Abstract
We have investigated mechanisms that contribute to reinforce the relationship between inflammation and cancer. Secreted phospholipase A2 group IIA (sPLA2-IIA) is a molecule relevant in inflammatory events and has been proposed as a marker for some of these. Previously, we reported the mitogenic properties of this sPLA2 in the human astrocytoma cell line 1321N1. Here, we go deeper into the mechanisms that link this inflammatory protein with proliferation in one of the most aggressive types of tumors. We found that phosphorylation of the extracellular regulated kinase (ERK) was preceded by the activation of the small GTPase Ras, and both failed to be activated by inhibiting protein kinase C (PKC). Fractionation and immunofluorescence studies revealed translocation of PKC alpha, delta, and epsilon to the membrane fraction upon stimulation with sPLA2-IIA. Immunoprecipitation analysis showed that sPLA2-IIA induces phosphorylation of the epidermal growth factor receptor (EGFR) through a PKC-dependent pathway. We found that phosphorylation of this receptor contributed to Ras and ERK activation and that inhibition of ERK, PKC, and EGFR blocked the mitogenic response induced by sPLA2-IIA. This study showed that sPLA2-IIA is able to bring into play EGFR to trigger its signaling and that PKC leads the distribution of resources. Interestingly, we found that this is not a cell-specific response, because sPLA2-IIA was also able to transactivate EGFR in MCF7 human breast cancer cells. Therefore, this mechanism could contribute to worsen the prognosis of a tumor in an inflammatory microenvironment. We also present more links of the tumor chain possibly susceptible to targeting.
Keywords: cancer, epidermal growth factor receptor, inflammation, secreted phospholipase A2-IIA, signal transduction
Although inflammation is part of the defense response, paradoxically it can lead to important health disorders. Since it was discovered in the 19th century, chronic inflammation has been proven to be a risk factor for cancer (for review see refs 1,2), both at the onset and as a prognostic marker. The tumor microenvironment is importantly orchestrated by inflammatory cells and mediators, which participate in key processes, such as proliferation, survival, and migration. Inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α, have been found to be increased in patients with metastatic cancer. This finding makes anti-inflammatory drugs, as well as mechanisms triggered by proinflammatory mediators, of great clinical interest in the potential prevention and treatment of some tumors.
Secreted phospholipase A2 type IIA (sPLA2-IIA) is an acute-phase protein expressed in response to a variety of proinflammatory cytokines by a number of different tissues and cell types. Increased sPLA2-IIA plasma levels have been reported in patients with various acute and chronic inflammatory conditions3,4 and are a marker for some of these.5,6
sPLA2-IIA expression and activity are increased in numerous cancers, including breast, pancreatic, skin, liver, and prostate cancers (for review see ref. 7). Several investigations have related this expression to prognosis, for example, in human breast cancer.8,9 sPLA2-IIA is also a marker of metastasis in patients with ovarian or gastrointestinal cancer,10 and in prostate adenocarcinoma the expression level increases along with the tumor grade.11 In mice, the sPLA2-IIA gene, Pla2g2a, has been identified as a susceptibility gene for cancers of the small and large intestines, although its role in this tumor type is still controversial.12 Also, subcutaneous injection of cells overexpressing PLA2g2a resulted in increased tumor size, and these cells were found to be more infiltrative than controls.13 The importance of these findings has drawn a claim for sPLA2-IIA and other PLA2 isoforms to be studied and considered as possible targets for anticancer drugs.7 Thus, there is interest in the mechanisms by which sPLA2-IIA influences the tumor, which could contribute to the design of more effective chemotherapeutic approaches.
For decades, sPLA2-IIA has been considered relevant in different pathologies owing to its capacity to release lipid mediators. In recent years, sPLA2-IIA has also been revealed as a signaling molecule itself, independent of its catalytic activity,14,15 and able to trigger different pathways resulting in opposing cellular responses, such as proliferation15,16 and apoptosis.17 In previous studies, we demonstrated that in the human astrocytoma cell line 1321N1, sPLA2-IIA triggered signaling cascades that involve members of the mitogen activated protein kinase (MAPK) family, resulting in proliferation of the astrocytoma cells.15
The astrocytoma capacity of growth and invasion is closely correlated with the expression of cell surface receptors that sense the signals present in the tumor microenvironment. One of the receptors found overexpressed in this tumor and many others is the epidermal growth factor receptor (EGFR).18 Also, overexpression of other signaling elements that can lead to proliferation, such as protein kinase C (PKC), has been reported and related to malignant progression.19 Both EGFR and PKC could have a role in the sPLA2-IIA–induced activation of extracellular regulated kinase (ERK) and mitogenesis. Therefore, we proposed to more deeply characterize the signaling machinery triggered by this inflammatory mediator, which ultimately leads to cell proliferation. This study may shed new light on the general machinery that modulates cancer and/or cancer risk, providing a better understanding of the linkages between inflammation and cancer.
Materials and Methods
Reagents
A C127 mouse fibroblast cell line stably transfected with the coding sequence of sPLA2-IIA from human placenta was used as a source of human recombinant enzyme (cell line kindly provided by Dr. Bereziat, France). sPLA2-IIA was purified as described previously,20 and the dose for all experiments was 1 μg/ml, as determined in previous studies.21 Farnesyl transferase inhibitor (FTI) 227, atorvastatin, PD98059, GF109203X, and AG1478 were from Calbiochem. Radiolabeled thymidine and antirabbit secondary HRP antibody were from GE Healthcare. Antimouse secondary HRP antibody was from Bio-Rad. Rabbit anti-ERK2 was from Zymed Laboratories. Rabbit phospho-ERK1/2 (Thr202/Tyr204), cytosolic PLA2 (cPLA2), and Ras were from Cell Signaling Technology. Antihuman actin antibody, antibodies against the different PKC isoforms (alpha, delta, epsilon, and zeta), and phospho-EGFR (Tyr-1173) antibody were all from Santa Cruz Biotechnology. Antiphosphotyrosine antibody, clone 4G10, was from Upstate (Millipore). EGFR antibody was a kind gift from Dr. Pandiella (Salamanca, Spain). Both antirabbit and antimouse FITC secondary antibodies, phorbol myristate acetate (PMA), and other chemicals were from Sigma.
Cell Culture and Transfection
1321N1, MCF7, and Calu-3 cells were cultured at 37°C under a 5% CO2 atmosphere in DMEM (1321N1 and MCF7) or RPMI (Calu-3) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin, 2 mM glutamine, and FCS (5% for 1321N1 and MCF7 cells or 10% for Calu-3 cells). The 1321N1 cells were transfected using Lipofectamine (Invitrogen):DNA (ratio 1.2:0.4) 24 hours before the assay, and cells were serum starved overnight.
Immunoblotting
Once cells reached semiconfluence, they were serum starved overnight. After stimulation, cells were harvested directly in Laemmli-dithiothreitol (DTT) loading buffer and boiled for 5 minutes. SDS-PAGE of all protein samples was then performed using polyvinylidene difluoride membranes, followed by detection using the GE ECL system.
Ras Activation Assay
Cells were seeded onto 100-mm plates, and when they reached semiconfluence, they were left in serum-free medium overnight. After stimulation with the agonists, they were harvested in 750 μL of lysis buffer (50 mM Tris pH 7.4, 200 mM NaCl, 5 mM MgCl2, 1% NP40, 15% glycerol, 10 μg/mL leupeptin, 5 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 100 mM orthovanadate). After clearing the lysates by centrifugation, we incubated the supernatants with GST-RBD-Raf-1 (kindly provided by Dr. J. L. Bos, UMC, Utrecht, The Netherlands) for 20 minutes at 4°C. After coupling, the GTP-bound form of Ras was recovered by centrifugation using GST beads and washed with a lysis buffer. The samples were then boiled in Laemmli-DTT buffer and analyzed by SDS-PAGE.
Cell Fractionation Assay
Membrane-enriched fractions were obtained after permeabilization of the cells with saponin.22 Briefly, cells were seeded onto 100-mm plates and harvested in 400 μL of Buffer A (150 mM KCl, 2 mM MgCl2, 20 mM HEPES, 10% glycerol, pH 7.2, 1 mM DTT, 1 mM EGTA, 1 mM EDTA, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin) containing 0.02% (w/v) saponin. After centrifugation, the supernatants (cytosolic fraction) were separated and boiled for 5 minutes in Laemmli-DTT buffer. Pellets (membrane fraction) were washed and resuspended in Buffer A containing Triton X-100 and then boiled in Laemmli-DTT buffer.
Immunofluorescence
Cells were grown on glass coverslips, serum starved overnight, and stimulated as indicated in each case. After the cells were washed with PBS, they were fixed and permeabilized with 4% PFA–0.1% Triton X-100 PBS and blocked with PBS 1% BSA. The cells were then incubated at 4°C overnight with the primary antibody. Following washing, coverslips were incubated with the secondary antibody for 1 hour; after thorough washing, they were mounted with Vectashield mounting medium for fluorescence (Vector Laboratories). Images were captured with a Nikon Eclipse TS100 microscope using a 20× or 40× objective lens.
EGFR Immunoprecipitation
Cell lysates were immunoprecipitated with 4 μL/mL anti-EGFR antibody at 4°C for 4 hours, as described previously.23 The immune complex was recovered using GammaBind G-Sepharose. After washing, the beads were resuspended in Laemmli-DTT loading buffer and subjected to SDS-PAGE. The extent of tyrosine phosphorylation of EGFR was determined by immunoblotting with an antiphosphotyrosine antibody.
Measurement of DNA Synthesis Reinitiation
Quiescent cells were treated in serum-free DMEM for 24 hours with sPLA2-IIA after preincubation with either the inhibitor or vehicle. Four hours before the cells were harvested, 0.5 μCi/mL [3H]thymidine was added to the medium. The incubation was then terminated by washing with ice-cold 0.1 M MgCl2, and the radioactivity incorporated into the trichloroacetic acid precipitable fraction was measured.
Results
sPLA2-IIA Activation of Ras is Essential for Full Activation of ERK
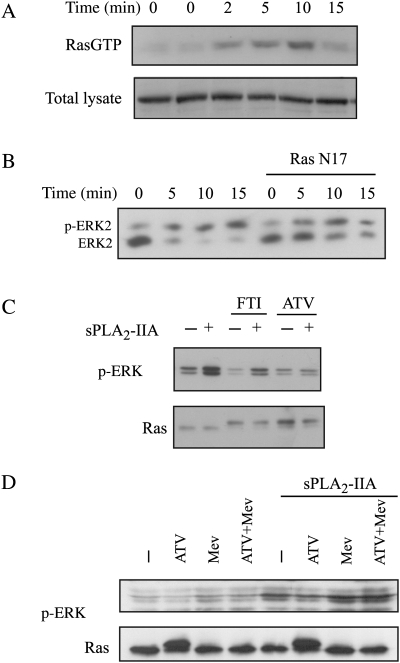
One of the best-known and well-described pathways that leads to ERK activation results from the activation of the small GTPase Ras. A pull-down assay, using a fusion protein with the Ras-binding domain of Raf-1 (GST-RBD), showed a transient activation of the small GTPase Ras after sPLA2-IIA incubation of 1321N1 cells (Fig. 1A). This activation was already visible after 2 minutes of stimulation and reached its peak between 5 and 10 minutes, which preceded the phosphorylation previously found in ERK by sPLA2-IIA in 1321N1 cells.
Fig. 1.
Ras participation in sPLA2-IIA–induced phosphorylation of ERK. (A) Serum-starved 1321N1 cells were stimulated with sPLA2-IIA for the indicated times. RasGTP was determined using the RBD pull-down assay (upper), and total lysates were also incubated with anti-Ras antibody (lower). (B) Cells were transfected with RasN17 and then serum starved and stimulated with sPLA2-IIA for the indicated times. ERK2 phosphorylation was checked by retardation on a Western blot. (C and D) Cells were preincubated overnight with 10-μM FTI, 10-μM atorvastatin, 100-μM mevalonate, or vehicle and then stimulated with sPLA2-IIA for 5 minutes. Western blots of the lysates were incubated with phospho-ERK or Ras antibodies (upper band, unprocessed Ras; lower band, processed Ras). The experiments shown are representative of the results obtained in at least 3 trials for each condition.
We proved the participation of Ras in the phosphorylation of ERK by transfecting the cells with a plasmid that encodes for Ras N17, a dominant negative mutant for Ras. A gel shift of ERK showed that the activation obtained in Ras N17-transfected cells was clearly lower than in mock-transfected cells (Fig. 1B). Either inhibition of the farnesyl transferase or the availability of its substrate would interfere.
Aware of the limits of dominant negative mutants and transient transfection itself, since the wild-type form is still functional, we further confirmed the Ras-ERK relationship using a different approach related to Ras processing. When newly synthesized, Ras GTPases are soluble cytosolic proteins that undergo prenylation to obtain the fully active enzyme. Either inhibition of the farnesyl transferase or inhibition of its substrate availability would interfere in the isoprenylation of Ras and, accordingly, in the processing to yield a functional protein.24 We used 2 different compounds for this purpose: FTI and atorvastatin, a permeable statin that inhibits the 3-hydroxy-3-methylglutaryl coenzyme A reductase and therefore inhibits synthesis of mevalonate and the production of farnesyl pyrophosphate. Preincubation with either of these compounds resulted in an augmentation of unprocessed Ras in the cytosol, unable to locate in the membrane (not shown). Unprocessed Ras runs slower on SDS-PAGE, and both the FTI- and atorvastatin-preincubated cells showed more abundant unprocessed Ras after overnight treatment (Fig. 1C). Phosphorylation of ERK by sPLA2-IIA was clearly diminished in the presence of either atorvastatin or FTI (Fig. 1C, upper panel), concordant with the Ras N17 assay mentioned above. When mevalonate was added to the culture medium at the same time as atorvastatin, the effect of atorvastatin was bypassed (Fig. 1D), while we found that in total protein the processed/unprocessed Ras levels had returned to basal (Fig. 1C).
PKC is Upstream of Ras and ERK
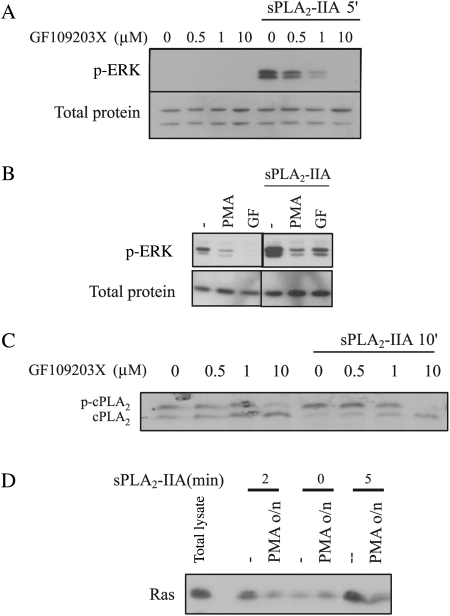
Another pathway that could be activating ERK, and Ras itself, comprises the PKC family. We preincubated 1321N1 cells with GF 109203X, a PKC inhibitor, for 30 minutes prior to stimulation with sPLA2-IIA. This inhibitor showed a clear dose-dependent inhibition of sPLA2-IIA–induced ERK phosphorylation (Fig. 2A). We also found this inhibition downregulating DAG-PKCs after overnight incubation with 1-μM PMA (Fig. 2B), again confirming PKC participation in ERK activation by sPLA2-IIA.
Fig. 2.
PKC participation in sPLA2-IIA–induced activation of ERK, cPLA2, and Ras. (A and C) 1321N1 cells were incubated with the indicated doses of GF109203X 30 minutes prior to stimulation with sPLA2-IIA. Western blots of total lysates were incubated with either phospho-ERK (upper A) or actin (lower A) antibodies or cPLA2 antibody (C). (B) 1321N1 cells were incubated with 1-μM PMA overnight or with 1-μM GF109203X for 30 minutes, prior to the addition of sPLA2-IIA for 5 minutes. Western blots of the lysates were incubated either with phospho-ERK (upper) or with actin antibody (lower). Samples shown belong to the same experiment and were run simultaneously but not consecutively; therefore, a lane represents the gap. (D) 1321N1 cells were incubated with 1-μM PMA overnight and then with sPLA2-IIA for the indicated times, and RasGTP was determined using the RBD pull-down assay. The first lane shows a sample of total lysate; the other lanes are Ras bound to the RBD, which represents RasGTP. In all cases, cells were serum starved overnight and the experiments shown are representative of the results obtained in at least 3 trials for each condition.
sPLA2-IIA activation of the cytosolic PLA2 (cPLA2) is ERK dependent in this cell line15; therefore, we checked whether PKC was, in turn, upstream of cPLA2. We incubated 1321N1 cells with different doses of GF109203X for 30 minutes prior to a 10-minute stimulation with sPLA2-IIA. The phosphorylated form of cPLA2 runs slower during electrophoresis, as shown in Fig. 2C, and the PKC inhibitor also blocked the activation of cPLA2 by sPLA2-IIA in a dose-dependent manner.
Next, we wondered whether Ras, which is necessary for full activation of ERK, also needed PKC to be activated by sPLA2-IIA. For that purpose, we incubated the cells with 1-μM PMA overnight and found that PMA downregulation of DAG-dependent PKCs impeded Ras activation by sPLA2-IIA (Fig. 2D). Thus, we can conclude that PKC activation precedes Ras, thereby suggesting that PKC activation is upstream of Ras and ERK in this system.
sPLA2-IIA Stimulation Induces PKC Translocation to the Membrane
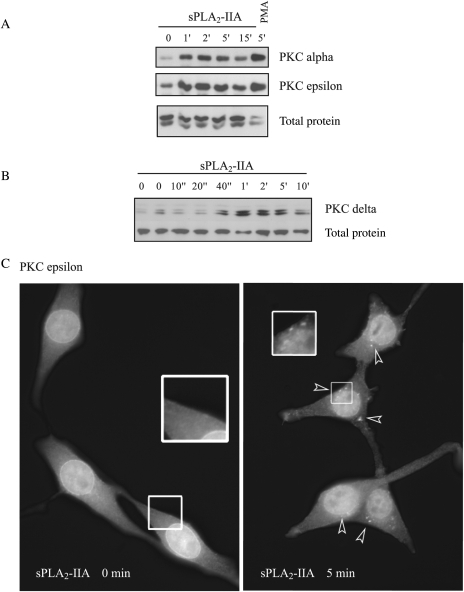
After activation, PKC translocates to the membrane to find its substrate. We examined PKC alpha, delta, epsilon, and zeta, and all expressed in 1321N1. After stimulating the cells with sPLA2-IIA, we performed fractionation studies. We found a quick and clear translocation of PKC alpha and epsilon to the membrane fraction in the presence of sPLA2-IIA (Fig. 3A). A 5-minute incubation with 1-μM PMA was used as a classical positive control. We performed a detailed time course for PKC delta (Fig. 3B) and found that translocation was already visible 40 seconds after sPLA2-IIA was added to the culture medium, and after 10 minutes it had reverted to basal levels. PKC translocation induced by sPLA2-IIA was not restricted to the outer membrane; by immunofluorescence analysis, we found that, upon stimulation, aggregates of PKC epsilon were formed in other membrane structures in the cell (in Fig. 3C, arrows indicate some of these aggregates).
Fig. 3.
PKC translocation to the membrane fraction. Serum-starved 1321N1 cells were stimulated with sPLA2-IIA or with 1-μM PMA (positive control) for the indicated times, and the membrane-enriched fraction was separated by centrifugation as described in the Methods section. SDS-PAGE was performed, and membranes were incubated with polyclonal antibodies against different PKC isoforms: PKC alpha and epsilon (A) and PKC delta (B). (C) Cells were fixed after stimulation with sPLA2-IIA or vehicle for the indicated times and incubated with a polyclonal antibody against PKC epsilon. Then an FITC secondary antibody was used, and the samples were analyzed and photographed using a Nikon Eclipse TS100 microscope at ×40. The experiments shown are representative of the results obtained in at least 3 trials for each condition.
Therefore PKC alpha, delta, and epsilon underwent a quick and transient translocation to the membrane, whereas PKC zeta remained unaffected (not shown), when 1321N1 cells were exposed to sPLA2-IIA.
sPLA2-IIA Transactivates EGFR, with the involvement of PKC
Because PKCs can also activate Ras and ERK through transactivation of other receptors and because we had found tyrosine phosphorylation by sPLA2-IIA,25 we investigated the possibility of transactivation of the EGFR upon sPLA2-IIA stimulation in 1321N1 cells.
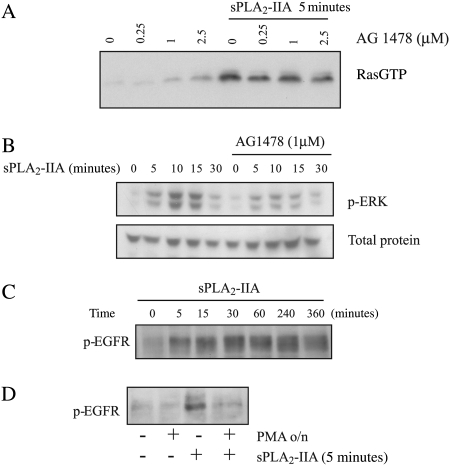
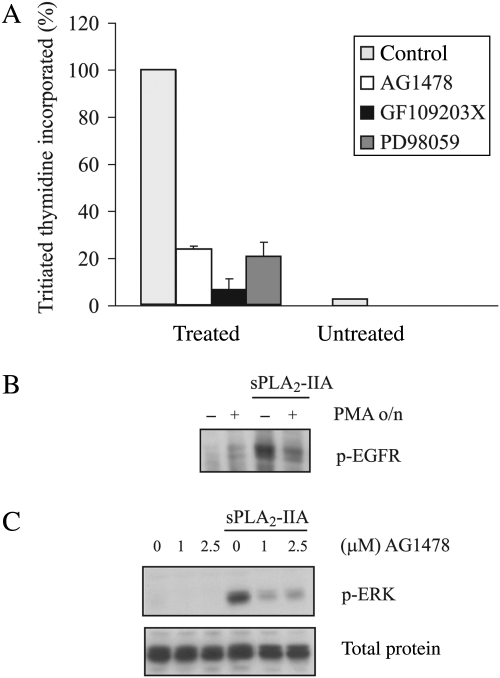
Tyrphostin AG1478 (AG) is an inhibitor of EGFR, so we preincubated 1321N1 astrocytoma cells with AG1478 prior to stimulation with sPLA2-IIA. The dose response of the inhibitor in a Ras pull-down assay suggested a role for EGFR in sPLA2-IIA activation of Ras, a partial inhibition of its activation (Fig. 4A). As expected, ERK behaved the same way (Fig. 4B), and only partial activation was achieved by sPLA2-IIA in the presence of AG1478.
Fig. 4.
EGFR activation and participation in sPLA2-IIA–induced Ras and ERK activation: role of PKC. Serum-starved 1321N1 cells were preincubated with the inhibitors, when indicated, and stimulated with sPLA2-IIA or vehicle for the indicated times. RasGTP was analyzed after a 30-minute preincubation with AG1478 (A), ERK phosphorylation was analyzed by Western blot with phospho-ERK antibody in a time course after preincubation with 1-μM AG1478 for 30 minutes (B), EGFR phosphorylation was also checked by Western blot with antiphospho-EGFR, (C) a time course of sPLA2-IIA, and (D) a 5-minute stimulation with sPLA2-IIA in the absence or presence of 1-μM PMA overnight. The experiments shown are representative of the results obtained in at least 3 trials for each condition.
EGFR undergoes tyrosine phosphorylation when activated, so we analyzed it by Western blotting using an antibody that recognizes phosphorylated EGFR. Results of this assay indicated that sPLA2-IIA induced a quick EGFR phosphorylation, which was sustained for about 6 hours and then diminished (Fig. 4C), returning to basal levels 12 hours after stimulation (not shown).
These results showed that the sPLA2-IIA signaling cascade activates EGFR and that this transactivation contributes to triggering Ras and ERK signaling.
We checked whether PKC was responsible for the phosphorylation of EGFR by sPLA2-IIA in 1321N1 cells. We downregulated DAG-PKCs with a 1-µM PMA treatment overnight and found that EGFR phosphorylation on tyrosine by sPLA2-IIA was considerably diminished in PMA-preincubated cells compared with cells preincubated with only vehicle (Fig. 4D). Therefore, we show a role for PKC in this transactivation.
sPLA2-IIA–Induced Proliferation Depends on EGFR, PKC, and ERK Activation
Proliferation is the cellular outcome after incubation of 1321N1 cells with sPLA2-IIA.15 All the members of the signaling cascades studied so far are closely related to mitogenic pathways in several systems. To check whether they were also involved in this system, assays with [3H]thymidine were performed in the absence or presence of their respective inhibitors: AG1478 for EGFR, GF109203X for PKC, and PD098059 for MAPK kinase (MEK) that consequently impedes activation of ERK.
We preincubated the cells with the indicated doses of the inhibitors for 30 minutes prior to the addition of sPLA2-IIA and found that the presence of each inhibitor resulted in a clear and dramatic reduction of the mitogenesis induced by sPLA2-IIA, as judged from the amount of [3H]thymidine incorporated (Fig. 5A).
Fig. 5.
sPLA2-IIA–induced proliferation in 1321N1 cells and sPLA2-IIA signaling induced in MCF7 cells. (A) Proliferation was measured as described in the Methods section. Serum-starved 1321N1 cells were stimulated with sPLA2-IIA for 24 hours after preincubation for 30 minutes with the indicated inhibitors (1-μM AG1478, 1-μM GF109203X, or 25-μM PD98059) or vehicle. [3H]Thymidine incorporated in sPLA2-IIA–stimulated cells was taken as 100%, and the rest of the values were related to this. Bars represent the means ± S.E. of 3 independent experiments performed in triplicate. (B and C) Serum-starved MCF7 cells were stimulated with sPLA2-IIA for 5 minutes after an overnight incubation with 1-μM PMA (B) or a 30-minute incubation with AG1478 (C) at the indicated doses. Total lysates were subjected to SDS-PAGE and incubated with phospho-EGFR (B) or phospho-ERK (C).
Therefore, the proliferation induced by sPLA2-IIA in this human astrocytoma cell line is triggered by a cascade depending on the activation of EGFR, ERK, and PKC.
sPLA2-IIA Also Transactivates EGFR in MCF7 Human Breast Cancer Cells
We wondered whether transactivation of this receptor by sPLA2-IIA could be considered as a possible mechanism to induce mitogenesis by sPLA2-IIA in other systems.
We performed our experiments in MCF7 cells, a human breast cancer cell line that expresses EGFR. Incubation with sPLA2-IIA induced the phosphorylation of EGFR in MCF7 cells (Fig. 5B), as was found for the astrocytoma cells. Similarly, downregulation of DAG-PKCs by overnight incubation of the cells with 1-μM PMA diminished the level of transactivation.
Not only did this result parallel the findings of our astrocytoma study, but we also found that sPLA2-IIA induced phosphorylation of ERK. This phosphorylation was not detected after preincubation of the cells with different doses of the EGFR inhibitor AG1478 (Fig. 5C), suggesting a role for EGFR in the signaling triggered by sPLA2-IIA in the human breast cancer cell line MCF7 as well.
Next, we wondered whether the cellular outcome after incubation with sPLA2-IIA would be similar to that for the astrocytoma cell line. We performed DNA synthesis reinitiation assays and found that [3H]thymidine incorporation was also greater in the presence of sPLA2-IIA than in the presence of a vehicle (100% vs 18%), and preliminary data indicated that sPLA2-IIA–induced proliferation was also both PKC and ERK sensitive (from 100% to 46% and 70%, respectively).
We also performed preliminary studies in the Calu-3 human lung adenocarcinoma cell line, which lacks expression of the EGFR,26 and these studies found that sPLA2-IIA was unable to induce a mitogenic response, resulting in comparable values of [3H]thymidine incorporated in the presence or absence of the enzyme. Likewise, no phosphorylation of ERK was found upon exposure of Calu-3 cells to sPLA2-IIA, indicating that it is unable to trigger this key signaling pathway. Further investigation should follow in order to certify a possible link between the level of EGFR expression and the proliferative properties of sPLA2-IIA.
Discussion
Although there is plenty of evidence to ascertain that inflammation can promote and influence cancer, the cause of this relationship is not fully understood. In this report, we showed how sPLA2-IIA, an acute-phase protein present in inflammatory events and a wide range of pathologies, including cancer, engages the EGFR, which is frequently overexpressed in tumors, to induce proliferation. We also showed how PKC, a key molecule of many signaling cascades, plays a determining role. Because both EGFR and PKC are important signaling elements in many tumor types, we consider this a relevant finding in the event of inflammation, which is frequently present in the tumor microenvironment.
We performed our studies in the 1321N1 human astrocytoma cell line, in which incubation with sPLA2-IIA induces proliferation.15 We wondered how sPLA2-IIA is able to elicit this response and the related mechanisms.
ERK is involved in the regulation of cell proliferation, survival, and differentiation; its aberrant regulation can contribute to cancer, and therefore, all the members of the Raf/MEK/ERK pathway have become classical targets in the field.27 sPLA2-IIA induces phosphorylation of ERK in 1321N1 astrocytoma cells15 and inhibition of this pathway stops proliferation.28 ERK is, therefore, a key element in sPLA2-IIA–induced mitogenesis; consequently, we concentrated on unravelling the upstream components that lead to ERK activation in this system.
Ras proteins have been found to be overexpressed or mutated in a wide variety of tumors, and they play a pivotal role in the transduction of several growth or differentiation stimuli (for review see refs 29,30). The switch of the small GTPase Ras from GDP to GTP can trigger the activation of the Raf/MEK/ERK cascade.31 We found activation of Ras when sPLA2-IIA was present, and it is necessary for full activation of ERK, as we showed by transfecting a dominant negative mutant of Ras or inhibiting Ras farnesylation with statins. In this situation, the presence of mevalonate restores Ras conformation and allows ERK full activation by sPLA2-IIA; therefore, we discard statins effects, other than the inhibition of farnesylation. Processing of other proteins can be also inhibited by the blockage of farnesylation; related to proliferation, we find the small GTPases Rheb (Ras homolog enriched in brain) and RhoB32 and the centromeric proteins CENP-E and CENP-F.33 Rheb has been described as regulating B-Raf, inhibiting its activation,34 whereas RhoB has been related to ERK but as an inhibitor.35 The centromere-associated proteins CENP-E and CENP-F are regulated during mitosis, and the inhibition of farnesylation results in accumulation of cells prior to metaphase.33 To our knowledge, there are no reports linking these farnesylated proteins with ERK phosphorylation, thereby reinforcing the role of Ras as an activator of ERK in this system.
The activation of Ras as well as ERK itself can also be achieved through the participation of members of the extended PKC family, which have been linked to cancer and represent a target for therapy.36 They could act on the cascade leading to ERK activation at different levels, activating Ras,37 Raf,38 or MEK39 or transactivating other receptors that trigger the cascade Ras/Raf/MEK/ERK.40
Several PKC isoforms were expressed and translocated to the membrane upon sPLA2-IIA stimulation of the astrocytoma cells: PKC alpha, PKC delta, and PKC epsilon. In all cases, we found a very quick and transient translocation. For PKC epsilon, we even found that sPLA2-IIA induces translocation to cell compartments other than the plasma membrane. PKC isoforms have been described as presenting a particular pattern of activation depending on the stimuli, undergoing translocation to different cellular compartments41 for a yet unknown purpose. The binding elements and precise localization of PKC epsilon demand further investigation. It has been stated that the expression and activity of various PKC isoforms are increased in malignant astrocytomas, suggesting that PKC activity contributes to tumor progression.19 In our system, inhibition of PKC with GF109203X blocked proliferation; therefore, PKC plays a determining role in the mitogenesis induced by sPLA2-IIA. Also, the inhibition of ERK blocked proliferation in 1321N1, and we found a link between both kinases: PKC is not only upstream of ERK and, as expected, of the cytosolic PLA2, cPLA2 (downstream effector of ERK15), but also of the activation of the small GTPase Ras. These facts propose a cascade in which PKC has a leading role and induces the activation of the Ras/Raf/MEK/ERK canonical pathway; we therefore considered the above-mentioned possibility of transactivation of other receptors.
Murakami et al.42 proposed the involvement of protein-tyrosine phosphorylation reactions in sPLA2-mediated cell activation in view of inhibitors’ ability to block this reaction. In our system, sPLA2-IIA also triggers a pathway dependent on tyrosine phosphorylation,25 supporting the possibility of transactivation of a growth factor receptor.
EGFR is one of the most important growth-stimulating receptors in a great variety of malignant tumors.18 Growth factor receptors activate ERK by a canonical mechanism: RTK-Shc-Grb2-Sos-Ras-Raf1-Mek-ERK.43 In this report, we showed that sPLA2-IIA induces a quick and sustained phosphorylation of EGFR. This activation is less strong than that induced by its natural ligand, epidermal growth factor, but similar in intensity, kinetics, and profile to those found for EGFR transactivation in other systems.44–46 We showed that this transactivation of the EGFR is necessary for sPLA2-IIA–induced proliferation in 1321N1 astrocytoma cells. The relevance of this mechanism becomes more apparent when we consider that the most frequent genetic alteration associated with glioblastoma is amplification of the EGFR gene, which results in overexpression of the receptor,47,48 believed to contribute to the aggressive phenotype seen in these tumors.49 Because Ras is not a frequent mutation in glioblastoma, the use of FTI inhibitors could be considered in the event of activation, as in this situation, through the transactivation of the EGFR by sPLA2-IIA or any other factor in the tumor microenvironment.
There are reports that state the relationship between EGFR and PKC in both directions, EGFR-activating PKC,50 and PKC-activating EGFR,40 as we find in this system. PKC can induce activation of EGFR by several mechanisms: ligand independent, through the activation of Src by a GPCR, or ligand dependent, by activating metalloproteinases, responsible for the release of extracellular membrane-bound epidermal growth factor–like ligands.51,52
How sPLA2-IIA is able to trigger all these pathways at the membrane level remains unknown. The role of this enzyme has been classically attributed to its catalytic activity, because it releases bioactive lipids, such as arachidonic acid, platelet-activation factor, and lysophosphatidic acid.53 In previous studies, we had shown that LPA is able to induce coupling of PLC to the receptor, but it does not modify the phosphorylation status of EGFR on its own in 1321N1 cells,23 either discarding LPA or stimulating the search of a signaling partner for LPA to accomplish the task. Nevertheless, when that study took place, we could not find sPLA2-IIA metabolites being released. Perhaps this finding should be revisited using the newer technologies available, even though sPLA2-IIA catalytic activity towards the cell membrane is apparently restricted to bacteria and modified eukaryotic membranes.53
On the other hand, the time course of EGFR phosphorylation shows an immediate response, discarding prostaglandins as the inducers of EGFR transactivation, as indicated in other reports.54 It is precisely the immediateness of the response that leaves the direct interaction of sPLA2-IIA with a membrane structure as the most plausible mechanism to trigger the signaling cascade of concern. Heparan sulfate proteoglycans (glypican, decorin, biglycan, M-type, N-type, or mannose receptors) are claimed to be binding motifs for sPLA2-IIA.55 Interestingly, a recent report has shown sPLA2-IIA induction of a mitogenic response linked to integrin engagement.16 The interaction of sPLA2-IIA with a specific receptor is also feasible, but not with those described so far, because the M-type sPLA2 receptor is not expressed in these cells and sPLA2-IIA has low affinity for the N-type receptor.55 The search for the binding partner of sPLA2-IIA, able to trigger the signaling cascades in these cells, is the subject of future investigations.
In this study, we identified key elements (PKC and EGFR) of the signaling cascade of sPLA2-IIA that lead to proliferation in astrocytoma cells, since inhibition of any of them blocks this cellular response. As noted in the introduction, sPLA2-IIA is present in a wide range of tumors of different origin. In breast cancer, it is now upgraded to the status of a prognostic marker,8,9 and also in MCF7 we find that sPLA2-IIA transactivates EGFR in a PKC-dependent manner. Again, sPLA2-IIA induces proliferation in these cells, which is abrogated by inhibition of PKC and ERK, conferring on EGFR a responsibility in the activation of ERK.
Regardless of evident cell-line differences, sPLA2-IIA triggers a similar cascade that results in a mitogenic response through the transactivation of EGFR. Further work could determine whether sPLA2-IIA mitogenic potential directly depends on the EGFR expression (preliminary work has shown that sPLA2-IIA is unable to induce proliferation in a human cell line that lacks EGFR) and also whether we are here presented with a general mechanism to be taken into consideration in tumors where sPLA2-IIA could be expressed due to an inflammatory environment. Inflammation and EGFR overexpression could occur in tandem to promote proliferation and worsen the prognosis, not only at the onset or development of the tumor. The resistance of some tumors to radiotherapy has been related to a higher expression of EGFR.56 The microenvironment of the tumor could be playing a role in this event because inflammation markers are found in patients receiving radiotherapy.57
The value of EGFR as a prognostic marker in gliomas is still under debate. Some studies point to differences regarding other factors,58 which might suggest considering additional factors not strictly confined to the plasma membrane. Treatments for cancer could possibly be made more effective by taking into account the characteristics of not only the tumor itself but also its microenvironment at each stage.
Conflict of interest statement. None declared.
Funding
This work was supported by the Ramón y Cajal Program (to M.H.), F.P.I. Program from the Autonomous Government of Castilla y León. (to R.M.), both cofunded by F.S.E and by the ISCIII, Red de Centros RECAVA, C03/01 (to P.M.H. and M.D.G.C.), and by grants SAF2005-01242 and SAF2009-08407 from the Spanish Ministry of Science and Innovation, and CSI11A08 from the Autonomous Government of Castilla y León.
Acknowledgments
The authors of this paper would like to thank the investigators mentioned in Materials and Methods for their generous gifts or reagents.
References
- 1.Aggarwal BB, Shishodia S, Sandur SK, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. doi:10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. doi:10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurt-Camejo E, Camejo G, Peilot H, et al. Phospholipase A2 in vascular disease. Circ Res. 2001;89:298–304. doi: 10.1161/hh1601.095598. doi:10.1161/hh1601.095598. [DOI] [PubMed] [Google Scholar]
- 4.Nevalainen TJ, Haapamaki MM, Gronroos JM. Roles of secretory phospholipases A2 in inflammatory diseases and trauma. Biochim Biophys Acta. 2000;1488:83–90. doi: 10.1016/s1388-1981(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 5.Kugiyama K, Ota Y, Takazoe K, et al. Circulating levels of secretory type II phospholipase A2 predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–1284. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- 6.Kugiyama K, Ota Y, Sugiyama S, et al. Prognostic value of plasma levels of secretory type II phospholipase A2 in patients with unstable angina pectoris. Am J Cardiol. 2000;86:718–722. doi: 10.1016/s0002-9149(00)01069-9. doi:10.1016/S0002-9149(00)01069-9. [DOI] [PubMed] [Google Scholar]
- 7.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochem Pharmacol. 2007;74:949–959. doi: 10.1016/j.bcp.2007.04.021. doi:10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Mannello F, Qin W, Zhu W, et al. Nipple aspirate fluids from women with breast cancer contain increased levels of group IIa secretory phospholipase A2. Breast Cancer Res Treat. 2008;111:209–218. doi: 10.1007/s10549-007-9779-1. doi:10.1007/s10549-007-9779-1. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita S, Yamashita J, Ogawa M. Overexpression of group II phospholipase A2 in human breast cancer tissues is closely associated with their malignant potency. Br J Cancer. 1994;69:1166–1170. doi: 10.1038/bjc.1994.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massing U, Kraft A, Stier B, et al. Examination of secretory phospholipase A2 (sPLA2) as a maker of metastasis. Eur J Cancer. 1997;33:S47–S48. doi:10.1016/S0959-8049(97)84591-1. [Google Scholar]
- 11.Graff JR, Konicek BW, Deddens JA, et al. Expression of group IIa secretory phospholipase A2 increases with prostate tumor grade. Clin Cancer Res. 2001;7:3857–3861. [PubMed] [Google Scholar]
- 12.Fijneman RJ, Cormier RT. The roles of sPLA2-IIA (Pla2g2a) in cancer of the small and large intestine. Front Biosci. 2008;13:4144–4174. doi: 10.2741/2998. doi:10.2741/2998. [DOI] [PubMed] [Google Scholar]
- 13.Belinsky GS, Rajan TV, Saria EA, et al. Expression of secretory phospholipase A2 in colon tumor cells potentiates tumor growth. Mol Carcinog. 2007;46:106–116. doi: 10.1002/mc.20271. doi:10.1002/mc.20271. [DOI] [PubMed] [Google Scholar]
- 14.Triggiani M, Granata F, Balestrieri B, et al. Secretory phospholipases A2 activate selective functions in human eosinophils. J Immunol. 2003;170:3279–3288. doi: 10.4049/jimmunol.170.6.3279. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez M, Burillo SL, Crespo MS, et al. Secretory phospholipase A2 activates the cascade of mitogen-activated protein kinases and cytosolic phospholipase A2 in the human astrocytoma cell line 1321N1. J Biol Chem. 1998;273:606–612. doi: 10.1074/jbc.273.1.606. doi:10.1074/jbc.273.1.606. [DOI] [PubMed] [Google Scholar]
- 16.Saegusa J, Akakura N, Wu CY, et al. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. J Biol Chem. 2008;283:26107–26115. doi: 10.1074/jbc.M804835200. doi:10.1074/jbc.M804835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagami T, Ueda K, Asakura K, et al. Human group IIA secretory phospholipase A2 induces neuronal cell death via apoptosis. Mol Pharmacol. 2002;61:114–126. doi: 10.1124/mol.61.1.114. doi:10.1124/mol.61.1.114. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. doi:10.1016/S0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 19.Benzil DL, Finkelstein SD, Epstein MH, et al. Expression pattern of alpha-protein kinase C in human astrocytomas indicates a role in malignant progression. Cancer Res. 1992;52:2951–2956. [PubMed] [Google Scholar]
- 20.Gijon MA, Perez C, Mendez E, et al. Phospholipase A2 from plasma of patients with septic shock is associated with high-density lipoproteins and C3 anaphylatoxin: some implications for its functional role. Biochem J. 1995;306(Pt 1):167–175. doi: 10.1042/bj3060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin R, Hernandez M, Ibeas E, et al. Secreted phospholipase A2-IIA modulates key regulators of proliferation on astrocytoma cells. J Neurochem. 2009;111:988–999. doi: 10.1111/j.1471-4159.2009.06377.x. doi:10.1111/j.1471-4159.2009.06377.x. [DOI] [PubMed] [Google Scholar]
- 22.Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez M, Barrero MJ, Crespo MS, et al. Lysophosphatidic acid inhibits Ca2+ signaling in response to epidermal growth factor receptor stimulation in human astrocytoma cells by a mechanism involving phospholipase C gamma and a G(alpha i) protein. J Neurochem. 2000;75:1575–1582. doi: 10.1046/j.1471-4159.2000.0751575.x. doi:10.1046/j.1471-4159.2000.0751575.x. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs JB, Pompliano DL, Mosser SD, et al. Selective inhibition of farnesyl-protein transferase blocks ras processing in vivo. J Biol Chem. 1993;268:7617–7620. [PubMed] [Google Scholar]
- 25.Hernandez M, Barrero MJ, Alvarez J, et al. Secretory phospholipase A2 induces phospholipase C gamma-1 activation and Ca2+ mobilization in the human astrocytoma cell line 1321N1 by a mechanism independent of its catalytic activity. Biochem Biophys Res Comm. 1999;260:99–104. doi: 10.1006/bbrc.1999.0832. doi:10.1006/bbrc.1999.0832. [DOI] [PubMed] [Google Scholar]
- 26.Friess T, Scheuer W, Hasmann M. Combination treatment with erlotinib and pertuzumab against human tumor xenografts is superior to monotherapy. Clin Cancer Res. 2005;11:5300–5309. doi: 10.1158/1078-0432.CCR-04-2642. doi:10.1158/1078-0432.CCR-04-2642. [DOI] [PubMed] [Google Scholar]
- 27.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. doi:10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 28.Martin R, Hernandez M, Ibeas E, et al. Secreted phospholipase A2-IIA modulates key regulators of proliferation on astrocytoma cells. J Neurochem. 2009;111:988–999. doi: 10.1111/j.1471-4159.2009.06377.x. doi:10.1111/j.1471-4159.2009.06377.x. [DOI] [PubMed] [Google Scholar]
- 29.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. doi:10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 30.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 31.Moodie SA, Willumsen BM, Weber MJ, et al. Complexes of Ras. GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. doi:10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 32.Lebowitz PF, Prendergast GC. Non-Ras targets of farnesyltransferase inhibitors: focus on Rho. Oncogene. 1998;17:1439–1445. doi: 10.1038/sj.onc.1202175. doi:10.1038/sj.onc.1202175. [DOI] [PubMed] [Google Scholar]
- 33.Ashar HR, James L, Gray K, et al. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem. 2000;275:30451–30457. doi: 10.1074/jbc.M003469200. doi:10.1074/jbc.M003469200. [DOI] [PubMed] [Google Scholar]
- 34.Im E, von Lintig FC, Chen J, et al. Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene. 2002;21:6356–6365. doi: 10.1038/sj.onc.1205792. doi:10.1038/sj.onc.1205792. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Sun J, Pradines A, et al. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J Biol Chem. 2000;275:17974–17978. doi: 10.1074/jbc.C000145200. doi:10.1074/jbc.C000145200. [DOI] [PubMed] [Google Scholar]
- 36.Ali AS, Ali S, El Rayes BF, et al. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev. 2009;35:1–8. doi: 10.1016/j.ctrv.2008.07.006. doi:10.1016/j.ctrv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Liu H, Coughlin J, et al. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood. 2005;105:3648–3654. doi: 10.1182/blood-2004-10-3916. doi:10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
- 38.Kolch W, Heidecker G, Kochs G, et al. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. doi:10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 39.Wen-Sheng W. Protein kinase C alpha trigger Ras and Raf-independent MEK/ERK activation for TPA-induced growth inhibition of human hepatoma cell HepG2. Cancer Lett. 2006;239:27–35. doi: 10.1016/j.canlet.2005.07.034. doi:10.1016/j.canlet.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Stewart JR, O'Brian CA. Protein kinase C-{alpha} mediates epidermal growth factor receptor transactivation in human prostate cancer cells. Mol Cancer Ther. 2005;4:726–732. doi: 10.1158/1535-7163.MCT-05-0013. doi:10.1158/1535-7163.MCT-05-0013. [DOI] [PubMed] [Google Scholar]
- 41.Buchner K, Adamec E, Beermann ML, et al. Isoform-specific translocation of protein kinase C following glutamate administration in primary hippocampal neurons. Brain Res Mol Brain Res. 1999;64:222–235. doi: 10.1016/s0169-328x(98)00324-6. doi:10.1016/S0169-328X(98)00324-6. [DOI] [PubMed] [Google Scholar]
- 42.Murakami M, Hara N, Kudo I, et al. Triggering of degranulation in mast cells by exogenous type II phospholipase A2. J Immunol. 1993;151:5675–5684. [PubMed] [Google Scholar]
- 43.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. doi:10.1016/S0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 44.Eguchi S, Numaguchi K, Iwasaki H, et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. doi:10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Zhang A, Ding G, et al. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. doi:10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- 46.Zwick E, Daub H, Aoki N, et al. Critical role of calcium-dependent epidermal growth factor receptor transactivation in PC12 cell membrane depolarization and bradykinin signaling. J Biol Chem. 1997;272:24767–24770. doi: 10.1074/jbc.272.40.24767. doi:10.1074/jbc.272.40.24767. [DOI] [PubMed] [Google Scholar]
- 47.Wong AJ, Bigner SH, Bigner DD, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. doi:10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphrey PA, Wong AJ, Vogelstein B, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988;48:2231–2238. [PubMed] [Google Scholar]
- 49.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. doi:10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen EE, Lingen MW, Zhu B, et al. Protein kinase C zeta mediates epidermal growth factor-induced growth of head and neck tumor cells by regulating mitogen-activated protein kinase. Cancer Res. 2006;66:6296–6303. doi: 10.1158/0008-5472.CAN-05-3139. doi:10.1158/0008-5472.CAN-05-3139. [DOI] [PubMed] [Google Scholar]
- 51.Fischer OM, Hart S, Gschwind A, et al. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. doi:10.1042/BST0311203. [DOI] [PubMed] [Google Scholar]
- 52.Shah BH, Farshori MP, Catt KJ. Neuropeptide-induced transactivation of a neuronal epidermal growth factor receptor is mediated by metalloprotease-dependent formation of heparin-binding epidermal growth factor. J Biol Chem. 2004;279:414–420. doi: 10.1074/jbc.M309083200. doi:10.1074/jbc.M309083200. [DOI] [PubMed] [Google Scholar]
- 53.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. doi:10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 54.Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261–270. doi: 10.1002/jcp.20560. doi:10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 55.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 56.Kelly MP, Lee ST, Lee FT, et al. Therapeutic efficacy of (177)Lu-CHX-A''-DTPA-hu3S193 radioimmunotherapy in prostate cancer is enhanced by EGFR inhibition or docetaxel chemotherapy. Prostate. 2009;69:92–104. doi: 10.1002/pros.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xanthinaki A, Nicolatou-Galitis O, Athanassiadou P, et al. Apoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary report. Support Care Cancer. 2008;16:1025–1033. doi: 10.1007/s00520-007-0379-8. doi:10.1007/s00520-007-0379-8. [DOI] [PubMed] [Google Scholar]
- 58.Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–1128. [PubMed] [Google Scholar]