Abstract
Over the last two decades, chemotherapy has been introduced in protocols for patients with intracranial germinoma with the objective of reducing the volume and the dose of irradiation without compromising survival rates. The aim of this work is to critically analyze the pattern of relapse in a cohort of patients with nonmetastatic germinoma prospectively treated with chemotherapy followed by focal field radiation. Data of all germinoma patients registered in the French protocol for intracranial germ cell tumors between 1990 and 1999 were reviewed. The pattern of relapse, management, and outcome were analyzed in 10 of 60 patients who developed a recurrence after initial treatment. In 9 patients, the site of recurrence was local or loco-regional, notably in the periventricular area for 8. One patient only had isolated distant leptomeningeal relapse. The review of the sites of relapse suggests that most recurrences could have been avoided with a larger ventricular field of radiation. Treatment at first relapse included chemotherapy (10 patients), high-dose chemotherapy and stem cell transplant (8 patients), and/or radiation therapy (4 patients). Five patients experienced a second relapse. At a median follow-up of 72 months since the first relapse, 8 patients are alive in second or third remission. This review identified an excess of periventricular relapses when the focal field of radiation is used in the combined management of germinoma. These relapses are predominantly marginal or outside radiation fields. Ventricular field radiation appears a logical alternative to decrease the incidence of such relapses. Future trials should aim at better identifying patients who may benefit from local and ventricular radiation, respectively.
Keywords: germinoma, high-dose chemotherapy, relapse, ventricular radiation
Germ cell tumors of the central nervous system (CNS) are a heterogeneous group of tumors that constitutes approximately 3% of primary pediatric CNS tumors in Europe and the United States but a far higher percentage in the Far East. Germinomas account for 70% of these tumors. Germinomas commonly develop within the suprasellar or pineal regions but may also be found in the basal ganglia, spinal cord, or nonmidline structures, with 15%–25% of germinomas arising simultaneously in the pineal and the suprasellar sites. If we consider these bifocal lesions as loco-regional rather than disseminated disease, most intracranial germinomas are nonmetastatic at the time of presentation. Until approximately the late 1980s, the standard management of germinoma was based on craniospinal irradiation (CSI) to a dose of 25–35 Gy with a 10–25-Gy boost to the primary tumor. Using this approach, the 5-year survival rates in retrospective and prospective series were above 80% demonstrating the radiosensitivity and high cure rate of intracranial germinoma with radiation therapy (RT) only.1–3 In an attempt to reduce the potential morbidity of CSI, cooperative groups have investigated the feasibility of combined modality treatment, using sequential chemotherapy followed by focal radiation for nonmetastatic germinoma and CSI with a boost to the tumor bed and metastatic sites for patients with disseminated germinoma. It was speculated that the high chemosensitivity of germinoma might allow RT to be given more selectively without increasing the risk of relapse; one of the theoretical advantages for giving pre-RT chemotherapy was the opportunity to reduce radiation doses and volumes. Excellent 5-year event-free survival rates above 80% have been obtained in these pilot studies.4–6 However, still approximately 15% of patients with germinomas experience local recurrence and/or leptomeningeal dissemination. In the absence of prospective randomized studies, comparisons between CSI and focal radiation for patients with nonmetastatic germinoma are speculative and may be influenced greatly by variations in patients’ characteristics. Retrospective reviews have suggested that focal radiation alone was associated with an increased risk of spinal relapses.7,8 More recently, it has been suggested that focal radiation may increase the risk of marginal failures.9–11 In an attempt to evaluate the relationship between radiation volume reduction and the risk of relapse in nonmetastatic germinoma patients, the Germ Cell Tumor Committee of the SFOP (Société Française d'Oncologie Pédiatrique) centrally reviewed all relapses from the prospective protocol SFOP TGM-TC-90.
Materials and Methods
The TGM-TC-90 Protocol
Pathological diagnosis of germinoma was required before initiation of the protocol. However, for patients with moderate human chorionic gonadotropin (HCG) secretion, histological confirmation of germinoma was recommended but not compulsory. Initial stage procedure included clinical examination, assessment of tumor markers in the serum and/or the cerebrospinal fluid (CSF) review of the operative notes, postoperative cranial computed tomography or magnetic resonance imaging (MRI) scan, MRI scan of the spine, and cytological examination of lumbar CSF when this was considered safe.
Details of the TGM-90 protocols have been described previously.6,12 Briefly, after diagnosis, 4 courses of chemotherapy were given at a 3-week interval: courses 1 and 3 included carboplatin (600 mg/m2) on day 1 and etoposide (150 mg/m2 per day) from day 1 to 3; courses 2 and 4: ifosfamide (1.8 g/m2 per day) from day 1 to 5 and etoposide (150 mg/m2 per day) from day 1 to 3. Response was assessed after 2 and 4 courses. Three to 4 weeks after completion of these 4 courses of chemotherapy, RT was initiated. RT was administered at a daily dose of 1.6–1.8 Gy with 5 weekly fractions up to a recommended dose of 40 Gy delivered over 4.5–5 weeks for patients with localized disease. The recommended safety margins were 2 cm around the initial tumor volume, regardless of tumor shrinkage during chemotherapy. RT guidelines recommended the use of a high-energy linear accelerator of 4–9 MeV with at least 2 parallel opposed fields (conventional planning). Patients with metastatic disease were treated with craniospinal radiation at a dose of 24 Gy and a local tumor boost of 16 Gy. All patients were followed clinically during and after treatment, and MRI scan of the head was recommended every 4 months during the first 2 years following the completion of therapy unless additional examinations were indicated clinically. Imaging studies were then performed at 6 monthly intervals for 5 years. In addition, serum tumor markers were regularly checked. Progression was defined as any new lesion detected in any of the examinations with or without an elevation of tumor markers. A complete restaging (CSF examination, MRI of the brain and the spine, tumor markers) was performed for relapsing patients.
Patient Data and Evaluation of Recurrences
The records of all assessable patients with nonmetastatic intracranial germinoma treated in the French TGM-90 study between August 1990 and March 1999 who experienced treatment failure were reviewed. Information obtained from their records included the following: date of birth, sex, date of diagnosis, initial therapeutic intervention, pattern of relapse, management of relapse, and outcome. The following data were obtained at presentation and at relapse: diagnostic imaging studies (brain and spine), CSF analysis, serum and CSF tumor markers (βHCG and α-fetoprotein), pathology when available, and details of surgical, chemotherapeutic, and radiotherapeutic interventions.
All MRI scans and radiation treatment parameters of these patients were reviewed centrally by a pediatric radiologist (H.B.), a radiation oncologist (C.A.), a physicist (G.G.), and a neuro-oncologist (E.B.). A multidisciplinary panel of radiation oncologists of the Société Française de Lutte contre les Cancers et Leucémies de l'Enfant et de l'Adolescent (SFCE) participated to the first review session coordinated by C.A. Both primary tumor and the first site(s) of relapse were positioned on simulator films. The margins of the initial radiation fields were reviewed, and the site(s) of relapse was analyzed with respect to its location in relation to the edge of the radiation field. Depending on the location, relapses were defined as in-field (in the target volume), at margins (<1.5 cm from the target volume or within the 50%–90% isodose curves), out-field proximal (still on beam trajectory, with radiation dose below the 50% isodose curves), or at a distance of radiation exposure.
Results
Between January 1990 and October 1999, 80 patients with a presumed or confirmed diagnosis of germinoma were registered in this prospective study of the SFOP. Fourteen patients had metastatic disease and 66 had nonmetastatic germinoma. Six patients received elective craniospinal radiation based on the physican's decision or at the patient/parents’ request and 60 received limited field radiation according to the protocol recommendations. Within this latter group, 24 patients had a suprasellar tumor, 30 had a pineal lesion, and 6 had a bifocal germinoma with both a suprasellar and a pineal component. Eight of 51 patients who had blood markers carried out had a slight (<50 mIU/ml) βHCG secretion in the serum, and 14 of 45 had positive βHCG or free βHCG in the CSF (6 had combined positive serum and CSF markers). Fifty-one patients underwent a biopsy, whereas 9 patients including 6 with positive βHCG were treated without histological confirmation. All 60 patients received 4 courses of chemotherapy followed by protocolar focal irradiation. Patients with bifocal tumors were treated with a large field encompassing both suprasellar and pineal primary tumor sites.
Relapses
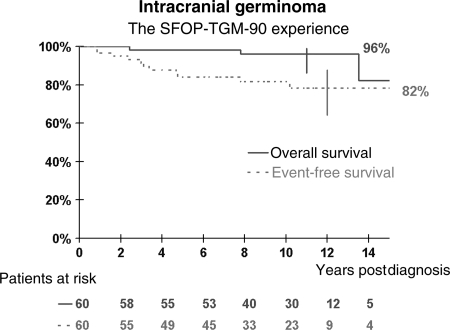
Data were censored in March 2008 or at the date of last recorded follow-up visit. The median follow-up for the whole cohort was 10 years, and 40 patients had a follow-up ≥8 years. The 5-year event-free and overall survival were 84.2% (CI: 72.5%–91.4%) and 98.2% (CI: 90.7%–99.7%), respectively. The 10-year event-free and overall survival were 81.8% (CI: 69.5%–89.9%) and 96% (CI: 86.3%–98.9%), respectively (Fig. 1). One patient with a pre-existing chronic condition died of unrelated causes. Ten of 60 patients relapsed. Characteristics of relapsing and nonrelapsing patients were not different with regard to sex distribution, age, tumor location, CSF diversion, tumor size, and presence of HCG secretion in the blood or in the CSF (Table 1). The median time to relapse was 32 months (10–121 months) with 3 patients developing late relapses at 57, 59, and 121 months, respectively.
Fig. 1.
Event-free and overall survival of patients with nonmetastatic germinoma treated with combined chemotherapy and focal radiation in the SFOP-TGM-TC-90 protocol.
Table 1.
Characteristics of patients with and without relapse
| Relapses | Nonrelapses | |
|---|---|---|
| Sex | ||
| Male | 8 | 36 |
| Female | 2 | 15 |
| Age | 14.2 (9.6–19) | 13 (5–24) |
| Site | ||
| Pineal | 6 | 24 |
| Suprasellar | 3 | 21 |
| Bifocal | 1 | 5 |
| CSF diversion (including shunt) | 7 (6) | 26 (18) |
| Median size of largest dimension (range) | 20 mm (15–30) | 27 mm (6–77) |
| HCG secretion (number available) | ||
| Blood + (51) | 2 | 6 |
| CSF + (45) | 2 | 12 |
| Both (45) | 2 | 4 |
| Neither (45) | 8 | 30 |
| CSF + Blood − (45) | 0 | 8 |
| CSF − Blood + (45) | 0 | 2 |
| Blood − (51) | 8 | 44 |
Abbreviations: CSF, cerebrospinal fluid; HCG, human chorionic gonadotropin.
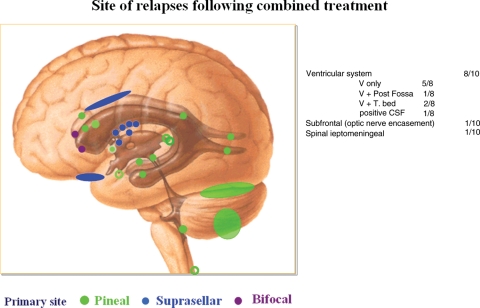
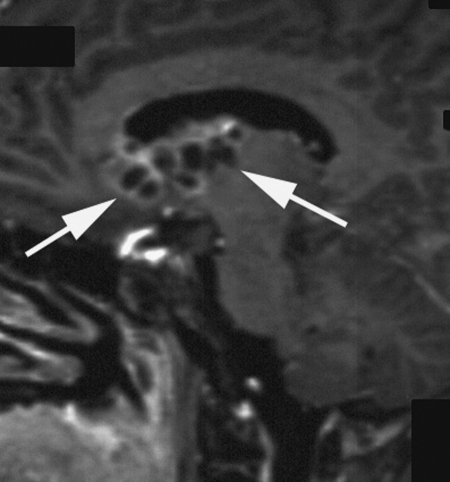
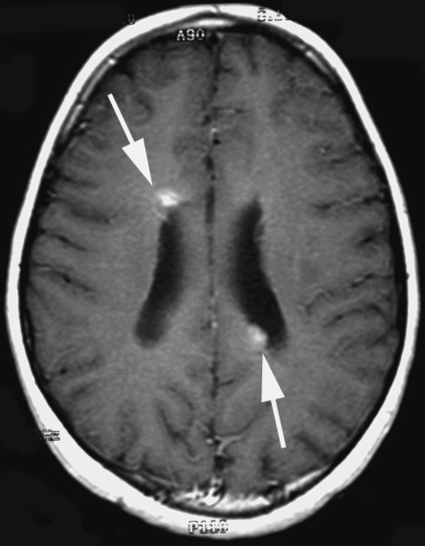
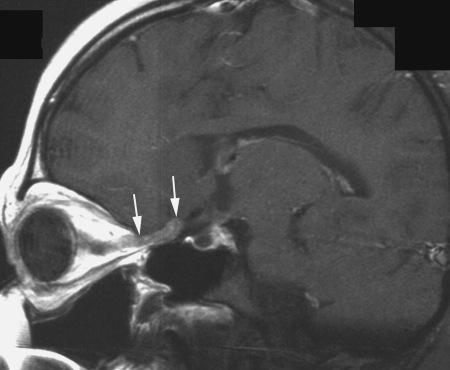
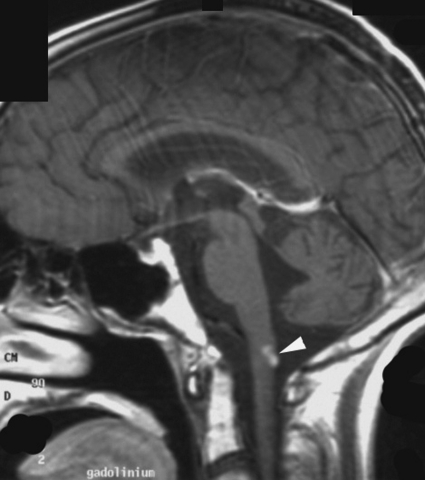
Relapses occurred predominantly in the periventricular subependymal area (lateral ventricles, third and fourth ventricle) in 8 of 10 patients (Table 2, Fig. 2). They were associated with evidence of relapse at the primary site in 2 patients and with infratentorial leptomeningeal dissemination in 1 patient (Figs 3 and 4). One patient with suprasellar primary (patient 1) had an isolated meningeal relapse at the margin of the radiation field located in the posterior part of the meningeal sheath of the right optic nerve (Fig. 5). This relapse was the latest, occurring 121 months from the time of diagnosis. One patient had a unifocal relapse located at the posterior face of the medulla oblongata (Fig. 6). One patient presented with distant isolated leptomeningeal relapse at the cervical and thoracic levels. For these 2 patients, the original MRI scans of the spine were reviewed to rule out metastatic seeding that could have been missed at diagnosis.
Table 2.
Patients characteristics, site, and type of relapse with regard to the radiation RT field
| Patient number | Primary site | Margins of radiation (technique) | Delay of relapse (months) | Type of relapse | Site of relapse/RT field | Treatment at first relapse | Treatment at second relapse | Outcome (follow-up from first relapse) |
|---|---|---|---|---|---|---|---|---|
| 1 | Suprasellar | 2 cm (2 fields) | 121 | Optic nerve meningeal sheath | Marginal | C-HDC | C | DOD (43) |
| 2 | Bifocal | 2 cm (2 fields) | 29 | Periventricular (frontal horns) | Marginal | C-HDC-RT | alive (54+) | |
| 3 | Pineal | 0.3–1.2 cm (4 fields) | 31 | Periventricular (peribulbar) | Marginal | C-HDC-CSI | alive (57+) | |
| 4 | Pineal | 1.5–2.5 cm (4 fields) | 57 | Periventricular (occipital horns) and leptomeningeal (infratentorial) | Marginal and out- field proximal | –C-HDC-S | C-CSI | alive (98+) |
| 5 | Pineal | 1–3 cm (4 fields) | 10 | Periventricular (left and right ventricles) | In-field and out-field proximal | C-HDC | alive (130+) | |
| 6 | Pineal | 0.7 cm (arc therapy) | 21 | Primary site and periventricular (frontal and temporal horns) | In-field and out-field proximal | C-HDC | C-S-CSI | alive (87+) |
| 7 | Pineal | 1–2.5 cm (4 fields) | 10 | Leptomeningeal spinal (C5, C7, T12) + CSF | Distant | –C-RT | C | DOD (20) |
| 8 | Pineal | 0.5–2 cm (2 fields) | 38 | Primary site, pituitary stalk, chiasm, and Periventricular nodules + CSF | In-field and out-field proximal | C-CSI | alive (177+) | |
| 9 | Suprasellar | 2 cm (4 fields) | 59 | Periventricular (frontal horn + third ventricle) | In-field and marginal | C-HDC | alive (54+) | |
| 10 | Suprasellar | >2 cm (4 fields) | 33 | Periventricular (frontal horns) | In-field and marginal | C-HDC | C-CSI | alive (94+) |
Abbreviations: C, chemotherapy; HDC, high-dose chemotherapy; S, surgery; RT, radiotherapy; CSI, craniospinal irradiation; DOD, dead of disease.
Fig. 2.
Distribution of all sites of first relapse: several patients had more than 1 site of failure; 2 sites in patients 2, 4, and 5; 4 sites in patient 6; multiple sites in patients 8 and 9; and continuous spread along the wall of lateral ventricles in patient 10.
Fig. 3.
Periventricular relapse (arrows) with nodular heterogeneous and pseudocystic pattern in patient 9. Postcontrast T1-weighted left parasagittal MR view.
Fig. 4.
Nodular periventricular relapse in patient 5 (arrows). Postcontrast T1-weighted axial MR view.
Fig. 5.
Meningeal relapse located in the posterior part of the meningeal sheath of the right optic nerve (arrows) in patient 1. Postcontrast T1-weighted sagittal MR view.
Fig. 6.
Distant meningeal relapse located at the posterior face of the medulla oblongata (arrowhead) in patient 3. Postcontrast T1-weighted sagittal MR view.
The review of the radiation fields did not identify any geographical miss. However, 3 patients had inadequate margins: in 2 patients (patients 3 and 8) treated with a conventional radiotherapy approach, the margin around the initial tumor volume was <1 cm at the anterior limit of lateral fields (3 and 5 mm, respectively), and for a third patient (patient 6), margins were intentionally reduced to 7 mm in the context of arc therapy. All 3 patients had an excellent response to preradiation chemotherapy. Overall, these 3 patients with inadequate margins all failed within the ventricular system and 2 (patients 6 and 8) failed at the primary site.
Salvage Treatment
First relapse.
After failing primary treatment, all patients received platinum-based chemotherapy (Table 2). Six patients were treated with a cisplatin–etoposide–ifosfamide (ICE) combination, 2 with a cisplatin–etoposide regimen, and 2 with a combination of etoposide and carboplatin. All responded to this salvage treatment, including 6 who achieved a complete response on imaging. Eight patients subsequently received high-dose chemotherapy with autologous stem cell rescue. For 7 patients, the conditioning regimen was a combination of etoposide and thiotepa, and 1 patient received 2 sequential courses of high-dose ICE followed by autologous stem cell transplant. Four patients then received adjuvant radiation treatment: focal irradiation in 1 patient and CSI in 3, including 2 who received an additional boost. Five patients are in continuous remission following salvage treatment (54–177 months after relapse): 2 patients were treated with chemotherapy followed by high-dose chemotherapy and stem cell transplant; 1 patient received conventional chemotherapy followed by CSI; and 2 patients were treated with conventional followed by high-dose chemotherapy and stem cell transplant and focal irradiation (1 patient) or CSI (1 patient). Out of the 6 patients who did not receive adjuvant radiation, only 2 did not experience further progression.
Second relapse.
Five patients experienced a second relapse at a median interval of 22.5 months after the first relapse (range: 9.5–35 months). Four of these 5 patients did not receive RT as part of their first salvage treatment. All received additional chemotherapy and 3 received craniospinal radiation with a boost. The patient who received craniospinal radiation as part of his first salvage treatment for a spinal relapse eventually developed new lesions and died of tumor progression. One patient who achieved a partial response on chemotherapy refused radiation treatment, progressed early after discontinuation of chemotherapy, and died of disease progression.
Overall, at a median interval of 72 months since the first relapse, 8 patients are alive in second (5 patients) or third remission (3 patients). All but one surviving patient had received high-dose chemotherapy as part of their salvage treatment and 6 surviving patients were reirradiated (at the time of first relapse in 3 and second relapse in 3).
Discussion
This long follow-up review of relapses in a prospective group of nonmetastatic intracranial germinoma patients homogeneously treated with chemotherapy followed by focal radiation showed that the majority of failures occur in the periventricular area. Indeed, in this experience, only 2 relapses occurred outside the ventricular system. Neither the original site of disease nor the size of the primary tumor appears to predict the risk of ventricular failure. The majority of ventricular relapses were located at the margins or outside the focal radiation field applied in this trial. Consequently, this pattern of relapse raises the question of the appropriate volume of radiation in patients with nonmetastatic germinoma.
A careful review of radiation fields in the present series identified reduced anterior margins (<1 cm) in 3 patients, whereas the recommended margins of 2 cm around the initial tumor volume were respected for all margins in 4 patients only. The significant shrinkage observed following preradiation chemotherapy may account for these protocol violations. However, as our review was limited to patients who presented treatment failure, the significance of these treatment violations is unknown.
From this experience, and in agreement with more recent observations of Japanese groups11,10 and the preliminary reports of the SIOP-96 trial,10,13 there appears to be a direct link between the volume of radiation and the pattern of relapse in patients with nonmetastatic germinoma treated with chemotherapy followed by focal radiation. In contrast, the dose of radiation does not seem to have the same impact on the risk of recurrence.14 In a meta-analysis of RT for patients with CNS germinoma, Rogers et al.9 found that the recurrence rate after focal radiation (with or without chemotherapy) was 23% compared with 8% when whole-brain or whole-ventricular irradiation was used. In an early report of the Japanese germ cell tumor group using chemotherapy followed by focal radiation, the incidence of treatment failure was significantly associated with the size of the radiation fields: most relapses developed outside the radiation volume and were associated with <1 cm margins.10 Our results are in agreement with these observations and suggest that a subset of patients with so-called localized germinoma might have in fact infraclinical subependymal disease that is not detected by conventional imaging techniques and that is not controlled by chemotherapy. The significance of this observation should be linked to neurosurgeons’ reports of endoscopically visible plaques of disseminated disease on the ventricles at the time of third ventriculostomy, which remained undetectable in MRI scanning.15–17 These findings illustrate the limitations of current MRI and CSF staging methods in the detection of regional periventricular disease in germinoma patients and identify the ventricular system as the area at risk of dissemination. Although the incidence of failure reported here (16%) and in the literature (23%) is limited to a subset of patients when focal radiation fields are used, our data suggest that most relapses might be avoided with a larger field of radiation. As long as we do not have tools to safely detect subependymal disease, this underscores the need for encompassing the ventricles rather than defining the volume of radiation according to the primary tumor site with an additional margin. In view of these results, the SIOP group has already recommended the use of ventricular radiation for patients with nonmetastatic germinomas.18 The definition of the ventricular volume should take into account the risk of subependymal recurrence in the lower part of the fourth ventricle as illustrated in our experience with patient 3; this suggests that consideration should be made to include the caudal edge of the fourth ventricle in the ventricular volume. This series did not include any patient with basal ganglia germinoma. Whole brain or craniospinal radiation have been traditionally used in this context.19,20
Although the decision to consider ventricular radiation for all patients may reduce the incidence of relapses, one should keep in mind that 80%–85% of patients with localized germinoma are successfully treated with the limited radiation field approach. The use of ventricular fields is therefore an alternative “by default” and the consequence of our inability to detect patients at a risk of periventricular failure. Current European and Japanese groups have adopted this approach, and the outcome of ongoing studies will determine whether these larger volumes will result in improved event-free survival. However, the use of larger volumes should be balanced against the short- and long-term toxicity of this strategy, and ideally efforts should be made to identify these patients (overall 15%–20%) who would benefit from these larger radiation volumes. Anecdotal observations of ventricular dissemination during endoscopy and the detection of tumor dissemination with 5-Aminolevulinic acid induced fluorescence suggest that identification of these patients at risk may be possible.15–17,21
The follow-up in this series is long, ranging from 1 to 16 years, with a median of 10 years. This long follow-up contributes to shed new lights on the pattern of relapse in germinoma, and particularly to point out the risk of late relapses, so far anecdotally reported in the literature.22,23 Three patients in this series experienced relapses >4 years after diagnosis, and the latest relapse occurred at 10 years, with a median time to relapse of 32 months. This suggests that early reports of prospective or pilot germinoma studies should be considered with caution when the follow-up is short, as late relapses in germinoma are not exceptional.
The salvage rate in this series is high, with 8 of 10 patients who are in second (5 patients) or third (3 patients) remission at the last follow-up. Two patients died despite salvage chemotherapy and craniospinal radiation in 1 patient and high-dose chemotherapy without radiation in the second patient whose family declined radiation. Such a high survival rate among patients who have suffered primary or secondary treatment failure is unique in the context of recurrent malignant brain tumors. The respective role of high-dose chemotherapy and radiation in the management of recurrent germinoma patients is still unclear, and the optimal treatment for these patients is undefined. Radiation is obviously the logical option for patients who fail initial treatment with chemotherapy only. Merchant et al.24 reported the outcome of 8 patients initially treated with chemotherapy only who were successfully retrieved with one course of high-dose cyclophosphamide followed by full neuraxis RT. All 8 patients were alive in complete second remission at the median follow-up after RT of 32 months. There is limited information on the management of patients who relapsed following combined modality therapy. Our experience shows that these patients can be reinduced with the same chemotherapy, in particular with platinum-containing regimens. Eight patients in this series received high-dose chemotherapy with autologous stem cell transplant. The largest experience of high-dose chemotherapy with autologous bone marrow or stem cell rescue was reported by Modak et al.,25 with 9 recurrent germinoma patients treated with thiotepa-based regimens, including 7 who were long-term survivors. However, only 1 patient in this experience did not receive radiation before or after high-dose chemotherapy. Similarly, in our experience, 6 of 8 long-term survivors did receive radiation as part of their retrieval, and 4 of 6 patients who were treated with high-dose chemotherapy without additional radiation experienced a second relapse. Both experiences suggest that RT is an important component of the management of recurrent germinoma. The role of high-dose chemotherapy is still unclear, and excellent outcomes have also been reported in patients with recurrent germinoma treated with conventional chemotherapy and wide-field low-dose radiation.22,26
Conclusion
This SFOP experience points out the risk of periventricular, marginal, and out radiation field relapse when a combined approach with focal RT is used in the treatment of nonmetastatic germinoma. Future trials should carefully assess the potential benefit of ventricular radiation in reducing the incidence of such occurrences. Ultimately, efforts should be made to better identify the risk of ventricular spread of pineal and/or suprasellar germinoma patients in order to tailor radiation fields and volumes accordingly.
Conflict of interest statement. None declared.
Funding
Supported by the Société Française d'Oncologie Pédiatrique.
References
- 1.Dearnaley DP, A'Hern RP, Whittaker S, Bloom HJ. Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962–1987. Int J Radiat Oncol Biol Phys. 1990;18(4):773–781. doi: 10.1016/0360-3016(90)90396-2. [DOI] [PubMed] [Google Scholar]
- 2.Merchant TE, Sherwood SH, Mulhern RK, et al. CNS germinoma: disease control and long-term functional outcome for 12 children treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2000;46(5):1171–1176. doi: 10.1016/s0360-3016(99)00375-2. [DOI] [PubMed] [Google Scholar]
- 3.Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17(8):2585–2592. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 4.Matsutani M. Combined chemotherapy and radiation therapy for CNS germ cell tumors—the Japanese experience. J Neurooncol. 2001;54(3):311–316. doi: 10.1023/a:1012743707883. [DOI] [PubMed] [Google Scholar]
- 5.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997;32(1):71–80. doi: 10.1023/a:1005732105727. [DOI] [PubMed] [Google Scholar]
- 6.Bouffet E, Baranzelli MC, Patte C, et al. Combined treatment modality for intracranial germinomas: results of a multicentre SFOP experience. Societe Francaise d'Oncologie Pediatrique. Br j cancer. 1999;79(7–8):1199–1204. doi: 10.1038/sj.bjc.6690192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Goss B, Ford J. CNS germ cell tumors: pattern of failure and effects of radiation volume. J Med Assoc Thailand. 2006;89(4):415–421. [PubMed] [Google Scholar]
- 8.Ogawa K, Yoshii Y, Shikama N, et al. Spinal recurrence from intracranial germinoma: risk factors and treatment outcome for spinal recurrence. Int J Radiat Oncol Biol Phys. 2008;72(5):1347–1354. doi: 10.1016/j.ijrobp.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 9.Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol. 2005;6(7):509–519. doi: 10.1016/S1470-2045(05)70245-X. [DOI] [PubMed] [Google Scholar]
- 10.Matsutani M. Treatment of intracranial germ cell tumors: the second phase II study of Japanese GCT study group. Thirteenth International Symposium on Pediatric Neuro-Oncology: June 29–July 2, 2008: Chicago. J Neurooncol. 2008;10:420. [Google Scholar]
- 11.Shirato H, Aoyama H, Ikeda J, et al. Impact of margin for target volume in low-dose involved field radiotherapy after induction chemotherapy for intracranial germinoma. Int J Radiat Oncol Biol Phys. 2004;60(1):214–217. doi: 10.1016/j.ijrobp.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Baranzelli MC, Patte C, Bouffet E, et al. Nonmetastatic intracranial germinoma: the experience of the French Society of Pediatric Oncology. Cancer. 1997;80(9):1792–1797. [PubMed] [Google Scholar]
- 13.Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of localized and metastatic germinoma treated according to SIOP CNS GCT 96. Thirteenth International Symposium on Pediatric Neuro-Oncology: June 29–July 2, 2008; Chicago. J Neurooncol. 2008;10:420. [Google Scholar]
- 14.Shim KW, Kim TG, Suh CO, et al. Treatment failure in intracranial primary germinomas. Childs Nerv Syst. 2007;23(10):1155–1161. doi: 10.1007/s00381-007-0394-6. [DOI] [PubMed] [Google Scholar]
- 15.Reddy AT, Wellons JC, 3rd, Allen JC, et al. Refining the staging evaluation of pineal region germinoma using neuroendoscopy and the presence of preoperative diabetes insipidus. Neurooncology. 2004;6(2):127–133. doi: 10.1215/S1152851703000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellons JC, 3rd, Reddy AT, Tubbs RS, et al. Neuroendoscopic findings in patients with intracranial germinomas correlating with diabetes insipidus. J Neurosurg. 2004;100(5 Suppl Pediatrics):430–436. doi: 10.3171/ped.2004.100.5.0430. [DOI] [PubMed] [Google Scholar]
- 17.Lafay-Cousin L, Millar BA, Mabbott D, et al. Limited-field radiation for bifocal germinoma. Int J Radiat Oncol Biol Phys. 2006;65(2):486–492. doi: 10.1016/j.ijrobp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Alapetite C, Ricardi U, Saran F, et al. Whole ventricular irradiation in combination with chemotherapy in intracranial germinoma: the consensus of the SIOP CNS GCT Group. International Society of Paediatric Oncology Porto, Portugal. MedPediatrOncol. 2002;39:248. [Google Scholar]
- 19.Villani A, Bouffet E, Blaser S, Millar BA, Hawkins C, Bartels U. Inherent diagnostic and treatment challenges in germinoma of the basal ganglia: a case report and review of the literature. J Neurooncol. 2008;88(3):309–314. doi: 10.1007/s11060-008-9568-7. [DOI] [PubMed] [Google Scholar]
- 20.Huh SJ, Kim IH, Ha SW, Park CI. Radiotherapy of germinomas involving the basal ganglia and thalamus. Radiother Oncol. 1992;25(3):213–215. doi: 10.1016/0167-8140(92)90271-u. [DOI] [PubMed] [Google Scholar]
- 21.Mishima K, Tachikawa T, Adachi J, Ishihara S, Nishikawa R, Matsutani M. Fluorescence detection of CNS germ cell tumors with 5-aminolevulinic acid. Second International Symposium on Central Nervous System Germ Cell Tumors, Los Angeles. Neurooncology. 2005;7(4):530. [Google Scholar]
- 22.Kamoshima Y, Sawamura Y, Ikeda J, Shirato H, Aoyama H. Late recurrence and salvage therapy of CNS germinomas. J Neurooncol. 2008;90(2):205–211. doi: 10.1007/s11060-008-9649-7. [DOI] [PubMed] [Google Scholar]
- 23.Von Rohr E, Gonner F, Schroth G, Cerny T. Relapse and subarachnoid dissemination of a pineal germinoma 14 years after radiation therapy. J Clin Neurosci. 1999;6(3):247–249. doi: 10.1016/s0967-5868(99)90514-3. [DOI] [PubMed] [Google Scholar]
- 24.Merchant TE, Davis BJ, Sheldon JM, Leibel SA. Radiation therapy for relapsed CNS germinoma after primary chemotherapy. J Clin Oncol. 1998;16(1):204–209. doi: 10.1200/JCO.1998.16.1.204. [DOI] [PubMed] [Google Scholar]
- 25.Modak S, Gardner S, Dunkel IJ, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol. 2004;22(10):1934–1943. doi: 10.1200/JCO.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 26.Sawamura Y, Ikeda JL, Tada M, Shirato H. Salvage therapy for recurrent germinomas in the central nervous system. Br J neurosurg. 1999;13(4):376–381. doi: 10.1080/02688699943475. [DOI] [PubMed] [Google Scholar]