Abstract
Although treatment of medulloblastoma has improved, at least 30% of patients with this tumor die of progressive disease. Unfortunately, many of the children who survive suffer long-term treatment-related morbidity. Previous studies have demonstrated the efficacy of using oncolytic viruses to eradicate brain tumors. The objective of this study was to test the efficacy of measles virus in treating medulloblastoma. To determine whether medulloblastoma cells are susceptible, 5 different human medulloblastoma cell lines were analyzed for the expression of the measles virus receptor CD46. Fluorescence-activated cell-sorting analysis confirmed expression of CD46 on all cell lines tested, with UW288-1 having the most prominent expression and D283med displaying the lowest expression. CD46 expression was also demonstrated, using immunohistochemistry, in 13 of 13 medulloblastoma tissue specimens. All 5 medulloblastoma cell lines were examined for their susceptibility to measles virus killing in vitro. A measles virus containing the green fluorescent protein (GFP) gene as a marker for infection (MV-GFP) was used. All cell lines exhibited significant killing when infected with MV-GFP, all formed syncytia with infection, all showed fluorescence, and all allowed viral replicaton after infection. In an intracerebral murine xenograft model, a statistically significant increase in survival was seen in animals treated with the active measles virus compared with those treated with inactivated virus. These data demonstrate that medulloblastoma is susceptible to measles virus killing and that the virus may have a role in treating this tumor in the clinical setting.
Keywords: bioluminescence, measles virus, medulloblastoma, oncolytic virus
Brain tumors are the most common solid tumors of childhood, occurring at a rate of 2–5 cases per 100 000 annually.1 Medulloblastoma accounts for 20% of these tumors.2 Traditionally, medulloblastomas have been treated with radical surgical resection and radiation therapy, resulting in 5-year survival rates of about 60%.3,4 As the devastating effects of radiation therapy to the pediatric brain have been recognized, emphasis has been placed on decreasing the dose of radiation used and adding systemic chemotherapy.5 Although these refinements in treatment have increased the 5-year survival to close to 70%,6 there still is significant mortality and morbidity associated with the treatment. Thus, there is an urgent need to investigate alternative therapeutic approaches that are more effective and have less toxic side effects.
Measles virus, a negative strand RNA virus, kills cells by inducing the formation of multinuclear cell aggregates known as syncytia, which result from the fusion of infected cells.7 The virus enters the cell by binding of its H glycoprotein to the membrane-bound cell receptor's signaling lymphocyte activation molecule (SLAM), found predominately on lymphocytes, or CD46, found on many cell types.8–10 CD46, or membrane cofactor protein, belongs to the family of membrane-associated complement regulatory proteins that provide protection against complement-mediated lysis and is frequently overexpressed in tumors.11 In contrast to the wild-type measles virus, vaccine strains are attenuated and have a remarkable record of safety, having been administered millions of times with a significant decrease in measles incidence, morbidity, and mortality.12
Vaccine strains of measles virus have been used to kill tumor cells in a number of tumor types, including multiple myeloma, ovarian carcinoma, lymphoma, and in the adult brain tumor, glioblastoma multiforme (GBM).13–18 Interestingly, the Edmonston vaccine strain of measles virus, unlike the wild-type virus, preferentially uses CD46 rather than SLAM as a receptor.18 This preference most probably explains the efficacy of Edmonston strains in killing tumor cells, given the high level of expression of CD46 in multiple tumor types.
In this study, we have investigated the ability of a modified Edmonston's strain of measles virus to kill medulloblastoma cells in vitro and in vivo. The medulloblastoma cell lines were examined for expression of CD46, as were human medulloblastoma. In vitro killing of medulloblastoma cells from established cell lines was investigated at various multiplicities of infection (MOIs). Using an orthotopic model of medulloblastoma, in which cells were injected into the brains of immunocompromised mice, the efficacy of measles virus in prolonging the life of the injected animals was determined. We have demonstrated potent antitumor activity of the virus against medulloblastoma in vitro and in vivo. Overall, the results reported here suggest that the use of modified measles virus may represent an effective new treatment for medulloblastoma.
Materials and Methods
Cell Culture
The Vero (African green monkey) cell line and the human medulloblastoma cell lines Daoy, D283med, and D341med were obtained from the American Type Culture Collection. Human medulloblastoma lines UW288-1 and UW426 were supplied by Dr. Francis Ali-Osman (University of Washington, Seattle, WA). Cells were grown in DMEM containing 10% fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 incubator. D283med and D341med were supplemented with 20% FBS.
Production of MV
The MV-GFP virus was rescued as previously described19 and propagated in Vero cells at an MOI of 0.01 for 2 hours at 37°C. Following incubation, the medium containing unabsorbed virus was replaced with DMEM supplemented with 10% FBS. Cells were incubated for 48 hours at 37°C and then kept at 32°C for 24 hours. The presence of EGFP-positive cells was verified by fluorescence microscopy. Medium was gently aspirated, and cells were collected in Opti-MEM (Invitrogen). Virus was harvested by 2 cycles of freezing and thawing. The titer of the virus was determined by 50% tissue culture infective dose (TCID50) titration on Vero cells.17
Determination of CD46 Expression by Flow Cytometry
The cells were harvested, washed in PBS containing 2% BSA, and then incubated with FITC-labeled mouse anti-human CD46 (PharMingen). The washed cells were fixed in PBS containing 0.5% paraformaldehyde and analyzed on a Becton Dickinson FACScan Plus cytometer. Analysis was performed using the CellQuest software (BD Biosciences).
Immunohistochemistry
Human brain sections were obtained from the Mayo Tissue Registry according to an IRB approved protocol. Each paraffin section was treated by incubation twice in xylene for 5 minutes and then placed through an ethanol gradient (100, 95, 80, 70, and 60%) for 5 minutes. The sections were then placed in 10 mmol/L of citrate buffer (pH 6.0) and microwaved twice for 2 minutes to improve staining by antigen unmasking. After the sections were washed and quenched of endogenous peroxidases, they were blocked and incubated with CD46 monoclonal antibody (1:750; Immunotech) overnight at 4°C. An immunoperoxidase procedure (Vectastain Elite ABC kit; Vector Laboratories) was carried out to visualize the peroxidase by reaction with diaminobenzidine and hydrogen peroxidase (Vector Laboratories) and counterstained with hematoxylin. Controls in which the primary antibody was omitted revealed no labeling.
Assessment of Cytopathic Effect In Vitro
Cells were plated in 6-well plates at a density of 1 × 105 cells per well. Twenty-four hours after seeding, the cells were infected with MV-GFP in quadruplicate at different MOIs from 0.1, 1, and 10 in 1 mL of Opti-MEM for 2 h at 37°C. Each MOI was performed in triplicate wells. At the end of the incubation period, the virus was removed and the cells were maintained in DMEM containing 10% FBS. The same number of uninfected cells in 6-well plates was used as controls. The number of viable cells in each well was counted using a hemocytometer at 2, 3, 5, and 7 days after infection. Viable cells were identified using trypan blue exclusion. The percentage of surviving cells was calculated by dividing the number of viable cells in the infected well by the number of viable cells in the uninfected well corresponding to the same time point. Infection was confirmed using fluorescent microscopy at the corresponding time points.
Generation of D283med Tumor Cells Stably Expressing Firefly Luciferase
D283med tumor cells were transduced with a lentiviral vector containing the firefly luciferase (fluc) gene under the control of a cytomegalovirus (CMV) promoter. Lentiviruses were obtained from SABiosciences as ready-to-transduce, replication incompetent, HIV-based, and VSV-G pseudotyped lentiviral particles. Cells were transduced at an MOI of 10 along with SureENTRY (SABiosciences) transduction reagent (4 µg/mL). Four days posttransduction, growth medium was supplemented with puromycin (1 µg/mL). Expression of Fluc was confirmed by measuring cellular luciferase activity (VICTOR3; Perkin Elmer).
Intracerebral In Vivo Tumor Model
D283med cells were injected into the right caudate putamen of 5-week-old Hsd:Athymic Nude-Foxn1nu mice (Harlan Laboratories) or 5-week-old cb17icr SCID mice (Charles River) using the small animal stereotactic frame (David Kopf Instruments). SCID mice were given 150 cGy total body irradiation 24 h prior to tumor implantation, using the small animal stereotactic frame (David Kopf Instruments). The injection coordinates were 2 mm to the right of bregma and 3 mm below the skull surface. Cells (1 × 106 per mouse) in PBS were injected over 10 min using a 26-gauge Hamilton syringe under isoflurane gas anesthesia. Prior to treatment, the mice were randomly divided into the following treatment groups: untreated, intratumoral (IT) administration of MV-GFP, and IT administration of UV-inactivated MV-GFP. Treatment was initiated 7 days after tumor implantation by IT injection using the same coordinates on the stereotactic frame as those used for implantation. The treatments were repeated every other day for a total of 5 doses (1 × 106 TCID50). The animals were euthanized if they developed neurologic deficits such as hemiparesis or lethargy. At the time of necropsy, brains were collected, fixed overnight with 10% formalin, paraffin embedded, cut into 5 µM tissue sections, and stained with H&E. All animal experiments were approved by the Nationwide Children's Hospital Institutional Animal Care and Use Committee.
In Vivo Bioluminescence Imaging
Bioluminescence imaging studies were conducted prior to each MV-GFP injection, then weekly thereafter using the Xenogen Ivis Spectrum (Caliper Life Sciences). Animals received an intraperitoneal injection of 4.5 µg Xenolight Rediject D-Luciferin (Caliper Life Sciences) and were continuously maintained under isoflurane gas anesthesia. Images were obtained 20 minutes after luciferin administration. The bioluminescence intensity was quantified using Living Image Software (version 3.1; Caliper). Signal intensity was quantified as the sum of detected photons per second within the region of interest.
Statistical Analysis
Survival curves were generated using the Kaplan–Meier method. The survival of mice in the different treatment groups was compared using the log-rank test. P < 0.05 was considered statistically significant. All statistical analyses were performed using Microsoft Office Excel (v.11.6560.6568 SP2) in Data Analysis using regression or paired 2-sample Student's t-test for means. Probabilities for the Student's t-test are listed as “P(T ≤ t) 2-tail” with an α of 0.05.
Results
Medulloblastoma Cell Lines and Clinical Specimens Express CD46
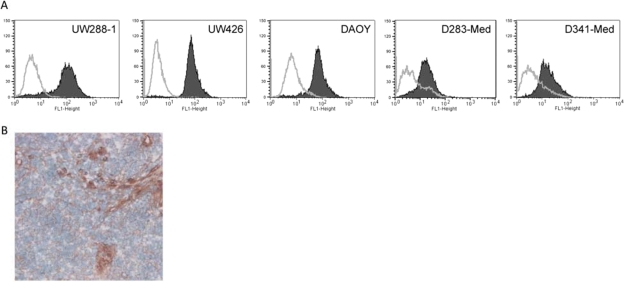
Measles virus utilizes the cell surface receptor CD46 to gain entry into susceptible cells. To determine whether medulloblastoma cells are susceptible, 5 different human medulloblastoma cell lines were analyzed for CD46 expression. Fluorescence-activated cell-sorting analysis confirmed expression of CD46 on all cell lines tested, with UW288-1 having the most prominent expression and D283med displaying the lowest expression (Fig. 1A). To determine the feasibility of killing medulloblastoma in patients, we examined a series of 12 newly diagnosed, primary medulloblastoma resection specimens for expression of CD46 using immunohistochemistry. All but 1 specimen was labeled with the anti-CD46 antibody, with the label localizing to the cell membrane (Fig. 1B). In all positive clinical samples, >95% of the tumor cells were CD46 positive as demonstrated by heavy labeling. Examination of normal brain specimens showed no CD46 expression (data not shown).
Fig. 1.
CD46 expression in medulloblastoma cell lines and tissue samples. (A) CD46 is highly expressed on the cell surface of medulloblastoma cells. Cells were stained with an anti-CD46 PE antibody (gray histogram) or an isotype control (black histogram) and analyzed by flow cytometry. (B) Immunohistochemical detection of CD46 in a medulloblastoma patient sample.
Measles Virus Replicates and Causes Significant CPE in Medulloblastoma Cell Lines In Vitro
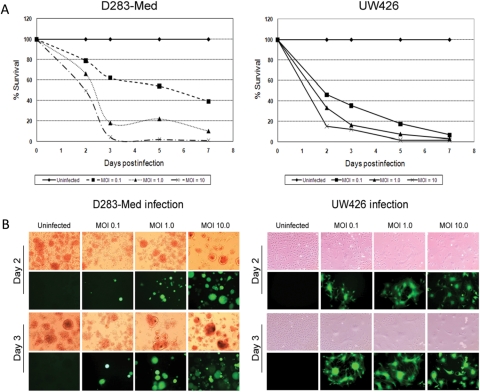
All 5 medulloblastoma cell lines were examined for their susceptibility to measles virus killing in vitro. A measles virus containing the GFP gene as a marker for infection (MV-GFP) was used. All cell lines exhibited significant killing when infected with MV-GFP, each displaying the presence of green fluorescent syncytia. Representative cell lines D283med and UW426 are demonstrated in Fig. 2. The killing of cells was time and dose dependent. When an MOI of 1 was used, all cell lines tested exhibited >80% cell death at 72 h. All cell lines had >90% cell death at 120 h when an initial MOI of 10 was used. As shown in Fig. 3, the significant syncytia formation and cytopathic effect demonstrates that MV-GFP infects and replicates efficiently in D283med and UW426.
Fig. 2.
Cytopathic effect of MV-GFP. (A) The viability of D283 and UW426 human medulloblastoma cells following administration of MV-GFP at an MOI of 0.1, 1, and 10.0. Less than 20% of the cells were viable at 72 h postinfection at an MOI of 1, and <1% of the cells were viable at 120 h postinfection at an MOI of 10. (B) The medulloblastoma cell line D283med was infected with MV-GFP at an MOI of 0.1, 1, and 10, and showed GFP expression and syncytium formation 48 and 72 h postinfection. The experiment was performed in quadruplicate, and a second independent experiment displayed similar results.
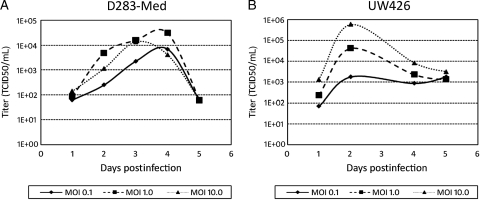
Fig. 3.
Replication of MV-GFP in D283med (A) and UW426 (B) cells is demonstrated by the titers obtained daily for 5 days at an MOI of 0.1, 1, and 10. Titration was done on Vero cells in a 96-well plate using the 50% end-dilution method.
To determine whether MV-GFP could replicate in medulloblastoma cells, titers of the virus produced from these cells were measured daily after infection. As shown in Fig. 4, D283med and UW426 supported replication of MV-GFP. Virus titers peaked 2 days postinfection in UW426 cells when an MOI of 0.1, 1, and 10 was used. Conversely, virus titers produced from D283med cells peaked at either 3 days when an initial MOI of 0.1 and 10 was used or 4 days when an initial MOI of 1.0 was used.
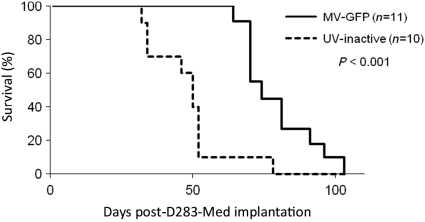
Fig. 4.
Antitumor effect of MV-GFP. D283 medulloblastomas were established in the right frontal lobe of female SCID mice (10–11 mice/group). Seven days after tumor implantation, the mice received a total of 107 pfu of MV-GFP or the same dose of UV inactivated MV-GFP. Mice treated with MV-GFP had significantly longer survival times than mice that received UV-inactivated MV-GFP (P < 0.01). A second independent experiment also demonstrated significant (P < 0.001) survival times of mice treated with MV-GFP when compared with mice that received UV-inactivated MV-GFP (data not shown).
Intratumoral Administration of Measles Virus Increases Survival of D283med Tumor-Bearing Mice
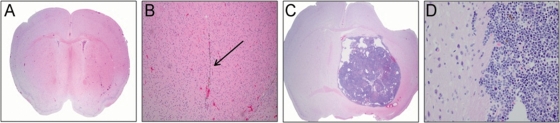
To determine the efficacy of MV-GFP in vivo, we established intracerebral D283med xenografts in athymic nude mice or whole-body irradiated SCID mice. D283med cells were stereotaxically injected into the right caudate putamen to establish tumors. Seven days following tumor injection, MV-GFP was injected intratumorally every other day for 10 days. Animals were monitored to assess survival. There was statistically significant prolongation of survival in MV-GFP–treated mice compared with UV-inactivated virus-treated controls (P > 0.001, athymic nude [Fig. 4]; P = 0.02, SCID [data not shown]). Upon pathological examination of the SCID brains, 8 of the 12 MV-GFP–treated animals revealed no signs of viable tumor cells (Fig. 5A). However, in these animals, there was evidence of the needle track, and it was typically associated with gliosis and hemosiderin-laden macrophages (Fig. 5B). The remaining 4 animals had evidence of tumor in the subarachnoid space, but there was no presence of tumor cells at the injection site. Pathological examination of the athymic nude animals revealed a similar tumor histology and spread (data not shown). In 8 of 11 MV-GFP–treated athymic nude animals, tumor cells were not detected at the injection site. However, tumor cells were observed in the subarachnoid space. Conversely, the brains of athymic nude and SCID animals treated with UV-inactivated MV-GFP contained large destructive tumors with mass effect (Fig. 5C). Leptomengeal spread was seen in many cases, which was not observed in the treated xenografts. Tumor cells were infiltrative into the adjacent brain (Fig. 5D) and were frequently mitotically active. Because the cause of death in animals treated with measles virus did not appear to be due to tumor progression or tumor dissemination, based on the absence of tumor in the brain or subarachnoid space, we attempted to determine the cause by examining other tissues in the animals at autopsy. We were unable to find any abnormalities in the lungs, heart, liver, kidneys, or GI tract of treated animals, so an underlying anatomic cause of death could not be determined.
Fig. 5.
Histological examination (H&E stain) of SCID brain following administration of MV-GFP (A and B) or UV-inactivated MV-GFP (C and D). There is no evidence of tumor in the MV-GFP treated animal (A, ×20 and B, ×100). The remnant of the needle tract (arrow; B, ×100) with associated gliosis and hemosiderin-laden macrophages is seen in the MV-GFP-treated animal. In a mouse treated with UV-inactivated MV-GFP, there is significant tumor growth and mass effect (C, ×20) with tumor infiltration into the adjacent normal brain (D, ×400).
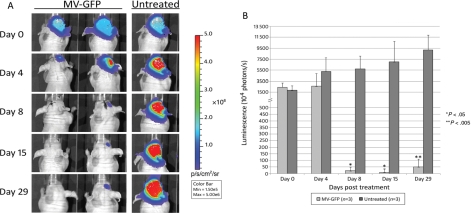
To facilitate noninvasive monitoring of intracerebral tumor growth and treatment response, D283med cells were engineered to stably express firefly luciferase (Fluc) (D283luc). Stereotaxic tumor implantation into athymic nude nice was confirmed in all animals by assessing a bioluminescent imaging signal 1 week after tumor implantation (Fig. 6A). Upon confirmation of successful tumor implantation, animals were randomized into 2 groups of 3 animals each. Animals were imaged prior to each MV-GFP treatment then weekly thereafter. Quantification of photons released by D283luc-implanted tumors demonstrated that mice treated with MV-GFP exhibited a significant decrease in tumor burden compared with untreated animals (Fig. 6B). One mouse treated with MV-GFP displayed complete tumor abolition 8 days after the initial MV-GFP administration (Fig. 6A), and 1 mouse had tumor aboliton by day 15. There was no re-occurrence of the tumor in the brain of either animal when they were examined microscopically after being euthanized on day 29. One additional animal treated with MV-GFP displayed significant tumor regression, peaking at 15 days after the initial MV-GFP administration (Fig. 6A). However, tumor burden increased thereafter in this animal as assessed by bioluminescent signal. When this animal was euthanized, microscopic examination of the brain revealed 2 small tumor deposits, 1 in the needle track and 1 in the meninges near the entry point of the needle into the brain. In those animals not treated with MV-GFP, the tumor burden as assessed by bioluminescent signal increased over time (Fig. 6A). When these animals were euthanized following bioluminescent imaging on day 29, pathological evaluation of their brains revealed large tumor masses at the injection site.
Fig. 6.
In vivo evaluation of antitumor activity of MV-GFP. (A) Bioluminescence images and corresponding (B) signal intensities obtained from mice containing D283Luc tumors. Animals were implanted with D283Luc cells 7 days prior to treatment. MV-GFP treatment was administered on days 0, 2, 4, 6, and 8. The bioluminescence signal was significantly decreased in MV-GFP–treated animals whereas the signal significantly increased in the untreated animals. (A) The MV-GFP–treated animal on the left exhibits complete tumor abolition, whereas the animal on the right exhibits significant regression (day 15), followed by an increase in tumor burden (day 29). Tumor regression was also observed in a third animal in the MV-GFP–treated group (animal not shown). Two additional untreated animals exhibited significant tumor increase (animals not shown).
Discussion
The results presented here demonstrate that established medulloblastoma cell lines express the measles receptor CD46. Importantly, we have also shown that medulloblastoma specimens removed from patients have high levels of CD46 expression. Having demonstrated receptor expression, experiments were then performed that revealed significant antitumor activity of the modified measles virus against medulloblastoma cell lines in vitro. Cell killing was accompanied, as expected, by syncytia formation. The cytotoxic effect was complete in 72 h. In addition, replication of the virus in the tumor cells was documented. Killing was seen at MOI as low as 0.1, and essentially complete killing was seen at an MOI of 1.0. Of note, killing of D283med, a suspension cell line, was documented at similar MOIs. This finding may have relevance to potential use of the modified measles virus for the treatment of tumor disseminated in the CSF, a possibility currently being explored in our laboratory.
The data presented here also show that modified measles virus significantly prolongs survival of measles-treated animals in an orthotopic model of medulloblastoma. While none of the animals survived long-term, we believe that the prolongation of survival is promising. Interestingly, when SCID-treated animals were autopsied, no tumor was found in their brains. Indeed, we were unable to determine the cause of death in these animals, despite histological examination of the lungs, heart, liver, kidneys, and GI tract, in addition to the brain. Upon consultation with a radiation oncologist, it was suggested that the whole-body irradiation given to the mice prior to tumor implantation may be the cause of the animal's premature death. Therefore, subsequent studies, including bioluminescent imaging, were performed in nonirradiated athymic nude mice.
Bioluminescent imaging analysis enhanced our ability to observe tumor growth and tumor regression following MV-GFP administration in vivo without having to sacrifice the animal. Pathological review of the animals confirmed the bioluminescent images in that 2 of the animals were free of tumor and the third had a very small amount of residual tumor. The complete abolition of tumor in 2 mice differed from the initial athymic nude mice study. In that study, all animals exhibited residual tumor at the time of pathological examination, although in 8 out of 11 mice the primary tumor was eradicated. It is highly plausible that tumor cells were able to gain access, either through direct injection or through infiltration, to the subarachnoid space via the right lateral ventricle before MV-GFP therapy was administered. It is our experience that when tumor cells gain access to the cerebral spinal fluid and the subarachnoid space, therapeutic efficacy is more challenging. MV therapy could be applied to the tumor bed following surgical resection to target microscopic residual disease. This approach potentially could ameliorate the need for radiation and chemotherapy. In the future, bioluminescent imaging analysis will allow us to better evaluate the effectiveness of our treatment regimen and perhaps tailor a better treatment schedule.
The virus used in these experiments is replication competent. We have demonstrated that virus is produced by infected cells and that infected cells express GFP, encoded by the infecting virus. Replication competent viruses have potential advantages over replication incompetent viruses. For example, in a trial of intrathecal administration of a nonreplicating p53 adenovirus, transgene expression was found only a short distance (5–8 mm) from the injection site, indicating that viral spread is very limited when the virus does not replicate.8 The lack of robust viral spread has profound implications for the treatment of brain tumors, where individual cells may migrate into the brain, away from the main tumor mass. Hopefully, the use of replicating virus will allow further spread of virus through the tumor.
The safety of vaccine strains of measles viruses in humans is well established. The effect of intracerebral injection of the virus in nonhuman primates has also been investigated. In the early 1970s, Albrecht et al20 found no clinical signs of encephalitis in rhesus monkeys injected with low passage Edmonston's strain. In 4 of 12 animals, virus could be isolated from Vero cells cocultured with brain sections for injected animals. No histological evidence of active measles infection, such as intranuclear inclusions or syncytia, could be found in any of the animals. Similarly, injection of the Edmonston strain virus into the thalamus or CSF via cisternal injection of grivet monkeys or cynomologuous monkeys had no clinical toxicity.21 The pathological findings in these monkeys, gliosis and inflammatory infiltrate, were no different from that seen in vehicle-only–injected animals. We have studied the effect of repeated injections of the Edmonston strain in rhesus monkeys previously vaccinated with the virus.22 In these experiments, the animals were extensively evaluated for virus and symptoms systemically and in the central nervous system. With 30 months of follow-up, no animal showed signs or symptoms of viral infection. No biochemical evidence of infection could be detected. Virus could not be recovered from the CSF, blood, or bucchal swabs. MR imaging at 4–5 months after injection showed no evidence of encephalitis. Taken together, these results strongly suggest that intracerebral injection of measles is safe.
A modified Edmonston strain measles virus has been investigated as a potential therapeutic agent for a number of tumor types. In particular, Galanis and co-workers7 have shown that the virus is effective against orthotopic models of GBM. In one study, 6 of 10 mice treated with modified measles virus survived more than 70 days compared with none of 8 surviving past 50 days when treated with UV-inactivated virus (P = 0.02).17,23,24 These studies have led to the opening of a clinical trial using modified virus for the treatment of adult patients with recurrent GBM. The results presented here differ from those seen in the GBM model in that all of the treated mice in these experiments died. Only one of the treated mice had detectable tumor on histological examination, suggesting that the virus is very effective against the tumor.
Other oncolytic viruses have been explored as possible treatment modalities for medulloblastoma. Lun et al28 reported on the effects of treatment with reovirus in vitro and in an orthotopic model. Reovirus requires activated Ras protein for tumor cell killing. Five of 7 medulloblastoma cells lines were susceptible to killing by reovirus in vitro, and, as expected, susceptibility was related to the level of activated Ras expression in the tumor cells. Similarly, these investigators demonstrated an increased survival in animals injected with Daoy into the cerebellum and cerebrum and subsequently treated with a single or multiple injections of reovirus into the same site. These investigators used Daoy in their in vivo studies because the cell line was the most sensitive to reovirus killing in vitro. We chose to use D283med in the in vivo experiments presented here because this cell line had lower levels of measles virus receptor expression when compared with other medulloblastoma lines tested. In addition, the molecular biology of Daoy is not typical of medulloblastoma, as the cell line is wild-type p53 null and has homozygous deletion of p16.25–27 Because all surgical medulloblastoma specimens expressed the measles virus receptor, measles virus may have some advantages over reovirus, where some tumors may be resistant to cell killing.
In a previous study the use of myxoma virus as a therapeutic agent for medulloblastoma was explored.28 In that study 9 of 10 medulloblastoma cell lines were effectively killed in vitro by myxoma virus. Similarly, they found that treatment of medulloblastoma with myxoma virus in an orthotopic murine xenograft model resulted in a significant prolongation of survival. Because sensitivity to myxoma virus seems related to activated Akt expression and because medulloblastomas frequently contain activated Akt, this virus has promise as a potential therapy. Indeed, concurrent treatment of tumor-bearing animals with rapamycin and myxoma virus resulted in a better survival than seen with drug or virus alone.
One major impediment to the successful use of MV as an anticancer therapy is preexisting antiviral antibodies in immunized (essentially all) patients. However, we do not believe that neutralizing antibodies to MV will have a major effect on the clinical applicability of a single dose of virus for the treatment of medulloblastoma, as the antiviral immune response will take a longer time to become active than the time it takes for the virus to kill the cells. Additionally, in peripheral regions of the tumor, the blood-brain barrier is intact, which is not permeable to antibodies. In a study of the effect of 2 injections of measles virus 1 week apart in immunized macaques, no evidence of brain toxicity was noted.22 In a study evaluating MV antibody status in children with cancer, the findings suggested that cancer and its associated therapy interfered with antibody production in these children.29 However, if multiple episodes of MV treatment are necessary, novel approaches have recently been developed to address this issue. Cell-based carriers, such as mesenchymal stem cells, have been evaluated and are currently being used to deliver MV to tumor cells while protecting them from antibody neutralization.30 If the efficacy of MV therapy is determined to be insufficient due to neutralizing antibodies, then a cell carrier may be an option to circumvent the antibody response.
In summary, we have demonstrated that a modified measles virus has therapeutic potential in the treatment of intracerebral medulloblastoma. These results provide initial data to be pursued with additional studies with the goal of using the virus in a clinical trial for the treatment of medulloblastoma.
Conflict of interest statement. None declared.
References
- 1.Bleyer WA. The impact in the United States and the world of central nervous system cancer during childhood. In: Packer RJ, Bleyer WA, Pochedly C, editors. Pediatric Neuro-oncology New Trends in Clinical Research. Vol. 3. Philadelphia, PA: Harwood Academic Publishers; 1992. pp. 1–7. [Google Scholar]
- 2.Humphreys R. Posterior cranial fossa brain tumors in children. In: Youmans JR, editor. Neurological Surgery. Philadelphia, PA: WB Saunders; 1982. pp. 2733–2752. [Google Scholar]
- 3.Allen JC, Bloom J, Ertel I, et al. Brain tumors in children: current cooperative and institutional chemotherapy trials in newly diagnosed and recurrent disease. Semin Oncol. 1986;13:110–122. [PubMed] [Google Scholar]
- 4.Hughes EN, Shillito J, Sallan SE, et al. Medulloblastoma at the joint center for radiation therapy between 1968 and 1984. The influence of radiation dose on the patterns of failure and survival. Cancer. 1988;61:1992–1998. doi: 10.1002/1097-0142(19880515)61:10<1992::aid-cncr2820611011>3.0.co;2-j. doi:10.1002/1097-0142(19880515)61:10<1992::AID-CNCR2820611011>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707–713. doi: 10.3171/jns.1989.70.5.0707. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Sutton LN, Goldwein JW, et al. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg. 1991;74:433–440. doi: 10.3171/jns.1991.74.3.0433. [DOI] [PubMed] [Google Scholar]
- 7.Galanis E, Bateman A, Johnson K, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12:811–821. doi: 10.1089/104303401750148766. doi:10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 8.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. doi:10.1200/JCO.2003.11.138. [DOI] [PubMed] [Google Scholar]
- 9.Wild TF, Malvoisin E, Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72:439–442. doi: 10.1099/0022-1317-72-2-439. doi:10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- 10.Tatsuo H, Ono N, Tanaka K, et al. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 11.Dorig RE, Marcil A, Chopra A, et al. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. doi:10.1016/0092-8674(93)80071-L. [DOI] [PubMed] [Google Scholar]
- 12.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurianz K, Ziegler S, Garcia-Schuler H, et al. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–939. doi: 10.1016/s0161-5890(99)00115-7. doi:10.1016/S0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 14.Cutts FT, Markowitz LE. Successes and failures in measles control. J Infect Dis. 1994;170(suppl.):S32–S41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 15.Grote D, Russell SJ, Cornu TI, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. doi:10.1182/blood.V97.12.3746. [DOI] [PubMed] [Google Scholar]
- 16.Peng KW, Ahmann GJ, Pham L, et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. doi:10.1182/blood.V98.7.2002. [DOI] [PubMed] [Google Scholar]
- 17.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 18.Peng KW, TenEyck CJ, Galanis E, et al. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 19.Duprex WP, McQuaid S, Hangartner L, et al. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albrecht P, Shabo AL, Burns GR, et al. Experimental measles encephalitis in normal and cyclophosphamide-treated rhesus monkeys. J Infect Dis. 1972;126:154–161. doi: 10.1093/infdis/126.2.154. [DOI] [PubMed] [Google Scholar]
- 21.Buynak EBPH, Creamer VMD, Goldner H, Hilleman MR. Differentiation of virulent from avirulent measles strains. Am J Dis Child. 1962;103:460–473. [Google Scholar]
- 22.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19:690–698. doi: 10.1089/hum.2008.035. doi:10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen C, Vongpunsawad S, Nakamura T, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. doi:10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 24.Paraskevakou G, Allen C, Nakamura T, et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- 25.Raffel C, Thomas GA, Tishler DM, et al. Absence of p53 mutations in childhood central nervous system primitive neuroectodermal tumors. Neurosurgery. 1993;33:301–305. doi: 10.1227/00006123-199308000-00018. discussion 305–306. [DOI] [PubMed] [Google Scholar]
- 26.Raffel C, Ueki K, Harsh GRT, et al. The multiple tumor suppressor 1/cyclin-dependent kinase inhibitor 2 gene in human central nervous system primitive neuroectodermal tumor. Neurosurgery. 1995;36:971–974. doi: 10.1227/00006123-199505000-00013. discussion 974–975. [DOI] [PubMed] [Google Scholar]
- 27.Raffel C. Medulloblastoma: molecular genetics and animal models. Neoplasia. 2004;6:310–322. doi: 10.1593/neo.03454. doi:10.1593/neo.03454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lun XQ, Zhou H, Alain T, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. doi:10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman S, Gigliotti F, Bockhold C, Naegele R. Measles and rubella antibody status in previously vaccinated children with cancer. Med Pediatr Oncol. 1988;16:308–311. doi: 10.1002/mpo.2950160504. doi:10.1002/mpo.2950160504. [DOI] [PubMed] [Google Scholar]
- 30.Mader EK, Maeyama T, Lin Y, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. doi:10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]