Abstract
The prognosis and clinical management of chronic liver diseases are highly dependent on the extent of liver fibrosis. Bigger the fibrosis, worse the prognosis; and bigger the risk of progression to cirrhosis. In current practice, liver biopsy is most frequently performed to assess the grade of inflammation and stage of fibrosis thereby providing prognostic information on which to base treatment decisions upon.
Liver biopsy is becoming more and more useless in the management of chronic liver disease due to large sampling error, consistent inter–observer disagreement, high emotional cost of patient, enormous health care commitment in case of rare but possible severe complications, the fact that it is a snapshot of a process that is everything but a frozen one. Therefore, every methodology that avoids performing this invasive procedure is welcome.
The purpose of this article is to present the noninvasive evaluation of patients with chronic liver disease as an alternative of liver biopsy in the assessment of hepatic structure and function.
Keywords: fibrosis, liver biopsy, serum markers, transient elastography, recurrency treatment
Hepatic fibrosis and cirrhosis are the endpoints of most types of chronic liver disease and the result of replacement of liver tissue with collagenous scar. The liver responds to injury with wound healing and, subsequently, fibrosis. This response occurs after essentially all kinds of injury (e.g. from virus, alcohol, iron, copper). Fibrosis is a result of an imbalance between fibrolytic and fibrogenic processes. Hepatic stellate cells (HCSs) are the key effector of cell fibrogenesis. In the normal liver HCSs are quiescent, but with liver injury they are activated and transform into myofibroblast–like cells, capable of proliferation. In addition, HSCs are an important source of metalloproteinase (TIMPs), matrix–degrading proteases with a central role in the remodeling of extracellular matrix.
Progressive scarring in response to a persisting liver insult leads to ‘cirrhosis’ characterized by fibrotic bands, parenchymal nodules and vascular distortion. Hepatic fibrosis and cirrhosis are morphologically defined and the pattern and extent of the morphological changes depend on the cause and stage of fibrosis. Accordingly, there is a wide spectrum in the degree of fibrosis and in the severity of clinical symptoms. Clinical presentation may vary widely, ranging from absent or nonspecific symptoms to life threatening ones. In most cases, no clear dividing line can be drawn between cirrhosis and the preceding liver disease because the transition is gradual and unapparent.
Indications for assessing liver fibrosis
It is important to have a safe and effective diagnostic tool for liver fibrosis for several reasons. Firstly, fibrosis is a central parameter of the severity of chronic liver disease associated with liver morbidity and mortality. Secondly, fibrosis is a key predictor for further progression to cirrhosis. Thirdly, advanced stage of fibrosis is the major criterion to start causal treatment.
For years, liver fibrosis was considered irreversible; however, there is accumulated clinical and experimental evidence to suggest that this axiom should be rejected. Reversal of fibrosis is a reality in some cases. Existing treatments, particularly those that treat the primary injury, can allow the complete resolution. When the underlying insult can be removed, it may soon be possible to offer patients specific antifibrotic therapy to reverse liver damage.
Assessing liver fibrosis is relevant for validation and monitoring any antifibrotic therapy. If compared to other prognostic parameters, fibrosis is definitely more important than liver inflammation and liver steatosis
Options for Liver fibrosis assessment
Percutaneous Liver biopsy
Limitations of liver biopsy
Although liver biopsy is often called the gold standard for assessment of liver disease, the true standard is the clinical outcome or what happens to the patient. Liver biopsy was initially developed as a diagnostic tool to help determine the cause of liver dysfunction. In some instances, liver biopsy is performed to determine the effect of treatment of known liver disease. It is an invasive procedure with certain unavoidable risks and complications. Significant complications occur in 1–5% of patients and the mortality rate is reported to be 1:1000 and 1/10.000 (Table 1). Despite these reservations, needle liver biopsy remains the primary tool in diagnosing liver diseases and in staging liver fibrosis (Fig 1).
Table 1.
Contraindications to percutaneous Liver Biopsy
| Uncooperative patient |
| Bleeding disorder |
| Infection of skin, pleura, right lower lung or peritoneum overlying the liver |
| Suspected liver abscess or vascular lesion |
| Difficulty in determining liver location, as with ascites |
| Severe extrahepatic obstruction |
Fig 1.

Percutaneous Liver Biopsy (Menghini)
Needle liver biopsy, however, removes only about 1/50.000 of the liver and so carries substantial sampling error. Both autopsy and laparoscopic studies have clearly shown that cirrhosis is missed on a single blind liver biopsy in 10–30% of cases. Both the size of the biopsy and the number of biopsies taken have a major effect on accuracy. An adequate biopsy should be at least 15 mm in length and contain more than 5 portal tracts. Studies have shown that biopsy specimens less than 25 mm in length can lead to underdiagnosis of cirrhosis; therefore, some investigators recommend larger biopsies. The problem of sampling error is compounded because liver biopsies are more and more often performed using the transjugular or radiographically guided approach, by which smaller samples are obtained.
Several studies have investigated the inter–observer and intra–observer variability in the histological and pathologic diagnosis of liver fibrosis based on biopsy specimens. Staging scores for fibrosis such as METAVIR, Ishak and Scheuer systems were created to standardize the evaluation of liver biopsies to minimize observer variation. Although not as great as the errors attributed to sampling variability, errors in disease staging for fibrosis with a 1 METAVIR stage appear occur in up to 20% of patients and a misdiagnosis of cirrhosis in 15% of patients. Staging errors especially for therapeutic decisions can lead to under treatment. This is particularly true for METAVIR stage 2 patients with chronic hepatitis C. Considering non–invasive tests, it is important to realize that the comparator liver biopsy is wrong in 20 % of cases, particularly where there is intermediate stage disease. More problems with histology are:
fibrosis progression in the majority of patients is slow, from normal to cirrhosis (stage 0 to stage 4 in >20 years).
follow–up biopsy is too insensitive to detect changes in fibrosis progression or regression within weeks to months or even years.
To quantify fibrosis more accurately, automated morphometry was investigated. Although the quantity of fibrosis detected with morphometry correlates to the stages of fibrosis, the nature of this relationship is not linear. As technology continues to develop, incorporation of topography and quantification of fibrosis may increase the value of this automated technology.
Noninvasive Tests
Noninvasive tests are an attractive alternative to hepatic biopsy in standardizing and monitoring chronic liver affections. This is the reason why the efforts to assess the lesions stage through non–invasive methods are justified. A noninvasive method which can provide the same information is also desired in the cases in which this technique cannot be possible. In a conventional way, lesion degree is assessed through tests that reflect the hepatic cells' permeability (transaminases) and the activity of hepatic cells synthesis (albumin, bilirubin, protrombin time). Noninvasive tests can be classified in several ways based on the modality of the test (serum blood tests or imaging) or the constituents of the tests (direct markers versus indirect markers of fibrosis). With the evolution of noninvasive tests, the performance can improve particularly with the use of combination or serial noninvasive tests.
Serological assays
A large number of serological markers of liver hepatic fibrosis have been studied for their accuracy in staging hepatic fibrosis. The ideal fibrosis test would have a high sensitivity and specificity, be relatively inexpensive, reflect fibrosis irrespective of cause and be easy to perform being reproducible and easily interpreted.
Direct serum markers of liver fibrosis
It has been suggested that measurement of direct serum markers of fibrogenesis, such as procollagen type Ⅲ N– terminal peptide (PIIINP) and direct serum marker of fibrolysis (MMP–1) might be helpful in evaluating liver fibrosis. Because of the lack of specificity, no marker of liver fibrosis has demonstrated test characteristics equivalent to liver biopsy(Fig 2).
Fig 2.
Serum markers of fibrosis
Abbreviations: TIMP–1, tissue inhibitor of metalloproteinase, GGT, gamma–glutamine transferase, ELFGA, European Liver Fibrosis Group algorithm, AST, aspartate aminotransferase, ALT, alanine aminotransferase (Table 2)
Table 2.
Serum indices of hepatic fibrosis
| FPI | Age, cholesterol, insulin resistance, past alcohol use,AST |
| Pohl Score | Platelet, AST, ALT |
| Fibrotest | alpha2 macroglobulin, GGT, age, sex, haptoglobin, total bilirubin, apolipolipoptrtein A1 |
| Forns Index | Age, platelet, GGT, cholesterol |
| APRI Index | AST, platelet |
| Hepa Score | Age, sex, hyaluronic acid, alpha2 macroglobulin, GGT |
| ELF | Propeptide Ⅱ collagen, haptoglobin, TIMP–1 |
| ELFGA | Age,amino–terminal propeptide of type Ⅲ collagen, haptoglobin, TIMP–1 |
| FibroSpect Ⅱ | Haptoglobin, TIMP–1, alpha2 macroglobulin |
The results of all these tests are similar to ROC that show an area under the curve (AUC) of approximately 0,80–0,85. The clinical utility of these tests is to rapidly screen patients for the presence of mild or significant liver disease. They can prevent the need for liver biopsy in 40% of patients and can be followed up over time.
Indirect markers of liver fibrosis
A variety of indirect markers of liver fibrosis have been evaluated. Several simple ratios and indices have been developed using aminotransferases, platelet count, prothrombine time and age, such as the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, the age–platelet index (AP index), the Pohl score, the cirrhosis determinant score (CDS) and the AST to platelet ratio index (APRI). Multivariate analyses have been directed at identifying markers of fibrosis among extracellular matrix molecules for combination into multicomponent serum panel or models with more testing capabilities. The most widely known of these are listed in biomarkers:
rather represent the whole liver;
only permit crude staging;
rather reflect liver function (secretion, endothelial uptake). The results the serological tests are shown in Table 3 (adapted from Lai and Afdhal).
Table 3.
Serological Tests for Liver Fibrosis; Abbreviations: PPV, positive predictive value, NPV, negative predictive value
| Patients | Serum Markers | AUROC (95%CI) | Sens. | Spec. | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Wai et all | 192 | APRI | 0,88 | 41% | 95% | 88% | 64% |
| Rosenberg et all | 1021 | ELF | 0,80 | 90,5% | 41% | 99% | 92% |
| Imbert–Bismut | 339 | Fibrotest | 0,87 | 87% | 59% | 63% | 85% |
| Castera et all | 183 | Fibrotest | 0,88 | NA | NA | NA | NA |
| Patel et all | 402 | Fibrospect | 0,831 | 77% | 73% | 74% | 76% |
| Adams et all | 221 | Hepascore | 0,82 | 63% | 89% | 88% | 95% |
Future studies will focus on using these tests for prediction of clinical outcomes and for the risk of disease progression.
Elastography
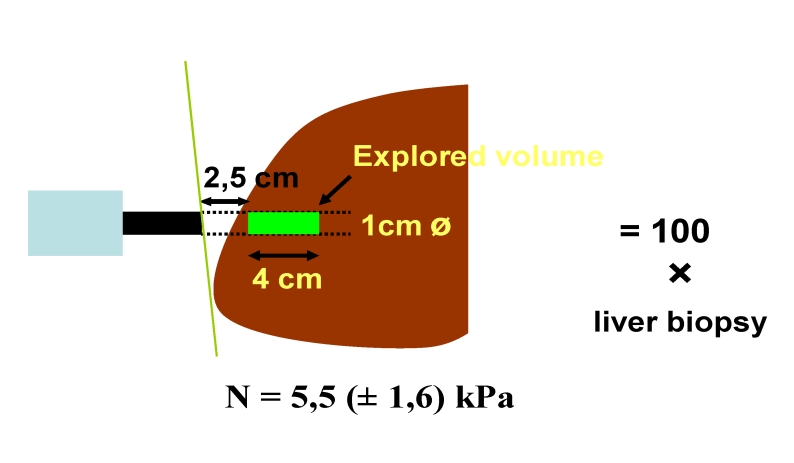
Transient elastography is more sensitive than currently available radiologic techniques for staging hepatic fibrosis. This technique uses a probe, (Fibroscan, Echosens), which includes an ultrasonic transducer, that creates a vibration of low frequency (50MHz) and amplitude, which is transmitted into the liver. The vibration wave induces an elastic shear wave that propagates through the liver. The velocity of the wave, as it passes through the liver, correlates directly with tissue stiffness or elasticity; the propagated wave travels faster with increasing fibrosis. A pulse–echo ultrasound allows measurement of the wave velocity and the results are presented as kilopascals (kPa). Stiffness is measured within a cylinder, measuring 1 cm in width and 4 cm in length, producing an estimated sampling area that is 100 times greater than biopsy. (Fig 3).
Fig 3.
Fibroscan ‘The stiffer the liver, the faster the shear wave propagates’
The elasticity result is given as the median of 10 accurate measurements; results range from 10 to 90 kPa in various stages of chronic liver disease. This technology demonstrates many features desirable for the non– invasive assessment of hepatic fibrosis. It is painless, quick (5 min), safe, can be performed bedside. A major advantage of transient elastography is the ability to take multiple measurements in the same liver. It provides immediate results and only short training is necessary.
The theoretical limitation of transient elastography are primarily mechanical factors that produce poor propagation of the wave, including the thickness and type of tissue separating the liver from the transducer (marked obesity and ascites), the ‘window’ quality (the rib space may be too narrow to allow good wave propagation) and some hepatic tissue characteristics, such as fatty liver or liver inflammation.
Ziol and collegues enrolled 327 patients with chronic hepatitis C in a multicenter study comparing METAVIR liver fibrosis stages on biopsy specimens with transient elastography. It is the largest study of hepatic elastography reported which concluded that elastography is a reliable tool to detect significant fibrosis or cirrhosis.
Castera and collegues studied 183 consecutive patients who had hepatitis C and compared the results of Fibroscan with FibroTest and the aspartate transaminase to platelet ratio (APRI) in their ability to detect cirrhosis. The investigators concluded that the tests had similar value in detecting cirrhosis, although the Fibroscan had the single best performance. The authors conclude that liver biopsy could be avoided in most patients with hepatitis C.
Another study, by Foucher et all., was carried out on 711 patients with chronic liver disease from all etiologies. Of the 711 patients, 354 patients had a liver biopsy. Foucher et all, found transient elastography to be of value in predicting fibrosis; it also correlates with complications of cirrhosis such as esophageal varices and bleeding, ascites and hepatocellular carcinoma. The results of many of these studies are shown in Table 4.
Table 4.
Performance of transient elastography; Abbreviations: HCV, hepatitis C virus, NAFLD, nonalcoholic fatty liver disease, signf., significant
| Study | Disease | Prevalence of sign of fibrosis | AUC | Threshold kPa | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Fraquelli et al | Mixed | 50 | 0,86 | 7,6 | 81% | 76% |
| Gomez–Dominiguez | Mixed | 82 | 0,74 | 4,0 | 94% | 33% |
| Chang et al. | Mixed | 44 | 0,86 | 9,0 | 83% | 85% |
| Castera et al. | HCV | 74 | 0,83 | 7,1 | 67% | 89% |
| Ziol et al. | HCV | 65 | 0,79 | 8,8 | 56% | 91% |
| Yoneda et al. | NAFLD | 49 | 0,87 | 6,6 | 83% | 81% |
An alternative technique is to use MR elastography which has the advantage of being able to examine more parts of the liver including both lobes although it is significantly more expensive and time consuming.
Future Trends
Proteomics, genomics, genetic risk profiling and breath tests are exciting new technologies under investigation. Incorporation of non–invasive tests into large natural history cohort studies and into therapeutic trials should be a priority in the next years.
Conclusions
Advanced fibrosis is the major predictor of morbidity and mortality of chronic liver disease.
Sampling variability limits the usefulness of liver biopsy to stage fibrosis.
Current biomarkers scores can spare up to 40% of patients with F0–F1 liver biopsy.
Clinical proof and monitoring the antifibrotic drug effects require better noninvasive tests for fibrosis and especially for the dynamics of fibrogenesis
Transient elastography is a very promising noninvasive method for the diagnosis of significant fibrosis in patients with chronic liver disease.
Combining transient elastography with serum markers (FibroTest) as first line assessment could avoid liver biopsy in the majority of these patients.
Transient elastography is currently the most accurate method for the diagnosis of cirrhosis.
Because of its excellent acceptance by patients, transient elastography could be useful for monitoring fibrosis.
Guidelines are needed for its use in clinical practice.
References
- 1.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laleman W, Van Landeghem L, Severi T. Both Ca21–dependent and –independent pathways are involved in rat hepatic stellate cell contraction and intrahepatic hyper responsiveness to methoxamine. Am J Physiol Gastrointest Live Physiol . 2007;292:G556–G564. doi: 10.1152/ajpgi.00196.2006. [DOI] [PubMed] [Google Scholar]
- 4.Vanheule E, Geerts AM, Reynaert H. Influence of somatostatin and octreotide on liver microcirculation in an experimental mouse model of cirrhosis studied by intravital fluorescence microscopy. Liver Int. 2008;28:107–116. doi: 10.1111/j.1478-3231.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 5.Melton AC, Datta A, Yee HF. Ca21]i–independent contractile force generation by rat hepatic stellate cells in response to endothelin–1 . Am J Physiol Gastrointest Liver Physiol. 2006;290:G7–G13. doi: 10.1152/ajpgi.00337.2005. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K, Koga Y, Sakai H. cGMP–dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase . Circ Res. 2007;101:712–722. doi: 10.1161/CIRCRESAHA.107.153981. [DOI] [PubMed] [Google Scholar]
- 7.Laleman W, Van Landeghem L, Van der Elst I. Nitroflurbiprofen, a nitric oxidereleasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats . Gastroenterology. 2007;132:709–719. doi: 10.1053/j.gastro.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 8.Thein HH, Yi Q, Dore GJ. Estimation of stage–specific fibrosis progression rates in chronic hepatitis C virus infection: a meta–analysis and meta–regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 9.Ryder SD, Irving WL, Jones DA. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. GUT. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the study of the liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 11.Regev A, Berho M, Jeffers LJ. Sampling error and intra–observer variation in liver biopsy in patients with chronic HCV infection . Am J Gastroenterol . 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 12.The French METAVIR Cooperative Study Group 12. Intra–observer and inter– observer variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 13.Bedossa P, Dargere D, Paradis V. Mutations in the p53 gene occur in diverse human tumour types. Hepatology. 2003;38:1449–1457. [Google Scholar]
- 14.Wright M, Thursz M, Pullen R. Quantitative versus morphological assessment of liver fibrosis: semi–quantitative scores are more robust than digital image fibrosis area estimation. Liver Int. 2003;23:28–34. doi: 10.1034/j.1600-0676.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- 15.Standish RA, Cholongitas E, Dhillon A. An appraisal of the histopathological assessment of liver fibrosis . Gut. 2006;55:569–578. doi: 10.1136/gut.2005.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks J. Evaluations of diagnostic and screening tests . BMJ. 2006 doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guha IN, Parkes J, Roderick P. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers . Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 18.Parkes J, Guha IN, Roderick P. OPerformance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462–474. doi: 10.1016/j.jhep.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Gebo KA, Herlong HF, Torbenson MS. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:161–172. doi: 10.1053/jhep.2002.36989. [DOI] [PubMed] [Google Scholar]
- 20.Kaul V, Friedenberg FK, Braitman LE. Development and validation of a model to diagnose cirrhosis in patients with hepatitis C. Am J Gastroenterol . 2002;97:2623–2628. doi: 10.1111/j.1572-0241.2002.06040.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Shiffman ML, Cheung RC. Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology. 2006;130:1679–1687. doi: 10.1053/j.gastro.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Morra R, Munteanu M, Bedossa P. CDiagnostic value of serum protein profiling by SELDI–TOF ProteinChip compared with a biochemical marker, FibroTest, for the diagnosis of advanced fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;26:847–858. doi: 10.1111/j.1365-2036.2007.03427.x. [DOI] [PubMed] [Google Scholar]
- 23.Poon TC, Hui AY, Chan HL. Prediction of liver fibrosis and cirrhosis in chronic hepatitis B infection by serum proteomic fingerprinting: a pilot study. Clin Chem. 2005;51:328–335. doi: 10.1373/clinchem.2004.041764. [DOI] [PubMed] [Google Scholar]
- 24.Callewaert N, Van Vlierberghe H, Van Hecke A. Noninvasive diagnosis of liver cirrhosis using DNA sequence–based total serum protein glycomics . Nat Med . 2004;10:429–434. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 25.Lim AK, Taylor–Robinson SD, Patel N. Hepatic vein transit times using a micro bubble agent can predict disease severity non–invasively in patients with hepatitis C. Gut. 2005;54:128–133. doi: 10.1136/gut.2003.030965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolkenius U, Hahn D, Gressner AM. OGlucocorticoids decrease the bioavailability of TGF–beta which leads to a reduced TGF–beta signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2004;325:1264–1270. doi: 10.1016/j.bbrc.2004.10.164. [DOI] [PubMed] [Google Scholar]
- 27.Coco B, Oliveri F, Maina AM. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of FibroScan in a prospective study of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 28.Cobbold JF, Taylor–Robinson sd. Transient elastography in acute hepatitis: all that's stiff is not fibrosis. Hepatology. 2008;47:370–372. doi: 10.1002/hep.22200. [DOI] [PubMed] [Google Scholar]
- 29.Foucher J, Castera L, Bernard PH. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations 5 . Eur J Gastroenterol Hepatol. 2006;18:411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 30. Romero–Gomez M, Gomez–Gonzalez E, Madrazo A. Optical analysis of computed tomography images of the liver predicts fibrosis stage and distribution in chronic hepatitis C . Hepatology. 2008;47:810–816. doi: 10.1002/hep.22112. [DOI] [PubMed] [Google Scholar]
- 31.Boulanger Y, Amara M, Lepanto L. Diffusion–weighted MR imaging of the liver of hepatitis C patients. NMR Biomed. 2003;16:132–136. doi: 10.1002/nbm.818. [DOI] [PubMed] [Google Scholar]
- 32.Girometti R, Furlan A, Bazzocchi M. Diffusion–weighted MRI in evaluating liver fibrosis: a feasibility study in cirrhotic patients . Radiol Med. 2007;112:394–408. doi: 10.1007/s11547-007-0149-1. [DOI] [PubMed] [Google Scholar]
- 33.Aguirre DA, Behling CA, Alpert E. Liver fibrosis: noninvasive diagnosis with double contrast material–enhanced MR imaging. Radiology. 2006;239:425–437. doi: 10.1148/radiol.2392050505. [DOI] [PubMed] [Google Scholar]
- 34.Lim AK, Patel N, Hamilton G. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788–794. doi: 10.1053/jhep.2003.50149. [DOI] [PubMed] [Google Scholar]
- 35.Huwart L, Sempoux C, Salameh N. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase– to–platelet ratio index. Radiology. 2007;245:458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 36.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferaseto– platelet ratio index for the prediction of hepatitis C–related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 37.Shaheen AA, Myers RP. Systematic review and meta–analysis of the diagnostic accuracy of fibrosis marker panels in patients with HIV/hepatitis C coinfection. HIV Clin Trials. 2008;9:43–51. doi: 10.1310/hct0901-43. [DOI] [PubMed] [Google Scholar]
- 38.Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C–related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol . 2007;102:2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 39.Poynard T, Imbert–Bismut F, Munteanu M. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guha IN, Parkes J, Roderick PR. Non–invasive markers associated with liver fibrosis in non–alcoholic fatty liver disease. Gut. 2006;55:1650–1660. doi: 10.1136/gut.2006.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talwalkar JA, Kurtz DM, Schoenleber SJ. Ultrasound–based transient elastography for the detection of hepatic fibrosis: systematic review and meta–analysis . Clin Gastroenterol Hepatol. 2007;5:1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 42.Sebastiani G, Vario A, Guido M. Stepwise combination algorithms of noninvasive markers to diagnose significant fibrosis in chronic hepatitis C. J Hepatol. 2006;44:686–693. doi: 10.1016/j.jhep.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Castera L, Vergniol J, Foucher J. Prospective comparison of transient elastography, FibroTest, APRI and liver biopsy for assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Ziol M, Handra–Luca A, Kettaneh A. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C . Hepatology. 2005;43:913–914. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]