start of trabedersen treatment. The other patient was in complete remission and died of an unrelated cause (myocardial infarction) more than 2 years after start of trabedersen treatment. Taken together, the full clinically relevant beneficial effects of trabedersen can be accurately assessed only at later time points (eg, after 14 months for response assessment and after 24 months for survival assessment), and this finding will be incorporated into the design of subsequent, confirmatory studies (see also below).
Despite the fact that the primary endpoint did not reach statistical significance, the results indicate that the optimal dose is 10 µM trabedersen, as both efficacy (tumor control rate, tumor progression rate, and survival rate) and safety results tended to be superior for the 10 µM dose compared with the 80 µM dose, despite the inferior prognosis (higher age and tumor volume) of the 10 µM group at baseline. Although higher efficacy of the lower dose may seem counterintuitive, in vitro assays in human GBM cells have shown that 10 µM trabedersen has a stronger inhibitory effect upon TGF-β2 secretion when compared with 80 µM (manuscript in preparation), although the mechanism for this is not fully understood.
There was an advantage in long-term survival for the 10 µM trabedersen group, especially when compared with standard chemotherapy. In the entire study population, the 2-year survival rate for 10 µM trabedersen was 39% vs 22% for standard chemotherapy. The beneficial effect of 10 µM trabedersen concerning the 2-year survival rate was even more pronounced in patients with GBM aged ≤55 years with KPS >80% (40% vs 13%) and patients with AA (83% vs 42%). Remarkably, the median survival of patients with AA treated with 10 µM trabedersen exceeded that of standard chemotherapy by 17.4 months (39.1 vs 21.7 months). However, it has to be taken into consideration that the sample sizes of the AA subgroup and the group with GBM patients aged ≤55 years with KPS >80% were small and, therefore, restrict generalization of the results. Despite the clinical relevance of the treatment effects observed with trabedersen and the consistency with findings from previous phase I/II studies,12 the hypotheses generated in this phase II study need to be confirmed in prospective clinical studies. To this end, a randomized and controlled phase III study in patients with AA has been initiated (see below).
In this study, the proportion of patients with AEs considered related or possibly related to the study drug was highest in the standard chemotherapy group and lowest in the 10 µM trabedersen group. The patients treated with trabedersen had a higher rate of NCI-CTC grade 3/4 AEs than patients in the standard chemotherapy group, mainly regarding neurological events. The exact reason(s) for this has not been fully clarified to date and will be closely examined in current and upcoming studies.
Part of the reason for the higher frequency of AEs in the trabedersen groups may be that there were more scheduled study visits for the patients (up to 23 visits) compared with those with standard chemotherapy (up to 9 visits), thus allowing more chances for the observation of AEs. Moreover, all patients of the trabedersen groups had biopsies taken before start of therapy and 20 patients at 3 months after start of therapy, while no biopsies were taken from patients of the control group. Generally, the frequency and nature of the reported events were typical for patients with primary brain tumors who usually have a large number of accompanying neurological disorders.25,26 One reason for more neurological AEs observed in the trabedersen groups may be the mode of administration of trabedersen through CED (ie, the infusion process through an implanted catheter).12 Although events related to the implantation and the procedure of drug administration cannot be completely avoided, those observed during this study were manageable, and their incidence can be further reduced by intensified training of the investigators.
The finding that in the 10 µM trabedersen group more patients were assessed with CR or PR at month 14 than at month 6 (Table 2) supports the hypothesis from preclinical and phase I/II data that trabedersen acts mainly by inducing an immune response against the tumor. This is further supported by the fact that these responses continue to increase long after discontinuation of trabedersen—for instance, from SD to partial and even CRs without further antitumor treatment (see Supplementary Material, Figures S1 and S2). The suppression of immune reactions is commonly observed in patients with high-grade glioma.27 A major factor contributing to the suppression of the immune system is TGF-β2, as it has multiple effects on immunoregulatory functions, including the inhibition of lymphokine-activated killer cells and T-lymphocyte development and interleukin-2–dependent growth of T cells.5,28–31 Thus, the transient reduction in TGF-β2 levels via the antisense compound trabedersen may reverse TGF-β2–mediated immunosuppression. This may lead to the induction of a comprehensive, adaptive anticancer immune response that eliminates even residual cancer cells and has the potential to translate into a beneficial long-term outcome for patients. An immuno-based anticancer response is also in line with the observation in a previous phase I/II study where 1 AA patient had several localizations of the tumor in both brain hemispheres. All these tumor lesions receded completely after local therapy of only 1 tumor lesion with trabedersen.12 This phenomenon was also observed in patients of this phase IIb study.
Pseudoprogression, a transient initial increase of the enhancing lesion,32 was seen in about 10% of trabedersen-treated patients in this study. In these patients, pseudoprogression was associated with a favorable survival (manuscript in preparation), as has been reported previously in high-grade glioma patients treated with radiotherapy and TMZ.33
In conclusion, 10 µM trabedersen represents the optimal dose for further clinical development of trabedersen. The 6-month intratumoral CED technique was found to be safe and well tolerated.
On the basis of the promising results of this study, the randomized, active-controlled phase III study SAPPHIRE (Efficacy and Safety of AP 12009 in Adult Patients with Recurrent or Refractory Anaplastic Astrocytoma [WHO grade III] as Compared to Standard Treatment with Temozolomide or BCNU: A Randomized, Actively Controlled, Open-label Clinical Phase III Study) in patients with recurrent or refractory AA has been initiated to compare the 14-month progression and 24-month survival rates and median OS of 10 µM trabedersen with standard chemotherapy. Additional survival and tumor response parameters as well as quality of life and safety will also be assessed in the study.
Supplementary Material
Supplementary Material is available at Neuro-Oncology online.
Acknowledgments
We thank all patients who participated in this study and all investigators and staff from the following centers: U. Bogdahn (Germany), S. Burnin (Russia), L. Diudin (Russia), W. Grisold (Austria), D. Koch (Germany), V. Leshinskiy (Russia), V. Loshakov (Russia), A.K. Mahapatra (India), M. Mehdorn (Germany), J. Meixensberger (Germany), C. Mouli (India), S. Nair (India), V. Oliushine (Russia), V. Parfenov (Russia), J. Pichler (Austria), I. Poverennova (Russia), D. Raghunadhrao (India), Z.H. Rappaport (Israel), K.V.R. Sastry (India), A. Savchenko (Russia), G. Schackert (Germany), T. Schneider (Germany), R. Shakarishvili (Georgia), A. Sharma (India), Y. Shulev (Russia), G. Stockhammer (Austria), N.K. Venkataramana (India), H. Wassmann (Germany), M. Weller (Germany), M. Zaaroor (Israel).
We also thank Dr. Barry Drees and Dr. Christian Seitz for their assistance in manuscript preparation.
Conflict of interest statement. P.J., S.L., S.S., and H.H. are employees of Antisense Pharma GmbH; H.H. holds 0.1% of the stock of Antisense Pharma; K.-H.S. is the CEO of Antisense Pharma and holds 16% of the stock; trabedersen is protected by patents in different countries as well as for use in treating glioma in specific concentrations used in the present study.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Chang SM, Theodosopoulos P, Lamborn K, et al. Temozolomide in the treatment of recurrent malignant glioma. Cancer. 2004;100:605–611. doi: 10.1002/cncr.11949. doi:10.1002/cncr.11949. [DOI] [PubMed] [Google Scholar]
- 4.Fontana A, Bodmer S, Frei K, Malipiero U, Siepl C. Expression of TGF-beta2 in human glioblastoma: a role in resistance to immune rejection? CIBA Found Symp. 1991;157:232–241. doi: 10.1002/9780470514061.ch15. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades HN. Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. J Neurosurg. 1992;76:799–804. doi: 10.3171/jns.1992.76.5.0799. doi:10.3171/jns.1992.76.5.0799. [DOI] [PubMed] [Google Scholar]
- 6.Kjellman C, Olofsson SP, Hansson O, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89:251–258. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. doi:10.1002/1097-0215(20000520)89:3<251::AID-IJC7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Jachimczak P, Bogdahn U, Schneider J, et al. The effect of transforming growth factor-beta 2-specific phosphorothioate-anti-sense oligodeoxynucleotides in reversing cellular immunosuppression in malignant glioma. J Neurosurg. 1993;78:944–951. doi: 10.3171/jns.1993.78.6.0944. doi:10.3171/jns.1993.78.6.0944. [DOI] [PubMed] [Google Scholar]
- 8.Jachimczak P, Hessdorfer B, Fabel-Schulte K, et al. Transforming growth factor-beta-mediated autocrine growth regulation of gliomas as detected with phosphorothioate antisense oligonucleotides. Int J Cancer. 1996;65:332–337. doi: 10.1002/(SICI)1097-0215(19960126)65:3<332::AID-IJC10>3.0.CO;2-C. doi:10.1002/(SICI)1097-0215(19960126)65:3<332::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. doi:10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, et al. Targeted tumor therapy with the TGF-beta2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–139. doi: 10.1016/j.cytogfr.2005.09.002. doi:10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Schlingensiepen R, Goldbrunner M, Szyrach MN, et al. Intracerebral and intrathecal infusion of the TGF-beta2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides. 2005;15:94–104. doi: 10.1089/oli.2005.15.94. doi:10.1089/oli.2005.15.94. [DOI] [PubMed] [Google Scholar]
- 12.Hau P, Jachimczak P, Schlingensiepen R, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant glioma: from preclinical to Phase I/II clinical studies. Oligonucleotides. 2007;17:201–212. doi: 10.1089/oli.2006.0053. doi:10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 13.Hall WA, Sherr GT. Convection-enhanced delivery: targeted toxin treatment of malignant glioma. Neurosurg Focus. 2006;20:E10. [PubMed] [Google Scholar]
- 14.Bidros DS, Liu JK, Vogelbaum MA. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 2010;6:117–125. doi: 10.2217/fon.09.135. doi:10.2217/fon.09.135. [DOI] [PubMed] [Google Scholar]
- 15.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. doi:10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 16.Kunwar S, Prados MD, Chang SM, et al. Direct Intracerebral Delivery of Cintredekin Besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. doi:10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 18.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Lamborn KR, Buckner JC, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro-Oncol. 2010;12:164–172. doi: 10.1093/neuonc/nop019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro-Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. doi:10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13:19–21. doi: 10.1634/theoncologist.13-S2-19. doi:10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 22.Farley J, Rose PG. Trial design for evaluation of novel targeted therapies. Gynecol Oncol. 2010;116:173–176. doi: 10.1016/j.ygyno.2009.09.046. doi:10.1016/j.ygyno.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Ratain MJ, Sargent DJ. Optimising the design of phase II oncology trials: the importance of randomisation. Eur J Cancer. 2009;45:275–280. doi: 10.1016/j.ejca.2008.10.029. doi:10.1016/j.ejca.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. doi:10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 25.Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557–564. doi: 10.1001/jama.293.5.557. doi:10.1001/jama.293.5.557. [DOI] [PubMed] [Google Scholar]
- 26.Chandana SR, Movva S, Arora M, Singh T. Primary brain tumors in adults. Am Fam Physician. 2008;77:1423–1430. [PubMed] [Google Scholar]
- 27.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–1647. doi: 10.1084/jem.136.6.1631. doi:10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuppner MC, Hamou MF, Bodmer S, Fontana A, De Tribolet N. The glioblastoma-derived T-cell suppressor factor/transforming growth factor beta 2 inhibits the generation of lymphokine-activated killer (LAK) cells. Int J Cancer. 1988;42:562–567. doi: 10.1002/ijc.2910420416. doi:10.1002/ijc.2910420416. [DOI] [PubMed] [Google Scholar]
- 29.Kuppner MC, Hamou MF, Sawamura Y, Bodmer S, De Tribolet N. Inhibition of lymphocyte function by glioblastoma-derived transforming growth factor beta 2. J Neurosurg. 1989;71:211–217. doi: 10.3171/jns.1989.71.2.0211. doi:10.3171/jns.1989.71.2.0211. [DOI] [PubMed] [Google Scholar]
- 30.Bodmer S, Strommer K, Frei K, et al. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222–3229. [PubMed] [Google Scholar]
- 31.Fontana A, Bodmer S, Frei K, Malipiero U, Siepl C. Expression of TGF-beta 2 in human glioblastoma: a role in resistance to immune rejection? Ciba Found Symp. 1991;157:232–241. doi: 10.1002/9780470514061.ch15. [DOI] [PubMed] [Google Scholar]
- 32.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. doi:10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 33.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. doi:10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]