Abstract
This multicenter phase I study aimed to establish the recommended dose (RD) of the epidermal growth factor receptor (EGFR) inhibitor erlotinib, given as monotherapy or with radiotherapy to children with malignant brain tumors. Group 1 included patients with refractory or relapsing brain tumors receiving erlotinib alone, and group 2 included newly diagnosed patients with brainstem gliomas receiving radiotherapy and erlotinib. A conventional 3 + 3 dose escalation and a continual reassessment method, respectively, were utilized in 4 dose levels: 75, 100, 125, and 150 mg/m2 per day. Fifty-one children were enrolled (30 and 21, respectively); 50 received treatment. The RD of erlotinib was 125 mg/m2 per day as monotherapy or in combination with radiotherapy. Overall, 230 adverse events in 44 patients were possibly treatment related (216, grades 1 and 2; 9, grade 3; 1, grade 4; 4, grade 5). Dermatologic and neurologic symptoms were common; intratumoral hemorrhage was confirmed in 3 patients. In group 1, 8 of 29 patients (28%) had stable disease with tumor regression approaching 50% in a malignant glioma and an anaplastic oligoastrocytoma. In group 2, overall survival was 12.0 months. EGFR overexpression by immunohistochemistry was found in 17 of 38 (45%) tumor samples analyzed, with a partial gain of 7p11.2 in 1 glioblastoma; phosphate and tensin homolog loss was frequent in brainstem glioma (15 of 19). Mean (95% CI) apparent clearance and volume of distribution for erlotinib were 4.0 L/h (3.4–4.5 L/h) and 98.6 L (69.8–127.0 L), respectively, and were independent of the dose level; mean half-life was 16.6 hours. Thus, erlotinib 125 mg/m2 per day has an acceptable tolerability profile in pediatric patients with brain tumors and can be combined with radiotherapy.
Keywords: brain tumor, EGFR biomarker, epithelial growth factor receptor inhibitor erlotinib, pediatric phase I, pharmacokinetics, pontine glioma, radiosensitization
Central nervous system (CNS) tumors account for 20% of all pediatric cancers and are the leading cause of cancer-related death and morbidity in children.1,2 Improved diagnostics and treatment have resulted in a 55% cure rate,3 but many malignancies remain difficult to treat and are associated with a poor prognosis, particularly in children with brainstem gliomas: overall survival (OS) remains approximately 9 months, and most patients die from the disease within 2 years.4 Treatment for pediatric CNS tumors comprises maximal feasible resection combined with irradiation and/or chemotherapy.1,2 In high-grade tumors, radiotherapy remains the first-choice treatment for older children; for younger children (eg, those with medulloblastoma or low-grade glioma), addition of cytotoxic chemotherapy may improve OS and reduce or delay exposure to irradiation.1,2,5,6
Improved knowledge of the biology of pediatric brain tumors, including evidence for the overexpression of the epidermal growth factor receptor (EGFR),7–10 suggests a potential for targeted therapies. Erlotinib (Tarceva) is a potent, human EGFR tyrosine-kinase inhibitor that is being evaluated in various CNS tumors.11–17 It has proven efficacy in adult patients with various EGFR-expressing solid tumors18,19 and has shown antitumor activity and radiosensitizing effects in CNS tumor xenograft models and cell lines.20–24 Studies have suggested that erlotinib is able to cross the blood–brain barrier.25,26
This phase I study was initiated to establish the recommended dose (RD; primary endpoint), safety, pharmacokinetics (PKs), and efficacy (including correlation with tumor biomarkers) of erlotinib, given as monotherapy or with radiotherapy to children with malignant brain tumors.
Patients and Methods
Patients
Children with histologically/cytologically confirmed malignant brain tumors refractory to, or relapsing after, first-line therapy, for whom no effective treatment exists, were enrolled into group 1. Children with newly diagnosed, histologically confirmed brainstem glioma (excluding pilocytic glioma) were enrolled into group 2. Eligibility criteria included: age 1 to ≤21 years; Eastern Cooperative Oncology Group (ECOG) performance status ≤1 or Lansky play scale ≥70% (except when disease-related motor paresis); adequate hematologic, renal, and hepatic function; life expectancy ≥8 weeks; no organ toxicity ≥grade 2 intensity, except disease-related neurologic symptoms; no radiotherapy or chemotherapy within 4 weeks (6 weeks for nitrosourea) prior to entry; no lung disease, severe cardiac pathology, or ophthalmologic abnormalities; no history of spontaneous intratumoral hemorrhage, excluding small post-biopsy hemorrhage; and written informed consent (patients or parents/guardians). The protocol (NCT00418327) and amendments were approved by independent ethics committees and complied with local laws/regulations and the Declaration of Helsinki.
Study design and treatment
This was an open-label, multicenter, dose-escalation study. Erlotinib tablets were administered orally once daily in 3-week cycles at 4 dose levels: 75 mg/m2, 100 mg/m2, 125 mg/m2, and 150 mg/m2. According to the recommendations, the starting dose was approximately 80% of the adult RD (150 mg/day).27 In group 2, patients received erlotinib and local brainstem radiation of 54 Gy over 6 weeks (1.8 Gy/fraction per day); the first erlotinib dose was administered within 4 h after the initial irradiation and continued thereafter as a single agent until tumor progression.
Conventional 3 + 3 dose-escalation methodology was utilized for group 1.28 It was planned that 10 patients would be treated at the RD. In group 2, dose-escalation decisions were made using a continual reassessment method (CRM) with likelihood-based inference in order to allow continuous inclusion.29 If a first dose-limiting toxicity (DLT) was reported, a single-parameter logistic model was used to estimate the probability of experiencing a DLT at each level. After each new observation, the model was reassessed using all previous collected data. The dose level recommended for the next patient was the one nearest to the 20% target percentile. In case a new patient fulfilled eligibility criteria before the previous one had been fully evaluated for toxicity, (s)he could still be enrolled at the same dose as the previous one. Hence, all patients were included at the best ongoing estimate of the RD. If data on all patients on the current dose level were not available, dose escalation was not permitted: new patients were enrolled at the current or a lower dose level. Eight patients were to be treated at the best estimate of the RD following the stopping rule and the decision-tree analysis proposed by O'Quigley and Reiner.30 This enabled us to have an 80% probability that the recommendation would be maintained if 5 more patients were included.
Patients on enzyme-inducing antiepileptic drugs (EIAEDs) were allowed in the study, and PKs were evaluated. Dosage escalation was based on only patients not receiving anticonvulsants or patients receiving anticonvulsants who experienced DLT. Dose interruption/reduction was allowed in the case of treatment-related adverse events (AEs). We computed the mean daily dose (mg/m2) on the whole-treatment duration by summing the daily dose divided by the body surface area, from the first day to the last day of administration, including days with modified doses or temporary interruption (dose equal to zero). The relative dose intensity was estimated as the ratio of the mean daily dose (mg/m2) and the initial prescribed dose (mg/m2). Treatment was continued until disease progression (DP), unacceptable toxicity, or withdrawal. Patients were followed up every 3 months until death or this analysis.
Safety evaluation
DLTs were assessed over a 3-week period in group 1 and over a 6-week period in group 2. DLTs included: grade 3/4 nonhematologic AEs, excluding grade 3 fever; transient hepatic toxicity; grade 3/4 nausea/vomiting without adequate prophylaxis; AEs related to DP; grade 4 neutropenia or thrombocytopenia for >7 days; and grade 3/4 thrombocytopenia requiring transfusion during a time interval of >7 days. AEs were assessed throughout using National Cancer Institute Common Toxicity Criteria (version 3.0). Dermatologic toxicity was assessed using a standardized survey form on days 7 and 21 of the first treatment cycle and every 3 weeks thereafter. Clinical and laboratory assessments were conducted at baseline and then at 3-week intervals.
Efficacy evaluation
Tumor response was evaluated after 6 weeks in group 1 and after 12 weeks in group 2 using WHO criteria.31 Best tumor response was evaluated in group 2 over the treatment duration. Tumor responses were reviewed by an independent radiologist. Progression-free survival (PFS) and OS were estimated using the Kaplan–Meier method. All eligible patients were included in the efficacy analyses.
PK evaluation
Serial blood samples were taken at various intervals during the first 6 treatment cycles to determine the PK profile of erlotinib and its principal metabolite (OSI-420) at an early and late steady state at the following time points: before study medication, 30 minutes, and 1, 2, 4, 6, 8, and 24 hours after the erlotinib dose for the first and the second (group 1) or the third (group 2) cycle and 24-hour post-dose for the following cycles.
The determination of plasma erlotinib and OSI-420 concentrations was done using a validated coupled liquid chromatography–mass spectrometry technique.32 The calibration range was 1–3000 ng/mL for erlotinib and 1–1000 ng/mL for OSI-420. The lower limit of quantification was 1 ng/mL for both analytes, using 0.2 mL of plasma aliquots. Plasma concentrations were analyzed using a nonlinear mixed-effects population approach (NONMEM version VI [level 1.0], Icon Development Solutions) and a first-order conditional estimation with the interaction method.33 Models were compared using a χ2 test.
Biomarker evaluation
Archived tumor samples and prestudy biopsies (group 2) were obtained for a central histologic review of diagnosis and analysis of biomarkers by 2 independent neuropathologists. Fixed, paraffin-embedded tissue sections were stained for EGFR (clone 3C6) using the BenchMark® flexible automation system (Ventana Medical Systems S.A.). For other biomarkers, sections were incubated following antigen heat retrieval and protein blocking with mouse monoclonal anti-EGFRvIII (clone L8A4, 1:100; provided by Dr D. Bigner, Duke University), anti-phosphate and tensin homolog (PTEN) (clone 6H2.1, dilution 1:400), and rabbit polyclonal anti-pHER2/neu (A0485, 1:500–1000; both DakoCytomation Denmark A/S). Protein expression was evaluated using the EnVision System horseradish peroxidase–labeled polymer antimouse and antirabbit revelation kits (Dako) and the R.T.U. Vectastain Universal Elite ABC kit for EGFRvIII (Vector Laboratories, Burlingame). Immunostaining was scored on a 4-point scale (0, 1, 2, or 3) according to the percentage of both positive cells and staining intensity, as adapted from Mizoguchi et al.34 Scores of 2 or 3 were considered positive.
The influence of biomarkers on outcome was assessed in group 2. The Cox regression modeling was used to estimate the hazard ratios (HRs) and associated 95% confidence intervals (CIs) for treatment failure and death. A logrank test was used to compare the survival curves of patients with expressing and nonexpressing tumors.
EGFR (c-ErbB1) gene amplification was assessed by fluorescence in situ hybridization, according to Cappuzzo et al.,35 on fixed, paraffin-embedded tissue sections using the Vysis LSI EGFR Spectrum Orange/CEP 7 Spectrum Green Probe (Abbott Molecular).
Results
Patients
Fifty-one patients (30 in group 1 and 21 in group 2) were enrolled from June 2005 to August 2007; 50 patients received treatment. In 1 patient in group 1, study treatment initiation had to be postponed during clarification of a serious AE in the prior patient and thus he received another treatment. Median ages at inclusion were 10 years and 6 years, respectively. Most patients in group 1 had glial tumors: 12 had malignant gliomas, 6 had infiltrative brainstem glioma, and 7 had ependymomas. In group 2, 2 patients were classified as having WHO astrocytoma grade II, 1 oligodendroglioma grade II, 1 oligoastrocytoma grade II, 1 astrocytoma grade III, 3 oligoastrocytoma grade III, 7 grade IV, and 6 infiltrative gliomas not otherwise specified. The baseline characteristics of enrolled patients are shown in Table 1.
Table 1.
Baseline patient characteristics
| Group 1 (n = 29) | Group 2 (n = 21) | |
|---|---|---|
| Male/female (n [%]) | 15 (52)/14 (48) | 7 (33)/14 (57) |
| Median age (y; range) | 10.0 (4–20) | 6.0 (2–16) |
| Median time since diagnosis (mo; range) | 22.0 (0.5–110.6) | 0.5 (0.2–1.8) |
| ECOG performance status/Lansky play scale (n [%]) | ||
| 0: 90%–100% | 10 (34) | 8 (38) |
| 1: 70%–80% | 19 (66) | 11 (52) |
| 2: <70% | 0 (0) | 2 (10) |
| Tumor histology (n [%]) | ||
| Glioblastoma | 5 (17) | 0 (0) |
| Oligodendroglioma | 2 (7) | 0 (0) |
| Anaplastic oligodendroglioma | 2 (7) | 0 (0) |
| Anaplastic oligoastrocytoma | 2 (7) | 0 (0) |
| Gliomatosis | 1 (3) | 0 (0) |
| Infiltrative brainstem gliomaa | 6 (21) | 20 (95) |
| Anaplastic ependymoma | 6 (21) | 0 (0) |
| Otherb | 5 (17) | 1 (5) |
| Patients with metastatic disease (n [%]) | 5 (17) | 0 (0) |
| Reason for inclusion (n [%]) | ||
| Refractory disease | 21 (72) | — |
| Relapsing diseasec | 7 (24) | — |
| No standard therapy | 1 (3) | — |
| Patients receiving prior radiotherapy (n [%]) | 28 (97) | — |
| Median number of prior chemotherapy lines (range)d | 1 (1–5) | — |
aIn group 1, 2 were reviewed as WHO grade IV and 2 as infiltrative gliomas not otherwise specified, and 2 were not reviewed. In group 2, 2 were WHO astrocytoma grade II, 1 oligodendroglioma grade II, 1 oligoastrocytoma grade II, 1 astrocytoma grade III, 3 oligoastrocytoma grade III, 7 grade IV, and 6 infiltrative gliomas not otherwise specified.
bIncludes medulloblastoma, cerebral primitive neuroectodermal tumor, myxopapillary ependymoma, choroid plexus papilloma, choroid plexus carcinoma, and exophytic brainstem glioma (n = 1 of each).
cMedian (range) number of relapses was 2 (1–8).
dn = 26; 2 patients received radiotherapy only and 1 received no prior treatment.
Dose-finding outcomes
Group 1
No DLTs were observed at dose level 1 (n = 3) or 2 (n = 3) (Table 2). One of 3 evaluable patients at dose level 3 (125 mg/m2) experienced grade 5 intratumoral hemorrhage; no DLTs were reported in 3 additional patients. At dose level 4 (150 mg/m2), one of the 3 evaluable patients experienced grade 3 asthenia; 1 additional patient was then recruited. Two patients then experienced severe intratumoral hemorrhage after the 3-week safety-assessment period, and the dose-escalation process was stopped and de-escalated to dose level 3. Twelve additional patients were recruited at dose level 3; 2 were not evaluable for DLT due to early progression and 2 had concomitant EIAED without experiencing DLT. One of the 8 evaluable patients experienced dose-limiting grade 3 hyperbilirubinemia. Thus, the RD of single-agent erlotinib is 125 mg/m2. The probability of experiencing a DLT at this dose was 14% (95% CI: 2%–43%).
Table 2.
Dose-limiting toxicities (DLTs) possibly related to erlotinib administration in groups 1 and 2
| Erlotinib dose level (mg/m2) | Patients treated | DLT/evaluable patients | DLT |
|---|---|---|---|
| Group 1 | |||
| 75 | 3 | 0/3 | |
| 100 | 3 | 0/3 | |
| 125 | 7 | 1/6 | Grade 5 intratumoral hemorrhage (day 4) |
| 150 | 4 | 2/4 | Grade 3 asthenia (day 18) and grade 3 hemorrhage (day 29). Grade 5 intratumoral hemorrhage (day 49)a |
| Extension at 125 | 12b | 1/8 | Grade 3 hyperbilirubinemia (day 8) |
| Group 2 | |||
| 75 | 6 | 1/6 | Grade 5 seizures (day 37) |
| 100 | 6 | 0/6 | |
| 125 | 9 | 1/8 | Grade 3 folliculitis and pruritis (day 7) |
aOwing to the severity of the event, it was considered for dose-limiting toxicity.
bTwo patients with concomitant EIAED who did not experience DLT were not considered for it.
Group 2
While the first 2 patients at dose level 1 (75 mg/m2) were being evaluated for toxicity, 4 additional patients were recruited at the same level. One of these 6 evaluable patients died due to seizures/pulmonary aspiration, which was classed as a DLT. The dose was then escalated to level 2 (100 mg/m2): after evaluation of 6 patients who did not experience a DLT, the CRM methodology recommended further dose escalation. Nine patients were enrolled at dose level 3 (125 mg/m2); 1 of the 8 evaluable patients developed dose-limiting grade 3 folliculitis/pruritus. Further dose escalation was not recommended, and the trial was halted. On the basis of these observations, the RD of erlotinib in combination with radiation is 125 mg/m2. The probability of experiencing a DLT at this dose was 16% (95% CI: 4%–45%).
Treatment duration
Median treatment duration was 1.3 months in group 1 (2.7, 1.5, 1.1, and 1.5 months at dose levels 1, 2, 3, and 4, respectively) and 4 months in group 2 (3.4, 7.7, and 3.0 months at dose levels 1, 2, and 3, respectively). The relative dose intensity varied from 0.66 to 1.03, with a mean equal to 0.97. There was no significant relationship between prescribed and relative dose intensity. Only 5 patients received a mean daily dosing of <90% of the prescribed dose during the whole-treatment period. Forty patients (80%) stopped treatment due to DP, 4 due to death, 3 due to toxicity, and 1 each due to protocol violation, noncompliance, and revised diagnosis (ie, exophytic brainstem glioma). In group 2, the median duration of radiotherapy was 43 days (range: 36–49 days). In total, 37 patients had concomitant medication with steroids (21 prednisolone, 6 dexamethasone, 9 both associated, and 1 prednisone). Nine patients received anticonvulsants during the first 2 cycles; 2 of them had anticonvulsants considered as EIAED (carbamazepine and phenytoin).
Safety
Overall, 230 AEs in 44 patients were considered to be potentially related to treatment. Most were dermatologic or gastrointestinal (Table 3); 216 of 230 were grades 1 and 2. Fourteen occurring in 12 patients were grades 3–5: 9 grade 3 (asthenia, erythema, pruritus, folliculitis, surgical intervention for cyst, interstitial pneumopathy, whitlow, radiodermatitis, and vomiting), 1 grade 4 (intracranial hypertension), and 4 grade 5 (intratumoral hemorrhage possibly also related to DP [n = 2], neurologic impairment [n = 1], and seizure with pulmonary aspiration [n = 1]). Dermatologic toxicity was reported in 45 patients, including folliculitis (n = 38), dry skin (n = 29), erythema (n = 28), and pruritis (n = 16). Only 4 patients described grade 3 dermatologic symptoms. Abnormal growth of eyelashes and frizzy hair (grades 1 and 2) were observed with prolonged treatment durations of more than 3 months.
Table 3.
Treatment-related adverse events (AEs observed in >5 patients with at least 1 grade 3–5 AE reported): maximal grade of the AEs observed per patient
| Main AEs | Group 1 (29 patients) |
Group 2 (21 patients) |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | ||
| Dermatologic symptoms | |||||||||||
| Acne/rash/folliculitis | 12 | 7 | 0 | 0 | 0 | 11 | 7 | 1 | 0 | 0 | 38 |
| Dry skin | 10 | 4 | 0 | 0 | 0 | 12 | 3 | 0 | 0 | 0 | 29 |
| Erythema | 8 | 5 | 0 | 0 | 0 | 10 | 4 | 1 | 0 | 0 | 28 |
| Pruritus | 4 | 1 | 1 | 0 | 0 | 9 | 1 | 0 | 0 | 0 | 16 |
| Prolonged eyelashes | 4 | 1 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 12 |
| Hypertrichosis | 2 | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 8 |
| Alopecia | 1 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 8 |
| Dermatologic infection | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Gastrointestinal symptoms | |||||||||||
| Diarrhea | 6 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 12 |
| Vomiting | 1 | 1 | 0 | 0 | 0 | 4 | 2 | 1 | 0 | 0 | 9 |
| Nausea | 2 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 6 |
| Abdominal pain | 1 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 6 |
| Neurologic symptoms | |||||||||||
| Intratumoral hemorrhage | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| Intracranial hypertension | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Neurologic impairment | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Seizures and pulmonary aspiration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Other | |||||||||||
| Asthenia | 3 | 4 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 11 |
| Conjunctivitis | 2 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 7 |
| Interstitial pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Surgical intervention for tumoral cyst | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Efficacy
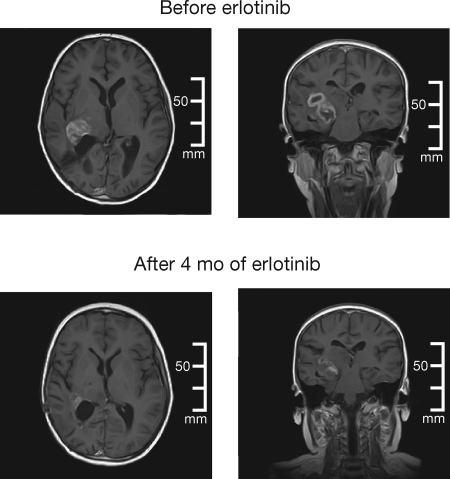
Eight of 29 evaluable patients (28%) in group 1 had stable disease (SD). Two SD patients, 1 with a malignant glioma and 1 with an anaplastic oligoastrocytoma (Fig. 1), had tumor regression of 44% and 47%, respectively, the latter having tumor stabilization for 8.8 months. Median PFS was 1.5 months, median OS was 4.1 months (95% CI: 1.9–6.8 months), and 6-month survival was 34% (95% CI: 20%–53%).
Fig. 1.
Magnetic resonance brain images in a 10-year-old boy with an anaplastic oligoastrocytoma who experienced 47% tumor regression while receiving single-agent erlotinib (at baseline [upper panels] and after 4 months of erlotinib treatment [lower panels]).
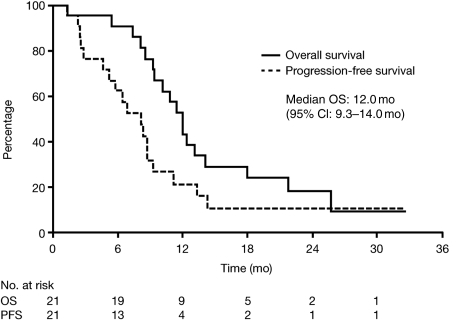
In group 2, 3 of the 18 evaluable patients (17%) had a partial response (PR) after 4 cycles of erlotinib plus radiotherapy and 9 of 18 (50%) had SD; 10 patients had tumor stabilization for ≥6 months. Analysis of best tumor response showed that 4 of the 18 patients (22%) had a PR and 8 of the 18 (44%) had SD. Median PFS was 8.0 months, median OS was 12.0 months (95% CI: 9.3–14.0 months), and 6-month survival was 90% (95% CI: 71%–97%; Fig. 2).
Fig. 2.
PFS and OS in patients with newly diagnosed brainstem glioma treated with erlotinib and radiotherapy (n = 21).
PK analyses
Erlotinib plasma concentrations were analyzed according to a one-compartment model with first-order absorption and elimination as described previously.33 Of note, the use of a 2-compartment model did not improve the goodness of fit. An additional compartment was used for analysis of the OSI-420 data. Inter-occasion variability was used for estimating specific PK parameters at cycle 1 and cycles ≥2. Among 46 evaluable patients, the daily measure of area under the plasma concentration–time curve (AUC0–24) ranged from 12.3 to 96.5 mg h/L for erlotinib and 1.0 to 14.9 mg h/L for OSI-420; the mean (95% CI) half-life of erlotinib was 16.6 hours (13.8–19.3 hours). Apparent oral clearance (CL/F) and apparent volume of distribution (Vd/F) for erlotinib and OSI-420 were independent of the dose level (Table 4). Mean (95% CI) population parameters for erlotinib were 4.0 L/h (3.4–4.5 L/h) for CL/F and 98.6 L (69.8–127.0 L) for Vd/F, whereas mean (95% CI) apparent CL/fm and Vd/fm (where fm is the fraction of erlotinib converted into OSI-420) for OSI-420 were 40.1 L/h (32.7–47.5 L/h) and 18.6 L (4.2–33.0 L), respectively. As expected, the 2 patients taking EIAED (ie, carbamazepine for 1 patient and phenytoin and carbamazepine for the other) had high values of apparent oral erlotinib clearance (9.6 and17.1 L/h, respectively).
Table 4.
Pharmacokinetic profile of erlotinib and its metabolite, OSI-420, by dose level
| Erlotinib dose level (mg/m2) | Cycle n (mean [95% CI]) | Erlotinib AUC0–24 (mg h/L) (mean [95% CI]) | Erlotinib CL/F (L/h) (mean [95% CI]) | Erlotinib Vd/F (L) (mean [95% CI]) | OSI-420 AUCm0–24 (mg h/L) (mean [95% CI]) | OSI-420 CL/fm (L/h) (mean [95% CI]) | OSI-420 Vd/fm (L) (mean [95% CI]) |
|---|---|---|---|---|---|---|---|
| 75 | 1 (n = 9) | 21.7 (17.2–26.3) | 3.5 (3.0–4.1) | 64.8 (45.9–83.8) | 2.1 (1.6–2.7) | 47.9 (36.8–59.0) | 18.1 (14.4–21.9) |
| ≥2 (n = 7) | 23.0 (14.1–31.9) | 3.8 (2.5–5.1) | 81.6 (45.7–117.5) | 1.7 (1.2–2.2) | 50.4 (34.0–56.7) | 20.2 (16.8–23.6) | |
| 100 | 1 (n = 8) | 26.8 (20.2–33.4) | 3.9 (3.1–4.8) | 85.5 (38.2–132.9) | 2.8 (0.4–5.2) | 45.1 (35.3–54.9) | 24.3 (19.6–29.2) |
| ≥2 (n = 8) | 27.5 (21.3–33.8) | 3.8 (2.9–4.8) | 107.9 (50.2–165.5) | 2.4 (1.7–3.1) | 44.9 (33.5–56.3) | 21.5 (18.6–24.4) | |
| 125 | 1 (n = 25) | 33.9 (25.6–42.1) | 5.0 (3.7–6.4) | 108.9 (85.9–132.0) | 3.6 (2.8–4.4) | 45.8 (36.7–54.9) | 19.2 (16.7–21.8) |
| ≥2 (n = 19) | 32.2 (24.6–39.8) | 5.1 (3.1–7.1) | 117.7 (87.2–148.2) | 4.2 (2.6–5.7) | 42.4 (29.7–55.0) | 18.6 (15.2–21.9) | |
| 150 | 1 (n = 4) | 42.4 (23.1–61.6) | 3.7 (1.6–5.8) | 108.3 (0–225.7) | 5.2 (0–15.6) | 38.8 (6.1–71.5) | 13.3 (6.1–20.6) |
| ≥2 (n = 4) | 40.0 (23.3–56.7) | 3.9 (1.8–6.1) | 123.1 (15.9–230.4) | 4.1 (1.5–6.6) | 41.7 (11.5–72.0) | 20.0 (17.5–22.5) |
Mean and 95% CI values are calculated from the post hoc parameter values (generated from individual predictions). n, number of patients with available data for pharmacokinetic analyses.
CL/fm, apparent metabolite clearance, where fm is the fraction of erlotinib converted into OSI-420. Vd/fm, apparent metabolite volume of distribution, where fm is the fraction of erlotinib converted into OSI-420.
Biomarker analyses
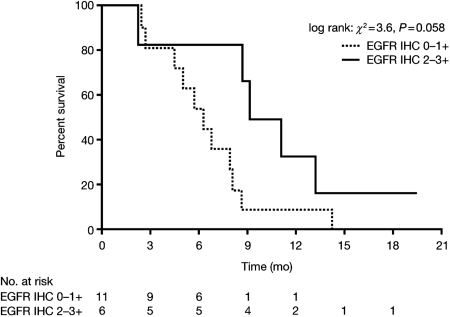
Seventeen of the 38 analyzed tumors (45%) were EGFR immunohistochemistry positive (IHC+; 9 in group 1 and 8 in group 2), and 18 of the 38 (47%) exhibited a loss of PTEN expression (5 in group 1 and 13 in group 2). High EGFR immunoexpression was most common in supratentorial gliomas (6 of 8), brainstem gliomas (8 of 20), and ependymomas (3 of 7) and was observed without EGFR gene amplification, although polysomy (3–5 copies) of the gene/chromosome 7 was found in 13 of 31 cases (3 supratentorial gliomas, 3 ependymomas, 1 medulloblastoma, and 1 brainstem glioma in group 1, and 5 brainstem gliomas in group 2). Monosomy of chromosome 7 was observed in 10 cases (3 supratentorial gliomas and 3 ependymomas in group 1 and 4 brainstem gliomas in group 2). One supratentorial glioblastoma with polysomy in 85% of cells had a gain (2/3 to 4/5) of the EGFR gene (7p11.2) in 35% of cells. Loss of PTEN was common in brainstem gliomas (total 15 of 19 samples; 2 of 2 in group 1 and 13 of 17 in group 2). EGFR immunoexpression in patients with brainstem glioma might be interpreted as marginally correlated with PFS (median of 10.1 months in EGFR IHC+ patients [n = 6] vs 6.3 months in EGFR IHC− patients [n = 11]; HR: 0.35; P = .058; Fig. 3), but not OS (HR: 0.47; P = .20). No correlation was found with outcome and PTEN loss.
Fig. 3.
EGFR overexpression by IHC (2+ and 3+) in patients with brainstem glioma might be interpreted as marginally correlated with PFS (HR: 0.35; P = .058).
Discussion
This study demonstrates that erlotinib has an acceptable tolerability profile in children with malignant brain tumors and can be combined with radiotherapy in children with newly diagnosed brainstem gliomas. Most AEs reported during treatment were mild or moderate. Dermatologic and gastrointestinal symptoms, particularly diarrhea, were the most common treatment-related AEs. These disorders are the known side effects of EGFR inhibitors, although they appear less common in children.17,36–38 As observed here, dermatologic AEs are usually grades 1 and 2 and can be managed effectively with symptomatic treatment17,36; more severe grade 3 cases can be managed with systemic antibiotics and dose reduction or treatment interruption.36,38
The RD of erlotinib in both subsets of patients was 125 mg/m2 per day, which is consistent with that in a separate phase I study of erlotinib and radiotherapy in 23 children, adolescents, and young adults (median age 10.7 years) with newly diagnosed, high-grade gliomas (120 mg/m2 per day).17 However, the dose is higher than that recommended in a phase I study of erlotinib with/without temozolomide in 46 children (median age 11.5 years) with various recurrent solid tumors, including CNS malignancies (85 mg/m2 per day).15 The difference in RD could be related to the lower age and different erlotinib formulation (tablet vs liquid) used in the present study. The RD of erlotinib was also higher than that used in adult patients with glioblastoma/malignant glioma (150–200 mg/day; administered alone or in combination with radiotherapy or chemotherapy).11–14,16 This probably relates to the lower incidence and severity of dermatologic and gastrointestinal AEs than in adult studies. Tolerability differences may be caused by hormonal changes during adolescence. When analyzing our data, we found that acne, erythema, and folliculitis occurred more frequently in adolescents (age ≥12 years) than in children (age <12 years) at each dose level. There was no significant difference in terms of either erlotinib or OSI-420 plasma concentrations between these 2 subgroups of patients. Indeed, PK parameters were correlated with body surface area, and erlotinib dose was calculated according to this morphologic parameter.
Twelve patients (24%) experienced severe or life-threatening adverse reactions, 4 of which led to death. Two of these latter resulted from intratumoral hemorrhage, 1 from a glioblastoma, and 1 from an anaplastic ependymoma. Grade 3 intratumoral hemorrhage was further observed in 1 patient with oligodendroglioma (all in group 1). Two other patients had neurologic deterioration that may have been related to the natural history of the disease rather than a reaction to the study drug. This illustrates the difficulty of assessing causality of neurologic events in the pediatric brain tumor population. Nevertheless, it might be possible that the patient who died at day 4 (who exhibited an EGFR IHC 3+ glioblastoma with a partial genomic gain within 35% of tumor cells and polysomy of the 7p chromosome) may have responded to erlotinib treatment with fulminant necrosis/bleeding rather than this being related to the rapid tumor progression. A review of 48 patients with diffuse brainstem glioma, treated at a US hospital over a 10-year period, found that symptomatic intratumoral hemorrhage occurred in nearly 20% of children within 12 months of initial diagnosis.39 Intratumoral hemorrhage was attributed to the tumor biology (necrosis), rather than the treatment received. Intratumoral bleeding has also been reported in children with brain tumors receiving gefitinib or imatinib mesylate, and this risk needs to be considered carefully when designing clinical trials with this type of agent, particularly in brain tumors.40,41
In contrast to current practice, all patients with newly diagnosed brainstem glioma underwent biopsy prior to receiving treatment. Two reports have shown that brain tumor biopsy in children is safe and associated with minimal morbidity.42,43 Importantly, biopsy allows confirmation of diagnosis and correlation of tumor biology with response. Biopsy also helped to elucidate the molecular characteristics of these tumors. In terms of safety, no cases of post-biopsy intratumoral hemorrhage were reported. These findings suggest that biopsy is both feasible and safe in patients with newly diagnosed brainstem glioma.
Erlotinib showed evidence of limited efficacy in both groups. Overall, 28% of patients in group 1 had SD, with 2 patients with malignant glioma and anaplastic oligoastrocytoma experiencing tumor regression approaching 50% with concurrent clinical improvement. Only the latter of these patients had available tumor material and exhibited high EGFR expression but no gene amplification. In group 2, the median OS of 12 months in patients with brainstem glioma compared favorably with historical values (approximately 9 months).4
The PK profiles of erlotinib and its metabolite OSI-420 were consistent with those reported previously in other pediatric studies.15,17 As also reported in these separate studies, there was wide interpatient variability in drug exposure, which increased in proportion with the administered dose and was likely due to pharmacogenetic and functional factors, together with possible PK interactions.
The exploratory analysis determined 6 of 8 supratentorial malignant gliomas and 6 of 20 brainstem gliomas with EGFR overexpression, which seems more frequent than so far observed in pediatric samples.7 However, the loss of PTEN expression was also reported in 15 of 19 brainstem gliomas. As the loss of PTEN expression may maintain activation of PI-3K/AKT pathways and has been reported to be a marker of poor survival in childhood glioma,44,45 further studies are required to investigate whether this biomarker influences the response to erlotinib. EGFR gene amplification was absent in all tumors, which is consistent with previous findings,7,46,47 although Bax et al.48 reported recently EGFR amplification in 11% of pediatric high-grade gliomas. A recent study found that 5 genes within the EGFR signaling pathway (STAT1, FKBP14, RAC1, PTGER4, and MYC) may modulate the response of adult glioblastoma to erlotinib.49 This suggests that the cross-talk of erlotinib-associated signaling pathways is complex and needs to be considered when designing new studies.
In conclusion, erlotinib has an acceptable tolerability profile in children with malignant brain tumors, including relapsing heavily pretreated patients and newly diagnosed, high-grade brainstem gliomas, although intratumoral, potentially life-threatening hemorrhage remains a substantial risk of this new therapeutic approach. The RD of erlotinib in children is higher than the RD for adults; further studies are required to define the efficacy of this treatment approach (including combining erlotinib with other targeted therapies) and to establish the impact of biomarkers (eg, by stratifying patients according to EGFR expression) on outcomes in pediatric glial tumors.
Funding
The study was funded by a grant from the Ligue Nationale Contre le Cancer within the framework of the project entitled Early Therapeutics Development in Pediatric Oncology, and F. Hoffmann–La Roche Ltd.
Acknowledgments
We thank all patients and their parents who participated in the trial, the teams of the treating European institutions, and Ms Sara Calmanti for editorial assistance.
Conflict of interest statement. None declared.
References
- 1.Hargrave DR, Zacharoulis S. Pediatric CNS tumors: current treatment and future directions. Expert Rev Neurother. 2007;7:1029–1042. doi: 10.1586/14737175.7.8.1029. [DOI] [PubMed] [Google Scholar]
- 2.Hargrave DR, Messahel B, Plowman PN. Tumours of the central nervous system. In: Pinkerton R, Plowman PN, Pieters R, editors. Paediatric Oncology. London, UK: Hodder Arnold; 2004. pp. 287–325. [Google Scholar]
- 3.Terracini B, Coebergh JW, Gatta G, et al. Childhood cancer survival in Europe: an overview. Eur J Cancer. 2001;37:810–816. doi: 10.1016/s0959-8049(01)00044-2. [DOI] [PubMed] [Google Scholar]
- 4.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 5.Gottardo NG, Gajjar A. Chemotherapy for malignant brain tumors of childhood. J Child Neurol. 2008;23:1149–1159. doi: 10.1177/0883073808321765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grill J, Bhangoo R. Recent development in chemotherapy of paediatric brain tumours. Curr Opin Oncol. 2007;19:612–615. doi: 10.1097/CCO.0b013e3282f03152. [DOI] [PubMed] [Google Scholar]
- 7.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–1792. [PubMed] [Google Scholar]
- 8.Khatua S, Peterson KM, Brown KM, et al. Overexpression of the EGFR/FKBP12/HIF-2alpha pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003;63:1865–1870. [PubMed] [Google Scholar]
- 9.Gilbertson RJ, Bentley L, Hernan R, et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8:3054–3064. [PubMed] [Google Scholar]
- 10.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 11.Krishnan S, Brown PD, Ballman KV, et al. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: results of North Central Cancer Treatment Group protocol N0177. Int J Radiat Oncol Biol Phys. 2006;65:1192–1199. doi: 10.1016/j.ijrobp.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neurooncology. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90:89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakacki RI, Hamilton M, Gilbertson RJ, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. J Clin Oncol. 2008;26:4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broniscer A, Baker SJ, Stewart CF, et al. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res. 2009;15:701–707. doi: 10.1158/1078-0432.CCR-08-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 19.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 20.Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 21.Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 22.Efferth T, Ramirez T, Gebhart E, Halatsch ME. Combination treatment of glioblastoma multiforme cell lines with the anti-malarial artesunate and the epidermal growth factor receptor tyrosine kinase inhibitor OSI-774. Biochem Pharmacol. 2004;67:1689–1700. doi: 10.1016/j.bcp.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Halatsch ME, Gehrke EE, Vougioukas VI, et al. Inverse correlation of epidermal growth factor receptor messenger RNA induction and suppression of anchorage-independent growth by OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in glioblastoma multiforme cell lines. J Neurosurg. 2004;100:523–533. doi: 10.3171/jns.2004.100.3.0523. [DOI] [PubMed] [Google Scholar]
- 24.Geoerger B, Gaspar N, Opolon P, et al. EGFR tyrosine kinase inhibition radiosensitizes and induces apoptosis in malignant glioma and childhood ependymoma xenografts. Int J Cancer. 2008;123:209–216. doi: 10.1002/ijc.23488. [DOI] [PubMed] [Google Scholar]
- 25.Meany HJ, Fox E, McCully C, et al. The plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite (OSI-420) after intravenous administration of erlotinib in non-human primates. Cancer Chemother Pharmacol. 2008;62:387–392. doi: 10.1007/s00280-007-0616-3. [DOI] [PubMed] [Google Scholar]
- 26.Broniscer A, Panetta JC, O'Shaughnessy M, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 2007;13:1511–1515. doi: 10.1158/1078-0432.CCR-06-2372. [DOI] [PubMed] [Google Scholar]
- 27.Smith M, Bernstein M, Bleyer WA, et al. Conduct of phase I trials in children with cancer. J Clin.Oncol. 1998;16:966–978. doi: 10.1200/JCO.1998.16.3.966. [DOI] [PubMed] [Google Scholar]
- 28.Ivanova A. Dose-finding in oncology—non-parametric methods. In: Ting N, editor. Dose Finding in Drug Development. New York, NY: Springer; 2006. pp. 49–56. [Google Scholar]
- 29.O'Quigley J, Shen LZ. Continual reassessment method: a likelihood approach. Biometrics. 1996;52:673–684. [PubMed] [Google Scholar]
- 30.O'Quigley J, Reiner E. A stopping rule for the continual reassessment method. Biometrika. 1998;85:741–778. [Google Scholar]
- 31.World Health Organization Geneva, Switzerland: World Health Organization; 1979. . WHO Handbook for Reporting Results of Cancer Treatment, WHO Offset Publication No. 48. [Google Scholar]
- 32.Zhao M, He P, Rudek MA, Hidalgo M, Baker SD. Specific method for determination of OSI-774 and its metabolite OSI-420 in human plasma by using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;793:413–420. doi: 10.1016/s1570-0232(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 33.Thomas F, Rochaix P, White-Koning M, et al. Population pharmacokinetics of erlotinib and its pharmacokinetic/pharmacodynamic relationships in head and neck squamous cell carcinoma. Eur J Cancer. 2009;45:2316–2323. doi: 10.1016/j.ejca.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Mizoguchi M, Betensky RA, Batchelor TT, et al. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65:1181–1188. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 35.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 36.Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008;12:283–290. doi: 10.1188/08.CJON.283-290. [DOI] [PubMed] [Google Scholar]
- 37.Tang PA, Tsao MS, Moore MJ. A review of erlotinib and its clinical use. Expert Opin Pharmacother. 2006;7:177–193. doi: 10.1517/14656566.7.2.177. [DOI] [PubMed] [Google Scholar]
- 38.TARCEVA® (erlotinib) Tablets, Oral. Full US Prescribing Information. OSI Pharmaceuticals, Inc. and Genentech, Inc. 2008 [Google Scholar]
- 39.Broniscer A, Laningham FH, Kocak M, et al. Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer. 2006;106:1364–1371. doi: 10.1002/cncr.21749. [DOI] [PubMed] [Google Scholar]
- 40.Daw NC, Furman WL, Stewart CF, et al. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2005;23:6172–6180. doi: 10.1200/JCO.2005.11.429. [DOI] [PubMed] [Google Scholar]
- 41.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium Report. Neurooncology. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pincus DW, Richter EO, Yachnis AT, et al. Brainstem stereotactic biopsy sampling in children. J Neurosurg. 2006;104((suppl 2):):108–114. doi: 10.3171/ped.2006.104.2.108. [DOI] [PubMed] [Google Scholar]
- 43.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(suppl 1):1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 44.Thorarinsdottir HK, Santi M, McCarter R, et al. Protein expression of platelet-derived growth factor receptor correlates with malignant histology and PTEN with survival in childhood gliomas. Clin Cancer Res. 2008;14:3386–3394. doi: 10.1158/1078-0432.CCR-07-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62:1118–1128. doi: 10.1093/jnen/62.11.1118. [DOI] [PubMed] [Google Scholar]
- 46.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg. 2006;105(suppl 5):418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 47.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bax DA, Gaspar N, Little SE, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res. 2009;15:5753–5761. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 49.Halatsch ME, Löw S, Hielscher T, Schmidt U, Unterberg A, Vougioukas VI. Epidermal growth factor receptor pathway gene expressions and biological response of glioblastoma multiforme cell lines to erlotinib. Anticancer Res. 2008;28:3725–3728. [PubMed] [Google Scholar]