Abstract
Vestibular schwannoma (VS) (or acoustic neuroma) accounts for about 5%–6% of all intracranial tumors; little is known about the etiology. We investigated the association between various sociodemographic indicators and VS in a cohort of 3.26 million Danish residents, with 1087 cases identified in 35 308 974 person-years under risk, with data accrued from 1993 to 2006. Complete ascertainment of cases was ensured by using population-based and clinical cancer registries. Information on sociodemographic indicators was obtained on an annually updated individual level from Statistics Denmark. Log-linear Poisson regression models were used to estimate incidence rate ratios (IRRs). Linear regression models were used to examine the association between sociodemographic indicators and tumor size. We found that IRRs decreased gradually with decreasing level of education, with values of 0.62 (95% CI: 0.49–0.78) for men and 0.62 (95% CI: 0.50–0.77) for women with a basic education compared with a higher education. Similar results were found for disposable income. Marital status was associated with a higher incidence of VS in men but not women; nonmarried men with a basic education had an IRR of 0.34 (95% CI: 0.23–0.50) compared with married men with a higher education. Lower incidence rates were also observed among unemployed or early-retirement pensioners, whereas there were no differences in incidence rates across the broad groups of occupations and across the types of districts. Sociodemographic indicators were not associated with the tumor size. The magnitude of the differences in incidence rates across the groups of different socioeconomic indicators suggests a high potential for earlier diagnosis of VS by improving the awareness of early symptoms.
Keywords: acoustic neuroma, cohort study, epidemiology, marital status, socioeconomic status, vestibular schwannoma
Vestibular schwannoma (VS), also termed acoustic neuroma, is a tumor that arises on the 8th cranial nerve leading from the brainstem to the inner ear and accounts for about 5%–6% of all intracranial tumors.1 VS occurs in a sporadic, mostly unilateral form and in a hereditary, mostly bilateral form, with the latter accounting for about 5%–10% of the VS cases.2 Little is known about the etiology of sporadic VS3; recent research has focused on loud noise and the use of mobile phones as possible causes, but no conclusive evidence is yet available.4,5 The incidence rate of VS has increased steeply over the last 30 years1,6; with easier access to MRI, the tumors are diagnosed in an earlier stage when still small.6 The increase in the number of diagnosed tumors is mainly explained by the diagnosis of intrameatal and small tumors among older persons who were not previously offered MRI examination.7
An examination of sociodemographic variables may provide clues to the etiology. Such variables are also a commonly used concept in health research, and general inequalities in health reflect social inequalities.8 However, there is again little reliable data for the association between sociodemographic variables and the risk for VS. One reason is that when brain tumors are investigated in aggregated data, few conclusions can be drawn with regard to associations in more rare subtypes of tumors within this tumor entity.9 Another reason is that if studies are of case–control design requiring active participation of subjects, results need to be interpreted with caution, as sociodemographic indicators may reflect participation rates.10,11
Here, we report results from the linkage of information on a variety of sociodemographic factors for more than 3 million Danes enrolled in a nationwide cohort study, with information on more than 1000 cases of VS diagnosed between 1993 and 2006. We obtained data from several sources: a nationwide population-based cancer registry, a nationwide population-based registry of continuously updated information on sociodemographic factors of each Danish resident, and a large clinical database covering all cases of VS, including detailed clinical information.
Subjects and methods
Details of the setup of the Danish nationwide cohort study on social inequality and cancer (“Cancer og ulighed,” CANULI) have been reported elsewhere.12 In brief, all residents born between 1925 and 1976 who resided in Denmark between 1993 and 2006, approximately 3.5 million persons, were enrolled in this study. Entry into the cohort was at age 30 years because younger persons might still be in the educational system and thereby in the process of establishing their socioeconomic position. Persons who immigrated to Denmark and their descendants were excluded because they compose an ethnically very heterogeneous group and data on their education, if acquired abroad, were not available. This left 3.26 million Danes for study. Dates of death or emigration were obtained for all cohort members through December 31, 2006.
Information on the socioeconomic status of the study cohort was obtained from the population-based Integrated Database for Labour Market Research in Statistics, Denmark, which contains yearly data since 1980. Three levels of education were defined as follows: basic school/high school (primary and secondary school education only), vocational education (formal occupational training following school), and higher education (university education). Disposable income was calculated based on household income after taxation and interest per person, adjusted for the number of persons in the household and for the value of the Danish krone in 2000, following a formula from the Danish Ministry of Finance. For categorical analyses, disposable income was grouped into low (1st quartile), middle (2nd and 3rd quartile), and high (4th quartile), based on separated distributions for men and women in the cohort for each study year, otherwise applied as a continuous variable.12 Categories for marital status were married, cohabitating, single, widow/widower, or divorced; for some analyses, marital status was aggregated into married and nonmarried. The type of district was classified into the capital area (Copenhagen), provincial cities, rural areas, and peripheral rural areas (being more than 40 km away from a local center with proper employment possibilities and having no border with a municipal center). The labor categories used were as follows: working, unemployed, pensioner due to age, and early-retirement pensioner; this last status is granted if a person is unable to work permanently due to mental or physical disability and this disability reduces the ability to work by at least 50%. The working group was further subdivided into manual (eg, construction, transportation, production workers), service (eg, nurses, hairdressers, caterers), agriculture (eg, farmers, fishermen), and creative (eg, researchers, designers, managers, lawyers, doctors, artists), following Florida's theory of the creative class as an emerging social class.13
Information on all cases of VS was obtained from two sources. In Denmark, VS patients are treated mainly in one clinical center, and the clinical database of this center was used as the first source.6 Additional clinical data from this source were also obtained to distinguish between the VS cases of intrameatal spread only and the cases of intra- and extrameatal spread. For the latter cases, tumor size was given as the extrameatal diameter in millimeters, measured at diagnosis. For the second information source, the cohort was linked with the nationwide population-based cancer registry to identify patients not registered in the clinical registry and vice versa.14 The follow-up for VS incidence was through 31 December 2006, to match the follow-up for vital status.
Incidence rates by 5-year age groups were calculated for the level of education and for martial status (married vs nonmarried). Log-linear Poisson regression models were used to estimate incidence rate ratios (IRRs), with 95% confidence intervals (CIs) between the levels of each factor, where 1 was chosen as the reference level.15 IRRs were always adjusted for period (1993–1999 and 2000–2006) and age (modeled as age and age2 in years); further adjustments are given as footnotes in the tables. We used the status of the sociodemographic indicator 2 years before the year of observation to avoid misclassification due to a change in the respective factor because of the presence of a yet-undiagnosed VS, with the following exception: for persons who had retired due to their age, we used if possible the latest recorded occupation for their labor status. Follow-up for the labor status analyses ended at age 69 years for all cohort members.12 Persons who had a diagnosis of cancer before being diagnosed with VS were censored at the date of diagnosis of their cancer and from then on excluded from the follow-up. Sex-specific logistic regression models were used to investigate whether spread of the VS (intrameatal only vs intra- and extrameatal) was associated with the education level or marital status (married vs nonmarried); these factors were entered one at a time, and all models were adjusted for the year of diagnosis and the age at diagnosis. Among patients with intra- and extrameatal VS, tumor size was examined with univariate characteristics (mean, median, and 1st and 3rd quartiles) in sociodemographic subgroups by education level and marital status, and differences were explored using the Kruskal–Wallis test. Sex-specific multiple linear regression analysis was used to additionally adjust for age (using age and age2 in the model) and year of diagnosis when investigating the association between tumor size and those sociodemographic factors.
Results
The study was based on 3 256 109 persons and 35 308 974 person-years of follow-up for VS. A total of 542 men and 545 women in the cohort had a diagnosis of VS without any previous cancer diagnosis.
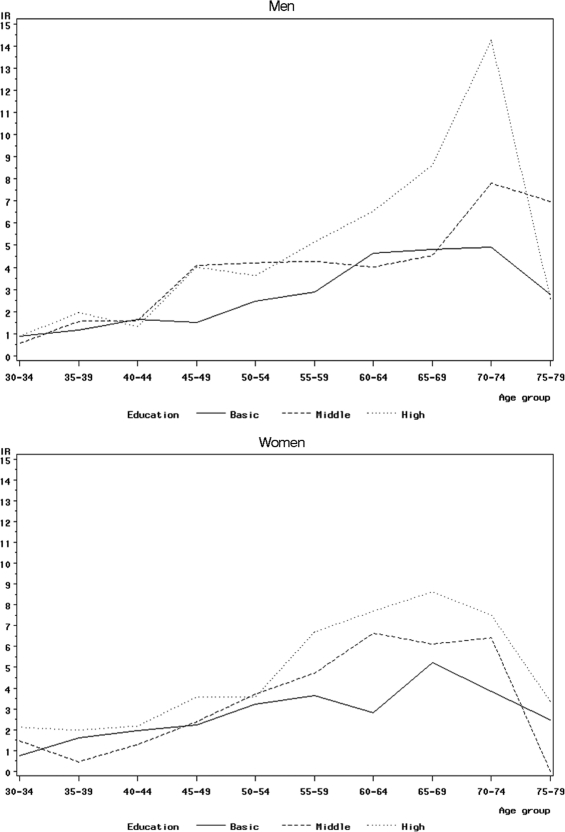
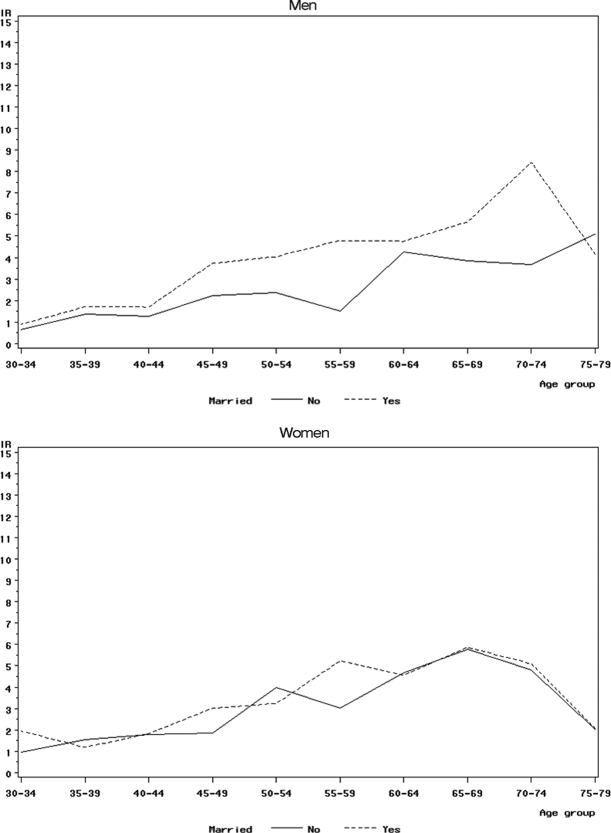
There was a gradient of decreasing IRRs with a decreasing level of education and decreasing disposable income observed in both men and women but slightly stronger in women (Table 1). With respect to disposable income, IRRs increased by a factor of 1.02 (CI: 1.01–1.04, P < .01) for men and of 1.03 (CI: 1.02–1.04, P < .01) for women with each additional 10 000 Danish kroner (∼€1340). Compared with married men, all other groups of nonmarried men had a lower incidence rate of VS, particularly so in singles and men cohabitating with their partner. A different picture was seen among women, where only divorced women had a decreased IRR compared with married women. There was no clear pattern with respect to the type of district. Compared with service class men, men classed as unemployed, pensioners, and early-retirement pensioners had a lower incidence rate of VS, whereas there were few differences across the various professional groups. Among women, only early-retirement pensioners had a distinctly lower incidence rate, whereas the IRR was slightly greater for creative professionals compared with service class women. After further adjustment for education level and disposable income, most IRRs changed very little; however, the association between creative class and VS in women was attenuated (Table 1). The higher incidence rate of VS with an increasing level of education in both men and women was seen across all ages, except in the 75–79-year age group (Fig. 1). Similarly, the higher incidence rate of VS in married men was also seen across all ages up to the age of 75 years, although it was less apparent in those aged between 30 and 39, and it seemed to be strongest in the 70–74-year age group (Fig. 2); comparable results were not seen for women. Because disposable income is also related to age, we investigated the joint effects of age and income, and the pattern was similar to that for education level (data not shown).
Table 1.
Sociodemographic indicators and risk for vestibular schwannoma in men and women, Denmark, 1993–2006
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | PYRS | IRR 1 IRR 2 | 95% CI | Cases | PYRS | IRR 1 RR 2 | 95% CI | |
| Level of educationa | ||||||||
| Higher | 137 | 3 681 202 | 1.00 | – | 146 | 4 008 134 | 1.00 | – |
| 1.00 | – | 1.00 | – | |||||
| Middle | 240 | 7 586 872 | 0.83 | 0.67–1.02 | 173 | 5 815 198 | 0.74 | 0.60–.93 |
| 0.86 | 0.69–1.07 | 0.78 | 0.62–0.97 | |||||
| Basic | 160 | 6 155 793 | 0.62 | 0.49–0.78 | 218 | 7 619 303 | 0.62 | 0.50–0.77 |
| 0.66 | 0.51–0.84 | 0.68 | 0.54–0.85 | |||||
| Disposable income | ||||||||
| Low (1st quartile) | 116 | 4 129 340 | 0.72 | 0.57–0.93 | 109 | 4 171 487 | 0.62 | 0.48–0.80 |
| 0.82 | 0.64–1.06 | 0.69 | 0.53–0.90 | |||||
| Middle (2nd/3rd quartile) | 251 | 9 003 191 | 0.82 | 0.67–0.99 | 256 | 8 947 180 | 0.77 | 0.64–0.94 |
| 0.88 | 0.72–1.08 | 0.83 | 0.68–1.01 | |||||
| High (4th quartile) | 175 | 4 552 955 | 1.00 | – | 180 | 4 504 821 | 1.00 | – |
| 1.00 | – | 1.00 | – | |||||
| Cohabitating status | ||||||||
| Married | 422 | 11 106 945 | 1.00 | – | 373 | 11 276 272 | 1.00 | – |
| 1.00 | – | 1.00 | – | |||||
| Cohabitating | 35 | 2 347 368 | 0.59 | 0.41–0.84 | 41 | 2 038 365 | 0.88 | 0.63–1.22 |
| 0.61 | 0.43–0.87 | 0.88 | 0.63–1.22 | |||||
| Single | 37 | 2 744 265 | 0.51 | 0.36–0.72 | 44 | 1 632 777 | 1.11 | 0.81–1.53 |
| 0.56 | 0.40–0.79 | 1.14 | 0.83–1.57 | |||||
| Divorced | 37 | 1 199 545 | 0.77 | 0.55–1.08 | 36 | 1 608 085 | 0.61 | 0.44–0.86 |
| 0.82 | 0.58–1.15 | 0.66 | 0.46–0.93 | |||||
| Widow/widower | 11 | 287 362 | 0.71 | 0.39–1.30 | 51 | 1 067 989 | 1.01 | 0.74–1.38 |
| 0.73 | 0.40–1.34 | 1.07 | 0.78–1.47 | |||||
| Type of district | ||||||||
| Capital region | 178 | 5 481 043 | 1.00 | – | 187 | 5 608 033 | 1.00 | – |
| 1.00 | – | 1.00 | – | |||||
| Provincial cities | 253 | 9 114 570 | 0.83 | 0.69–1.01 | 278 | 9 074 568 | 0.90 | 0.75–1.09 |
| 0.87 | 0.72–1.06 | 0.96 | 0.80–1.16 | |||||
| Rural | 82 | 2 172 627 | 1.11 | 0.86–1.44 | 51 | 2 065 994 | 0.73 | 0.53–0.99 |
| 1.20 | 0.92–1.57 | 0.79 | 0.58–1.08 | |||||
| Peripheral rural | 29 | 917 246 | 0.90 | 0.61–1.33 | 29 | 874 893 | 0.94 | 0.64–1.40 |
| 0.99 | 0.67–1.47 | 1.05 | 0.71–1.56 | |||||
| Affiliation to the work marketb | ||||||||
| Working | ||||||||
| Manual | 115 | 4 553 754 | 0.83 | 0.64–1.09 | 13 | 769 763 | 0.63 | 0.36–1.10 |
| 0.87 | 0.66–1.14 | 0.69 | 0.39–1.22 | |||||
| Service | 101 | 3 310 394 | 1.00 | – | 209 | 7351705 | 1.00 | – |
| 1.00 | – | 1.00 | – | |||||
| Agriculture | 24 | 544 838 | 1.21 | 0.77–1.89 | 5 | 148 135 | 0.98 | 0.40–2.38 |
| 1.36 | 0.86–2.15 | 1.10 | 0.45–2.68 | |||||
| Creative | 116 | 3 111 903 | 1.12 | 0.86–1.46 | 70 | 1 790 118 | 1.34 | 1.02–1.76 |
| 1.05 | 0.79–1.39 | 1.15 | 0.86–1.55 | |||||
| Unknown | 29 | 897 235 | 1.00 | 0.66–1.51 | 18 | 822 425 | 0.68 | 0.42–1.11 |
| 1.05 | 0.69–1.60 | 0.71 | 0.44–1.16 | |||||
| Unemployed | 20 | 1 378 064 | 0.45 | 0.28–0.72 | 51 | 2 096 248 | 0.79 | 0.58–1.07 |
| 0.49 | 0.30–0.80 | 0.87 | 0.63–1.19 | |||||
| Early retirement | 21 | 907 859 | 0.51 | 0.32–0.82 | 47 | 1 463 171 | 0.69 | 0.49–0.98 |
| 0.59 | 0.36–0.97 | 0.81 | 0.56–1.16 | |||||
| Pensioner | 37 | 827 717 | 0.67 | 0.43–1.05 | 61 | 851 407 | 1.14 | 0.78–1.67 |
| 0.74 | 0.47–1.16 | 1.26 | 0.85–1.86 | |||||
Abbreviations: PYRS, person-years under risk; IRR 1, incidence rate ratio adjusted for period and age; IRR 2, incidence rate ratio adjusted for period, age, level of education, and disposable income.
aUnkown education in men: 5 cases in 261 619 person-years (IRR: 0.48, 95% CI: 0.20–1.18); in women: 8 cases in 180 853 person-years (IRR: 1.03, 95% CI: 0.51–2.11)
bTime period 1994–2006 and age range 30–69 years
Fig. 1.
Age-specific incidence rates (IRs) of vestibular schwannoma in the period 1993–2006 among men and women by the educational level (basic/high school, vocational education, and higher education) in a cohort of 3.26 million persons in Denmark.
Fig. 2.
Age-specific incidence rates (IRs) of vestibular schwannoma in the period 1993–2006 among married and nonmarried men and women in a cohort of 3.26 million persons in Denmark.
Nonmarried men (aggregating single, cohabitating, divorced, and widowed) had a substantially lower VS incidence rate than married men, whereas nonmarried women showed only a tendency for a lower incidence rate (Table 2). Simultaneous examination of marital status and education level showed that both effects persisted among men. Among both married and nonmarried women, there was also a decreasing risk for VS with a decreasing level of education, but little difference by marital status among the 3 education-level groups.
Table 2.
Marital status and highest attained education and risk of vestibular schwannoma in men and women, Denmark, 1993–2006
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | PYRS | IRR | 95% CI | Cases | PYRS | IRR | 95% CI | |
| Marital status | ||||||||
| Married | 422 | 11 106 945 | 1.00 | – | 373 | 11 276 272 | 1.00 | – |
| Not married | 120 | 6 578 540 | 0.62 | 0.50–0.76 | 172 | 6 347 216 | 0.87 | 0.73–1.05 |
| Education and marital status | ||||||||
| Highest/married | 109 | 2 502 334 | 1.00 | – | 101 | 2 526 375 | 1.00 | – |
| Middle/married | 186 | 5 027 426 | 0.83 | 0.66–1.06 | 125 | 3 962 324 | 0.73 | 0.56–0.95 |
| Basic/married | 125 | 3 453 857 | 0.73 | 0.56–0.95 | 141 | 4 692 998 | 0.60 | 0.46–0.78 |
| High/not married | 28 | 1 178 867 | 0.75 | 0.49–1.14 | 45 | 1 481 758 | 0.84 | 0.59–1.19 |
| Middle/not married | 54 | 2 559 446 | 0.63 | 0.45–0.87 | 48 | 1 852 874 | 0.64 | 0.46–0.91 |
| Basic/not married | 35 | 2 701 936 | 0.34 | 0.23–0.50 | 77 | 2 926 305 | 0.55 | 0.41–0.75 |
Abbreviations: PYRS, person-years under risk; IRR, incidence rate ratio.
Information about the spread and size of the tumor was available for 1083 (99.6%) of the 1087 cases. Overall, 298 (27.4%) of all VS were intrameatal only, and this proportion varied little by the education level (basic: 28.9%, middle: 25.6%, higher: 28.6%) and marital status (married: 28.1%, nonmarried: 25.7%). Sex-specific logistic regression analysis with adjustment for the year of diagnosis and the age at diagnosis confirmed the absence of an association between the spread of the tumor and education as well as marital categories. With regard to the subjects’ level of education, middle (P = .31) and higher education (P = .89) did not differ from basic education with regard to the risk for extrameatal spread. Likewise, the risk for extrameatal spread was not influenced by marital status (P = .96).
Among the 785 patients with an intra- and extrameatal VS, the mean and median diameters were 17.1 and 14 mm, respectively. Mean and median diameters in most groups by education level or marital status were similar to these overall values (Table 3), although tumors tended to be larger among women with higher education and among nonmarried men. These findings were confirmed by sex-specific multiple linear regression analyses adjusted for age and year of diagnosis. The strongest predictor for tumor size was the year of diagnosis, with P-values well below .01 in all models, showing decreasing tumor size with recency of diagnosis.
Table 3.
Size of vestibular schwannoma with intra- and extrameatal spread in relation to the level of education and marital status
| Size (diameter in mm) | n | Mean | Median | 1st quartile | 3rd quartile | Punia | Pmultb |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Level of education | .77 | .58 | |||||
| Highest | 91 | 17.6 | 15 | 10 | 25 | ||
| Middle | 171 | 17.7 | 15 | 10 | 24 | ||
| Basic | 109 | 16.6 | 15 | 10 | 21 | ||
| Marital status | |||||||
| Married | 289 | 16.8 | 14 | 10 | 22 | .08 | .09 |
| Not married | 85 | 19.4 | 17 | 10 | 27 | ||
| Women | |||||||
| Level of education | .05 | .04 | |||||
| Highest | 111 | 19.1 | 18 | 10 | 27 | ||
| Middle | 134 | 16.5 | 14 | 10 | 22 | ||
| Basic | 159 | 15.6 | 12 | 9 | 22 | ||
| Marital status | .35 | .32 | |||||
| Married | 279 | 17.1 | 14 | 10 | 24 | ||
| Not married | 132 | 16.2 | 13 | 9 | 23 | ||
aThe Kruskal–Wallis test.
bMultiple linear regression analysis additionally adjusted for age (age and age2) and year of diagnosis.
Discussion
In this large, nationwide cohort study in Denmark with over 35 million person-years of follow-up, we observed clear associations between education level and disposable income and the occurrence of VS, with higher incidence rates of VS in men and women with higher education and higher income. We did not observe a clear association between tumor size and those sociodemographic indicators that showed the strongest associations with tumor incidence.
One reason for the increasing incidence of VS with increasing education and income might be a different risk profile in affluent persons, but little is known about the etiology of VS. Loud noise has been suggested as a risk factor in some but not all studies.4,15–18 Incidence rates across broad occupational categories in our study were relatively similar, providing further evidence that occupational exposures are unlikely to cause the distinct social gradient. Weak associations with mobile phone use have been observed in some case–control studies3 but not confirmed by others.19 During the time period of our study of 1993–2006, the long-term use of mobile phones of 10–15 years was not very frequent, particularly among women, and thus even if mobile phones were a risk factor, this cannot explain the social gradient observed in our study. If lifestyle factors play a role in the etiology of VS, it would thus be a healthy lifestyle posing a risk, for which there is little evidence from previous studies. However, there was an inverse association with smoking in a recent international case–control study.20 The same study reported an increased risk for parous women compared with nulliparous women, putting forward the hypothesis that sex hormone levels may play a role in the etiology of VS.
Alternately, one needs to consider issues related to the awareness of the tumor as well as diagnosis and detection patterns.21,22 VS is a slowly growing tumor and is often asymptomatic or minimally symptomatic, with hearing difficulties or hearing loss as a major first detectable symptom.23 Interviews with patients have demonstrated that many VS patients had hearing problems for years before the diagnosis, resulting in a long period during which early detection of the tumor is possible.24 Such earlier detection might be more common among better educated and more affluent persons because of increased awareness of hearing problems in higher social classes due to a higher dependency on communication skills. However, a decreased “doctor's delay” might also play a role. Interestingly, a previous study using the same cohort as the current study found no or very weak associations between education and income and all types of brain tumors combined, suggesting that the observed pattern is unique for VS, probably owing to the distinct symptomatology.9 In a recent case–control study in the United States involving 782 brain tumor cases (including 96 cases of VS) and 799 controls, by far the largest social gradient in all brain tumor types was also observed for VS (an estimate of an increased VS risk of 5.3 for graduate/professional compared with less than high school), but in contrast to the above-mentioned findings in Denmark, an association was also seen with low-grade glioma, another slowly growing tumor.11 Given the healthcare system in the United States, which may involve factors such as lack of medical insurance, availability of medical care, and the ability to afford medical care, discussion is needed in relation to underdiagnosis,25 but this does not play a role in the Danish system of free and equal access to public medical care. Therefore, it is more likely that it is the patient's delay, specifically the awareness and recognition of symptoms, the time elapsed before seeking medical help, and the time lag between going to general medical practice and being referred to specialized diagnostic centers, which might play a key role in explaining our results. An under-diagnosis might also be presumed among the unemployed or early-retirement pensioners, but such an assumption is of a more speculative nature.
Along these lines, it is a curious finding to observe a significantly higher incidence rate of VS in married men of all ages compared with nonmarried men but a weaker association among women. It might be that women are more likely to point out symptoms, such as hearing loss in men, than vice versa. Another possibility is that women are more insistent in advising men to seek medical help in the case of symptoms than vice versa. It remains unexplained, though, why married men also have a higher incidence rate compared with men cohabitating with their partner, and one may only speculate about the effect of the characteristics of such relationships. Pensioners did not show significantly lower incidence rates than most occupational groups, but high awareness of hearing problems following retirement is an empirically well-known phenomenon for hearing loss-related diagnoses other than VS.
Tumor spread and size were not associated with educational level and marital status, and there was even a tendency for larger tumors in women with a higher educational level. These findings raise some doubts as to whether the observed association with the incidence can be attributed entirely to early detection. However, in a Finnish case series of 91 VS patients, diagnostic delay had no effect on the VS size at the time of diagnosis,26 and in clinical practice, it is observed that hearing loss is not directly associated with tumor size, ie, a huge tumor may be found in a person with almost normal hearing and a small tumor may induce deafness.
Our study has some methodological advantages, including the size of the study, which utilized access to the entire Danish population with annual updates of the sociodemographic indicators and complete ascertainment of cases of a benign tumor that is usually under registered in most cancer registries. The combination of data from the nationwide population-based cancer registry with a clinical database established on the same premises using the unique Danish central population registry number of each person enabled us to obtain high-quality diagnostic information, including the size of the tumor, and reduced to a minimum the risk for misclassification of the outcome under study. Likewise, the use of these data sources established for administrative reasons and years before the current hypothesis was established reduced the risk for selection and information bias. Finally, the conduct of the study in a country characterized by a tax-paid public health system with equal access to health services at all levels secured a high degree of reliability of the data. To the best of our knowledge, our study is unique with regard to the study of the association between sociodemographic factors and the risk for VS. We cannot rule out that some associations are owing to chance, and we did not formally adjust for multiple comparisons; however, the internal consistency of our findings argues against the role of chance.
In conclusion, we observed that married Danish citizens characterized as having a high education and a high income were at a significantly increased risk for VS. Our results indicate that gender differences may play a role when it comes to noting the first symptoms of disease in a partner. The magnitude of the effect caused by sociodemographic factors suggests a potential for the earlier diagnosis of VS by improving the awareness of early symptoms in the population overall and specifically in the less-educated and less-affluent groups in society.
Funding
Our work was supported by the Danish Cancer Society.
Authors' contribution
All authors contributed to the design of the study and to writing the paper.
Acknowledgments
We thank the steering committee of the Danish cohort study on social inequality and cancer (Cancer og social ulighed – CANULI) for providing the cohort data.
Conflict of interest statement. None declared.
References
- 1.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neurooncology. 2006;8(1):27–37. doi: 10.1215/S1522851705000323. doi:10.1007/BF02672073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanser MJ, Sussman SA, Frazer K. Epidemiology, pathogenesis, and genetics of acoustic tumors. Otolaryngol Clin North Am. 1992;25(3):499–520. doi:10.1007/BF00935980. [PubMed] [Google Scholar]
- 3.Ahlbom A, Feychting M, Green A, Kheifets L, Savitz DA, Swerdlow AJ ICNIRP (International Commission for Non-Ionizing Radiation Protection) Standing Committee on Epidemiology. Epidemiologic evidence on mobile phones and tumor risk: a review. Epidemiology. 2009;20(5):639–652. doi: 10.1097/EDE.0b013e3181b0927d. doi:10.1139/g95-128. [DOI] [PubMed] [Google Scholar]
- 4.Edwards CG, Schwartzbaum JA, Nise G, et al. Occupational noise exposure and risk of vestibular schwannoma. Am J Epidemiol. 2007;166(11):1252–1258. doi: 10.1093/aje/kwm217. [DOI] [PubMed] [Google Scholar]
- 5.Corona AP, Oliveira JC, Souza FP, Santana LV, Rêgo MA. Risk factors associated with vestibulocochlear nerve schwannoma: systematic review. Braz J Otorhinolaryngol. 2009;75(4):593–615. doi: 10.1016/S1808-8694(15)30501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tos M, Stangerup SE, Cayé-Thomasen P, Tos T, Thomsen J. What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg. 2004;130(2):216–220. doi: 10.1001/archotol.130.2.216. [DOI] [PubMed] [Google Scholar]
- 7.Stangerup SE, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004;118(8):622–627. doi: 10.1258/0022215041917989. doi:10.1006/anbo.1995.1085. [DOI] [PubMed] [Google Scholar]
- 8.Dalton SO, Schüz J, Engholm G, et al. Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: summary of findings. Eur J Cancer. 2008;44(14):2074–2085. doi: 10.1016/j.ejca.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt LS, Nielsen H, Schmiedel S, Johansen C. Social inequality and incidence of and survival from tumours of the central nervous system in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44(14):2050–2057. doi: 10.1016/j.ejca.2008.06.015. doi:10.1006/anbo.1999.1071. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. doi:10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- 11.Inskip PD, Tarone RE, Hatch EE, et al. Sociodemographic indicators and risk of brain tumours. Int J Epidemiol. 2003;32(2):225–233. doi: 10.1093/ije/dyg051. doi:10.1023/A:1006344508454. [DOI] [PubMed] [Google Scholar]
- 12.Dalton SO, Steding-Jessen M, Gislum M, Frederiksen K, Engholm G, Schüz J. Social inequality and incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: background, aims, material and methods. Eur J Cancer. 2008;44(14):1938–1949. doi: 10.1016/j.ejca.2008.06.010. doi:10.1016/j.pbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Florida R. The Rise of the Creative Class. New York: Basic Books; 2002. [Google Scholar]
- 14.Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry—history, content, quality and use. Dan Med Bull. 1997;44(5):535–539. doi:10.1007/s001220050774. [PubMed] [Google Scholar]
- 15.Preston-Martin S, Thomas DC, Wright WE, Henderson BE. Noise trauma in the aetiology of vestibular schwannomas in men in Los Angeles County, 1978–1985. Br J Cancer. 1989;59(5):783–786. doi: 10.1038/bjc.1989.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards CG, Schwartzbaum JA, Lönn S, Ahlbom A, Feychting M. Exposure to loud noise and risk of vestibular schwannoma. Am J Epidemiol. 2006;163(4):327–333. doi: 10.1093/aje/kwj044. doi:10.1534/genetics.108.092304. [DOI] [PubMed] [Google Scholar]
- 17.Schlehofer B, Schlaefer K, Blettner M, et al. Environmental risk factors for sporadic vestibular schwannoma (Interphone Study Group, Germany) Eur J Cancer. 2007;43(11):1741–1747. doi: 10.1016/j.ejca.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Hours M, Bernard M, Arslan M, et al. Can loud noise cause vestibular schwannoma? Analysis of the INTERPHONE study in France. Occup Environ Med. 2009;66(7):480–486. doi: 10.1136/oem.2008.042101. doi:10.1111/j.1469-8137.1994.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 19.Schüz J, Jacobsen R, Olsen JH, Boice JD, Jr, McLaughlin JK, Johansen C. Cellular telephone use and cancer risk: update of a nationwide Danish cohort. J Natl Cancer Inst. 2006;98(23):1707–1713. doi: 10.1093/jnci/djj464. doi:10.1093/aob/mci005. [DOI] [PubMed] [Google Scholar]
- 20.Schoemaker MJ, Swerdlow AJ, Auvinen A, et al. Medical history, cigarette smoking and risk of vestibular schwannoma: an international case–control study. Int J Cancer. 2007;120(1):103–110. doi: 10.1002/ijc.22272. [DOI] [PubMed] [Google Scholar]
- 21.Lin D, Hegarty JL, Fischbein NJ, Jackler RK. The prevalence of “incidental” vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2005;131(3):241–244. doi: 10.1001/archotol.131.3.241. doi:10.1139/g93-004. [DOI] [PubMed] [Google Scholar]
- 22.Anderson TD, Loevner LA, Bigelow DC, Mirza N. Prevalence of unsuspected vestibular schwannoma found by magnetic resonance imaging. Otolaryngol Head Neck Surg. 2000;122(5):643–646. doi: 10.1016/S0194-5998(00)70189-6. doi:10.1534/genetics.105.041632. [DOI] [PubMed] [Google Scholar]
- 23.Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol. 1998;255(1):1–6. doi: 10.1007/s004050050012. doi:10.1139/G07-083. [DOI] [PubMed] [Google Scholar]
- 24.Christensen HC, Schüz J, Kosteljanetz M, Poulsen HS, Thomsen J, Johansen C. Cellular telephone use and risk of acoustic neuroma. Am J Epidemiol. 2004;159(3):277–283. doi: 10.1093/aje/kwh032. doi:10.1139/G08-043. [DOI] [PubMed] [Google Scholar]
- 25.Curry WT, Jr, Barker FG., 2nd Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93(1):25–39. doi: 10.1007/s11060-009-9840-5. doi:10.1093/aob/mcf008. [DOI] [PubMed] [Google Scholar]
- 26.Teppo H, Heikkinen J, Laitakari K, Alho OP. Diagnostic delays in vestibular schwannoma. J Laryngol Otol. 2009;123(3):289–293. doi: 10.1017/S0022215108003113. [DOI] [PubMed] [Google Scholar]