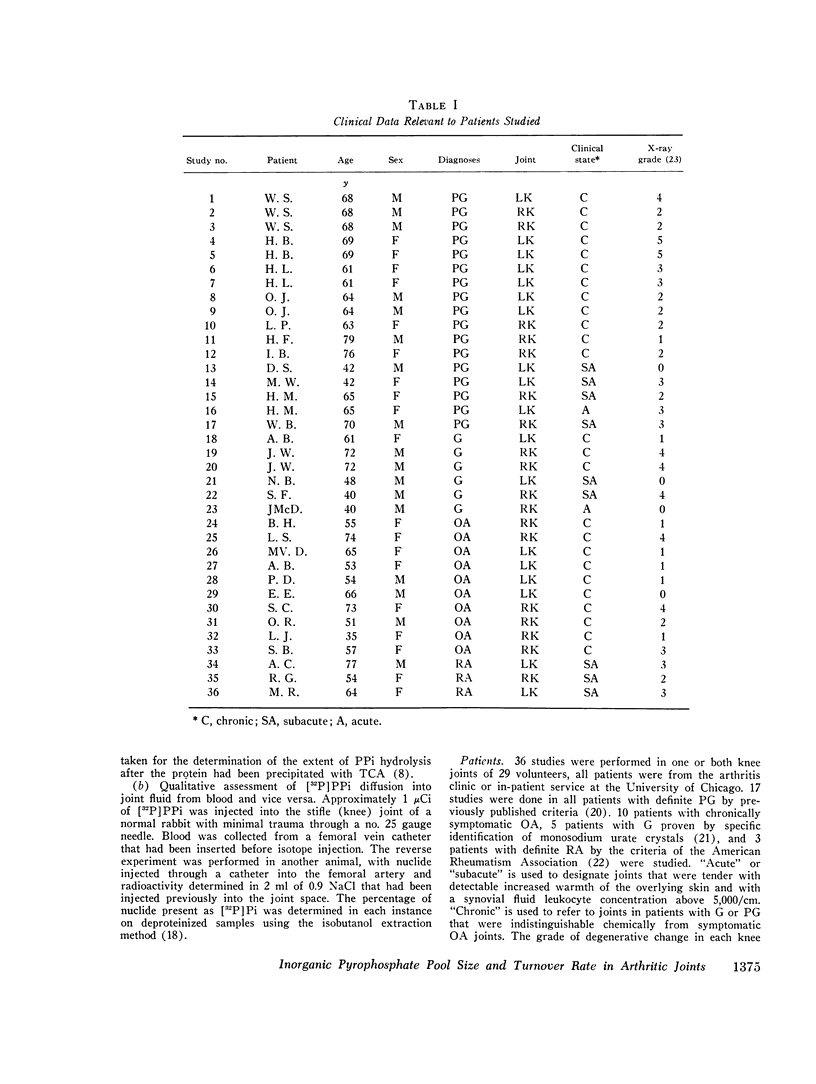
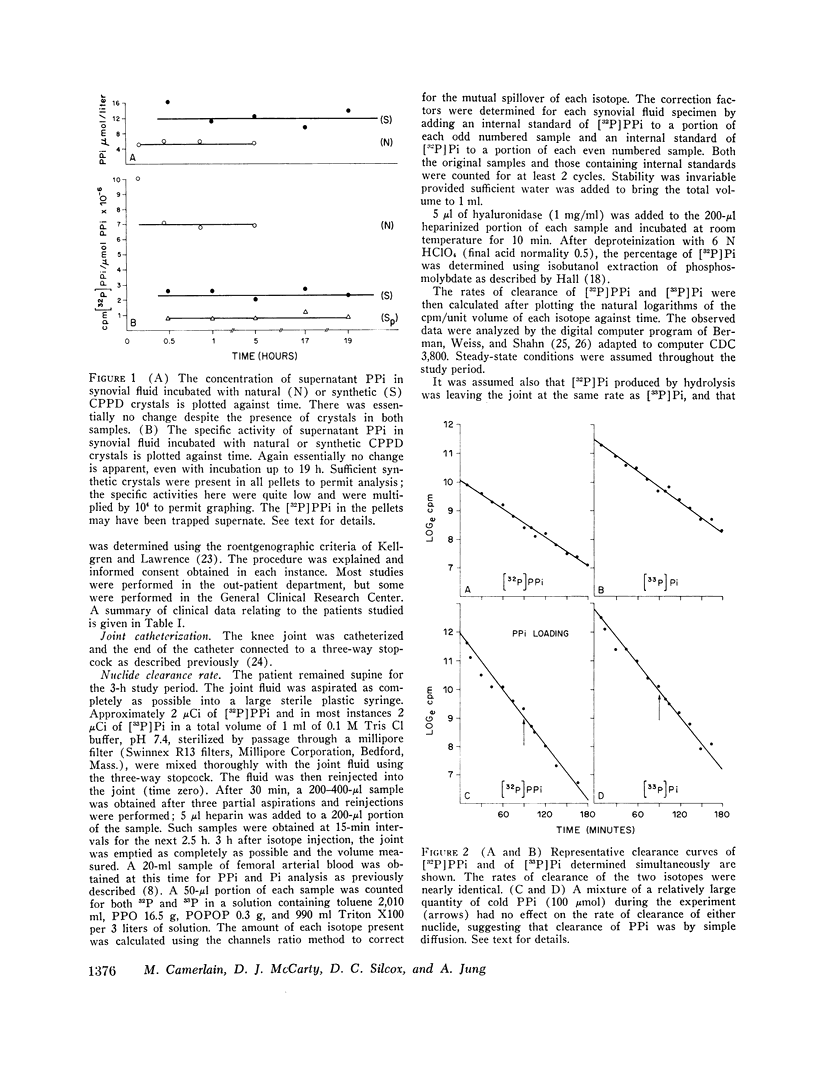
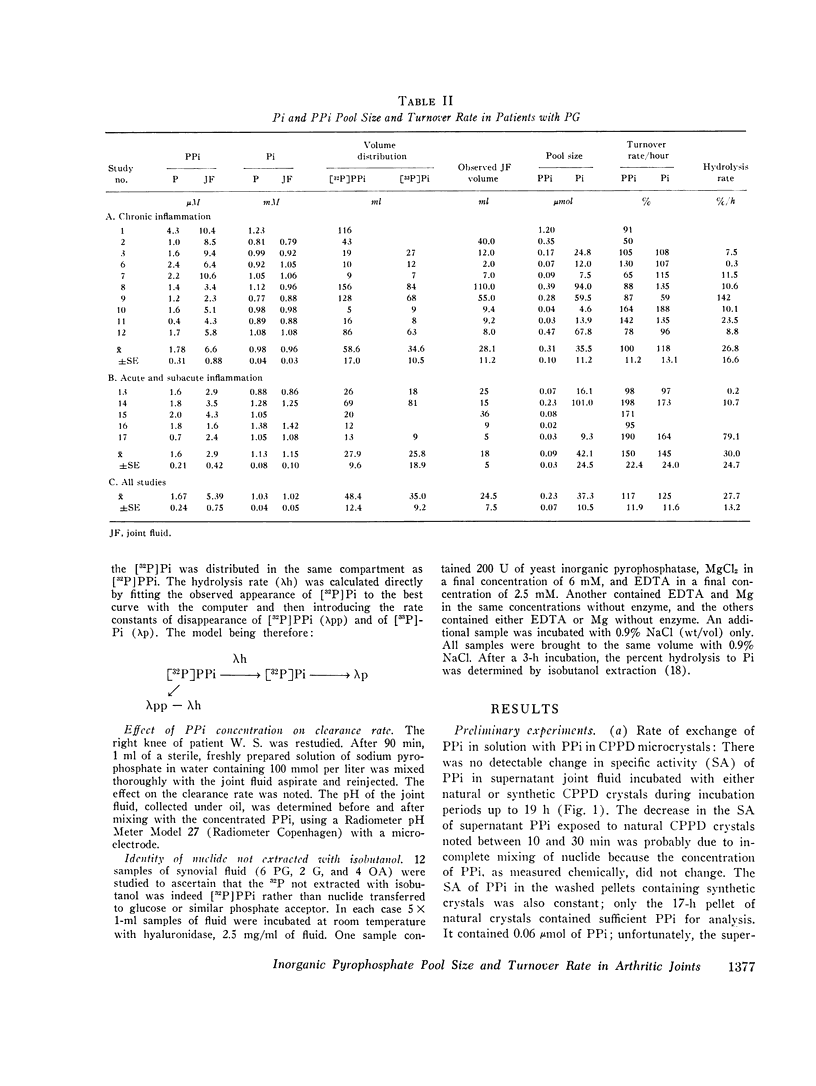
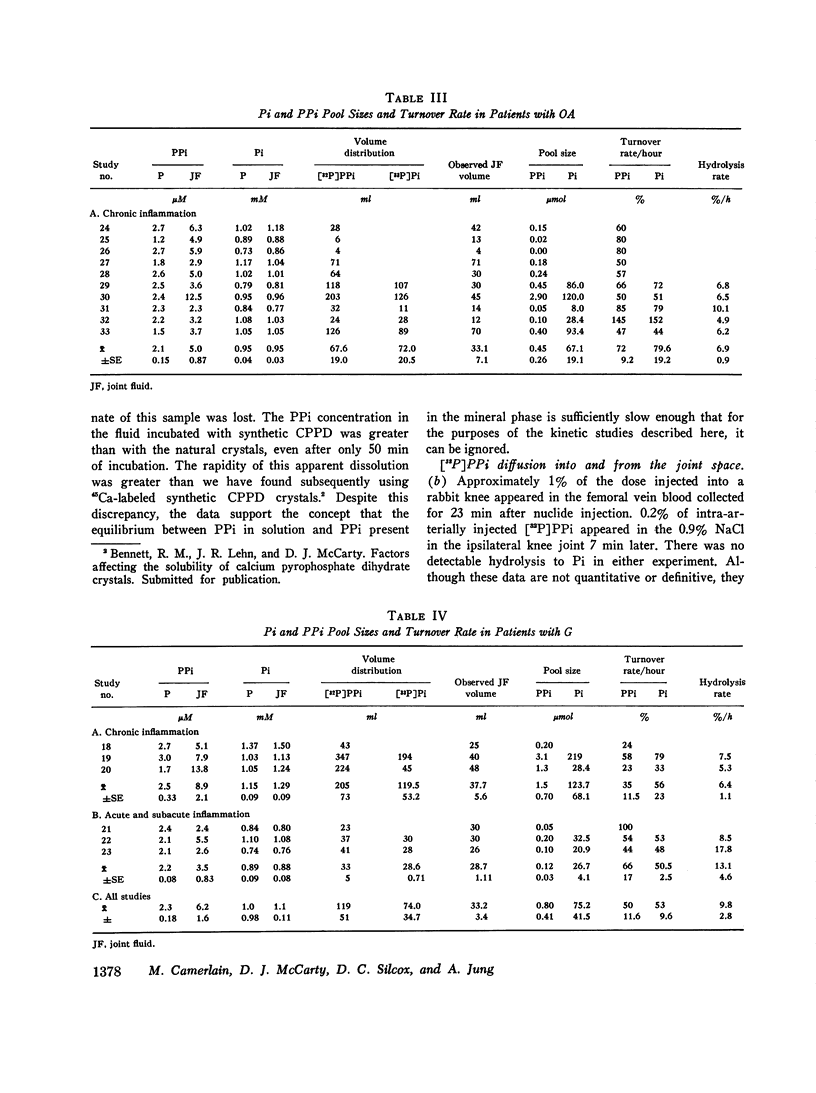
Abstract
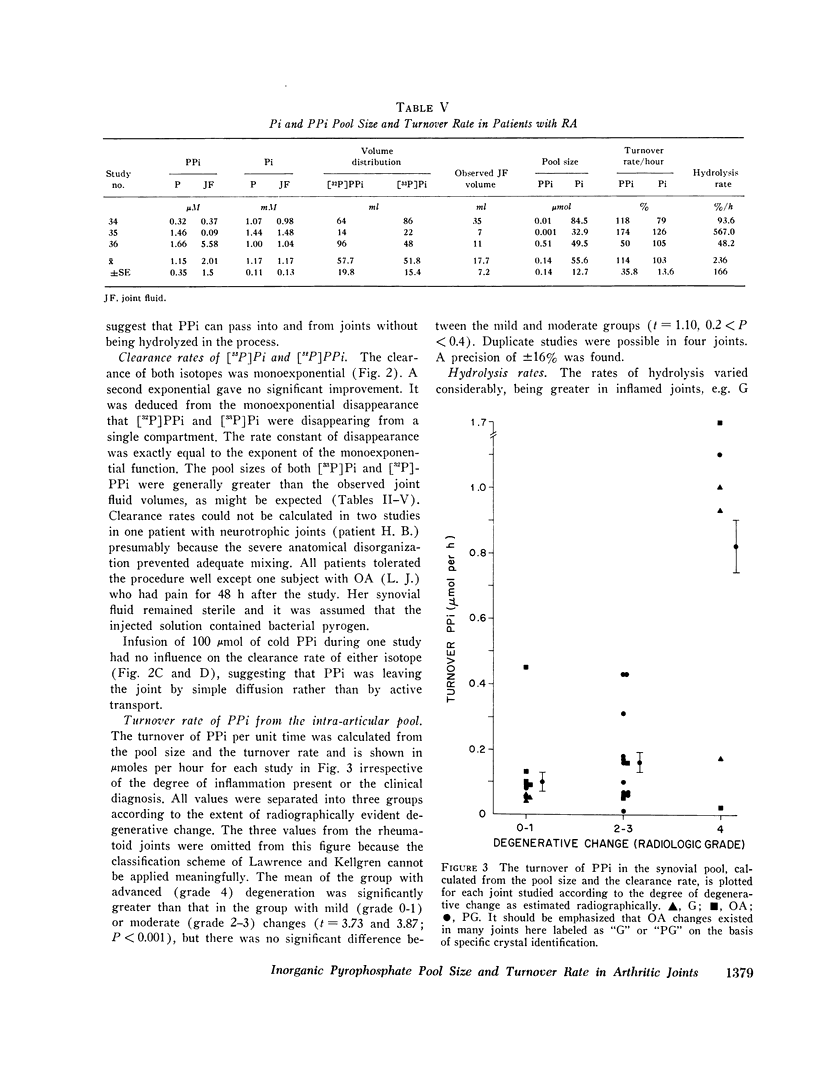
Recent studies have shown elevated inorganic pyrophosphate (PPi) levels in most knee joint fluid supernates from patients with pseudogout (PG) or osteoarthritis (OA) and more modestly elevated levels in some supernates from patients with gout or rheumatoid arthritis (RA) relative to PPi levels found in the venous blood plasma of normal or arthritic subjects. We measured the intraarticular PPi pool and its rate of turnover to better understand the significance of the joint fluid-plasma PPi gradient. Preliminary studies in rabbits showed that (32-P)PPi passed from joint space to blood and vice versa without detectable hydrolysis. Incubation of natural or synthetic calcium pyrophosphate dihydrate (CPPD) microcrystals with synovial fluid in vitro in the presence of (32P)PPi tracer showed no change in PPi specific activity in the supernate over a 19-h period so that exchange of PPi in solution with that in CPPD microcrystals could be ignored. Clearance rates of (32P)PPi and of (33P)Pi, as determined by serially sampling the catheterized knee joints of volunteers with various types of arthritis over a 3-h period, were nearly identical. The (32P)PPi/(32P)Pi was determined in each sample. A mixture of a large excess of cold PPi did not influence the clearance rate of either nuclide. The quantity of PPi turned over per hous was calculated from the pool size as determined by isotope dilution and the turnover rate. The residual joint fluid nuclide was shown to be (32P)PPi. The PPi pool was generally smaller and the rate of turnover was greater in clinically inflamed joints. The mean plus or minus SEM pool size (mu-moles) and turnover rate (percent/hour) in PG knees was 0.23 plus or minus 0.07 and 117 plus or minus 11.9, hydrolysis rate (%/h) to Pi was 27.7 plus or minus 13.2; in OA knees: 0.45 plus or minus 0.26 and 72 plus or minus 9.2, hydrolysis 6.9 plus or minus 0.9; in gouty knees: 0.8 plus or minus 0.41 and 50 plus or minus 11.6, hydrolysis 9.8 plus or minus 2.8; and in RA knees: 0.14 plus or minus 0.14 and 114 plus or minus 35.8, hydrolysis 236 plus or minus 116. PPi turnover (mumoles/hour) correlated with the degree of OA change present in the joint as graded by radiologic criteria irrespective of the clinical diagnosis. Mean PPi turnover in joints with advanced OA was greater than in those with mild or moderate changes (P smaller than 0.001), but the mild and moderate groups showed no significant difference. We conclude that synovial PPi turnover and elevated PPi fluid concentrations are not specific for PG patients, and that these factors alone cannot be the only determinants of CPPD crystal deposition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. D., Muniz O. E., Pita J. C., Howell D. S. Articular chondrocalcinosis. Microanalysis of pyrophosphate (PPi) in synovial fluid and plasma. Arthritis Rheum. 1973 Mar-Apr;16(2):171–178. doi: 10.1002/art.1780160206. [DOI] [PubMed] [Google Scholar]
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN M., WEISS M. F., SHAHN E. Some formal approaches to the analysis of kinetic data in terms of linear compartmental systems. Biophys J. 1962 May;2:289–316. doi: 10.1016/s0006-3495(62)86856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCHER J., MANKIN H. J., BERK R. N., RODNAN G. P. PREVALENCE OF CALCIFIED MENISCAL CARTILAGE IN ELDERLY PERSONS. N Engl J Med. 1965 May 27;272:1093–1097. doi: 10.1056/NEJM196505272722103. [DOI] [PubMed] [Google Scholar]
- Bywaters E. G. Calcium pyrophosphate deposits in synovial membrane. Ann Rheum Dis. 1972 May;31(3):219–221. doi: 10.1136/ard.31.3.219-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywaters E. G., Hamilton E. B., Williams R. The spine in idiopathic haemochromatosis. Ann Rheum Dis. 1971 Sep;30(5):453–465. doi: 10.1136/ard.30.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchuk K. H., Goetzl E. J., Kulka J. P. Respiratory gases of synovial fluids. An approach to synovial tissue circulatory-metabolic imbalance in rheumatoid arthritis. Am J Med. 1970 Aug;49(2):223–231. doi: 10.1016/s0002-9343(70)80078-x. [DOI] [PubMed] [Google Scholar]
- Geerards J., van der Korst J. K. Inheritance of primary articular chondrocalcinosis. Ann Rheum Dis. 1973 Jan;32(1):87–87. doi: 10.1136/ard.32.1.87-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Falchuk K. H., Zeiger L. S., Sullivan A. L., Hebert C. L., Adams J. P., Decker J. L. A physiological approach to the assessment of disease activity in rheumatoid arthritis. J Clin Invest. 1971 Jun;50(6):1167–1180. doi: 10.1172/JCI106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good A. E., Rapp R. Chondrocalcinosis of the knee with gout and rheumatoid arthritis. N Engl J Med. 1967 Aug 10;277(6):286–290. doi: 10.1056/NEJM196708102770603. [DOI] [PubMed] [Google Scholar]
- Grahame R., Sutor D. J., Mitchener M. B. Crystal deposition in hyperparathyroidism. Ann Rheum Dis. 1971 Nov;30(6):597–604. doi: 10.1136/ard.30.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL R. J. An improved method for the micro-determination of inorganic phosphate in small volumes of biological fluids. J Med Lab Technol. 1963 Apr;20:97–103. [PubMed] [Google Scholar]
- Johnson J. C., Shanoff M., Bass S. T., Boezi J. A., Hansen R. G. An enzymic method for determination of inorganic pyrophosphate and its use as an assay for RNA polymerase. Anal Biochem. 1968 Oct 10;26(1):137–145. doi: 10.1016/0003-2697(68)90037-7. [DOI] [PubMed] [Google Scholar]
- KELLGREN J. H., LAWRENCE J. S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTY D. J., HOLLANDER J. L. Identification of urate crystals in gouty synovial fluid. Ann Intern Med. 1961 Mar;54:452–460. doi: 10.7326/0003-4819-54-3-452. [DOI] [PubMed] [Google Scholar]
- MCCARTY D. J., Jr Crystal-induced inflammation; syndromes of gout and pseudogout. Geriatrics. 1963 Jun;18:467–478. [PubMed] [Google Scholar]
- MOSKOWITZ R. W., KATZ D. CHONDROCALCINOSIS COINCIDENTAL TO OTHER RHEUMATIC DISEASE. Arch Intern Med. 1965 Jun;115:680–683. doi: 10.1001/archinte.1960.03860180052009. [DOI] [PubMed] [Google Scholar]
- McCarty D. J., Solomon S. D., Warnock M. L., Paloyan E. Inorganic pyrophosphate concentrations in the synovial fluid of arthritic patients. J Lab Clin Med. 1971 Aug;78(2):216–229. [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Reginato A., Valenzuela F., Martinéz V., Passano G., Daza S. Polyarticular and familial chondrocalcinosis. Arthritis Rheum. 1970 May-Jun;13(3):197–213. doi: 10.1002/art.1780130301. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Bisaz S., Donath A., Morgan D. B., Fleisch H. Inorganic pyrophosphate in plasma in normal persons and in patients with hypophosphatasia, osteogenesis imperfecta, and other disorders of bone. J Clin Invest. 1971 May;50(5):961–969. doi: 10.1172/JCI106589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. G., Bisaz S., Fleisch H., Currey H. L., Rubinstein H. M., Dietz A. A., Boussina I., Micheli A., Fallet G. Inorganic pyrophosphate in plasma, urine, and synovial fluid of patients with pyrophosphate arthropathy (chondrocalcinosis or pseudogout). Lancet. 1970 Oct 31;2(7679):899–902. doi: 10.1016/s0140-6736(70)92070-2. [DOI] [PubMed] [Google Scholar]
- Silcox D. C., McCarty D. J., Jr Elevated inorganic pyrophosphate concentrations in synovial fluids in osteoarthritis and pseudogout. J Lab Clin Med. 1974 Apr;83(4):518–531. [PubMed] [Google Scholar]
- Silcox D. C., McCarty D. J. Measurement of inorganic pyrophosphate in biological fluids. Elevated levels in some patients with osteoarthritis, pseudogout, acromegaly, and uremia. J Clin Invest. 1973 Aug;52(8):1863–1870. doi: 10.1172/JCI107369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge R. A., Dick W. C., Bell G., Boyle J. A. Radioactive xenon (133Xe) disappearance rates from the synovial cavity of the human knee joint in normal and arthritic subjects. Ann Rheum Dis. 1968 Mar;27(2):163–166. doi: 10.1136/ard.27.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele A. D., McCarty D. J., Jr An experimental model of acute inflammation in man. Arthritis Rheum. 1966 Jun;9(3):430–442. doi: 10.1002/art.1780090308. [DOI] [PubMed] [Google Scholar]



