Abstract
Aims
Using intravascular ultrasound (IVUS), we sought to characterize coronary morphology in women with chest pain without major epicardial obstructive coronary artery disease (CAD). We have previously observed an unexpectedly high rate of adverse outcomes among women with chest pain and normal or insignificant obstructive CAD. Information about the presence and characteristics of coronary atherosclerosis in these women could provide insight into the mechanisms related to increased risk, as well as improved diagnosis, prevention, and treatment.
Methods
Women (n=100) with suspected ischemia without obstructive CAD (>50% stenosis) underwent IVUS of a left coronary segment with measurements by a core lab masked to clinical and angiographic findings.
Results
Angiograhic core lab analysis found 69.6% of patients had no (≤20%) and 30.4% had minimal (20–<50%) CAD. IVUS segmental images were interpretable by the core lab in 92 women, with 19 (21%) having no atherosclerosis (intimal-medial thickness <0.5 mm). In the remaining 73 women (79%), percent atheroma volume was 27±8% and mean maximum plaque thickness was 0.53±0.22 mm. Thirty-eight women with atherosclerosis (53%) had >30% of interrogated vessel involved. The average vessel involvement was 40%, and the maximum plaque thickness was 1.27 mm. The number of risk factors strongly correlated with percent atheroma volume (r=0.53, p<0.0001) and percent vessel involvement (r=0.51, p<0.0001), with the strongest independent predictor of both being age. Remodeling was assessed in 59/73 women (81%), and 73% had evidence of positive remodeling.
Conclusions
In symptomatic women without significant luminal obstructive CAD, we observed a very high prevalence of atherosclerosis with positive remodeling and preserved lumen size. These findings may help explain increased risk and emphasize need for improved diagnostic and treatment options for women with concealed CAD.
Keywords: chest pain in women, intravascular ultrasound, atherosclerosis, coronary artery disease
Introduction
Coronary artery disease (CAD) continues to be the single leading cause of morbidity and mortality among women in the United States.1 The Women’s Ischemia Syndrome Evaluation (WISE) is a National Heart, Lung and Blood Institute–sponsored study with the goal of improving the understanding of ischemic heart disease in women. The WISE was further designed to extend our understanding of the pathophysiologic mechanisms underlying ischemic heart disease in women and assess the role of new diagnostic modalities.2
We have previously emphasized that in patients undergoing coronary angiography for acute coronary syndromes, noncritical or non–flow-limiting disease is more often seen in women than in men.1 WISE and other studies have confirmed that many women referred for coronary angiography with signs and symptoms of suspected chronic stable ischemic heart disease do not have significant CAD by angiography. Approximately half of these women will have normal coronary angiograms, and the remainder will have only insignificant luminal irregularities (<50% stenosis).3 Despite prior reports suggesting a benign prognosis, we and others have observed that women experiencing chest pain in the absence of obstructive CAD are at increased risk of adverse events.4–8 WISE has shown that many women in this cohort have myocardial ischemia9 and either coronary endothelial dysfunction10 or microvascular dysfunction,11, 12 or both, which further predicts adverse events in follow-up. The mechanisms responsible for the apparent dissociation between lack of angiographic findings and increased risk of adverse events are not completely understood. Previous studies have suggested that patients (usually men) with normal coronary angiograms frequently have evidence of atherosclerosis by intravascular ultrasound (IVUS) imaging.13, 14 Whether the coronary arteries of women presenting with chest pain are truly normal, as is the general perception, or indeed have angiographically concealed disease is unclear. The presence, degree, and pattern of atherosclerotic plaque are unknown in such women, as is the presence or absence of remodeling. The aims of this exploratory analysis were to assess the presence and extent of atherosclerosis in a sample of women with ischemic symptoms in the absence of angiographically defined obstructive epicardial coronary artery lesions and to determine the relationship of atherosclerosis to risk conditions and possible remodeling by using IVUS imaging. This information could yield important new insights for the diagnosis, prevention, and treatment of ischemic heart disease in women.
Methods
Subjects
As part of the previously published WISE protocol,2 women referred to the cardiac catheterization laboratory for clinically-suspected ischemia were screened for eligibility. The WISE protocol was approved by the local institutional review board at each participating center, and all women provided written informed consent. Briefly, women were eligible if they were at least 18 years old and were undergoing a clinically indicated coronary angiogram as part of their standard medical care. Major exclusion criteria included a recent acute coronary syndrome, prior revascularization, pregnancy, a comorbidity compromising one-year follow-up, New York Heart Association class IV congestive heart failure, significant valvular or congenital heart disease, and a significant language barrier.
Patients have been enrolled in the main WISE protocols in three phases. The IVUS substudy was approved by institutional review board at the University of Florida and overlapped the first and second phases of main study enrollment. During the IVUS substudy participants in the main study were offered enrollment and provided additional informed consent. Patients in the substudy were eligible to complete the IVUS examination if no evidence (<20% diameter stenosis) or minimal evidence (>20% but <50% diameter stenosis) of CAD was found by angiography. Angiograms were assessed by the WISE Coronary Angiographic Core Laboratory at Brown University using quantitative and qualitative methods as described elsewhere.15
IVUS and Assessment of Atherosclerosis
The IVUS interrogated the left anterior descending (preferred sample site) or circumflex coronary artery if the left anterior descending was technically inaccessible. Following administration of intravenous heparin and intracoronary nitroglycerin, a 30 MHz,2.6 Fr (0.87 mm) single element beveled IVUS transducer rotating at 1800 rpm (Ultracross;Boston Scientific Scimed Inc, Maple Grove, MN) was advancedinto the vessel over a guidewire, and the transducer was positioned distally. An automated pullback device progressively withdrew the transducer at a speed of 0.5 mm/sec. During pullback, images were obtained at a rate of 30 frames/sec and recorded on videotape. Recordings were masked to clinical and angiographic data and forwarded to the IVUS Core Lab at the Cleveland Clinic Foundation for image quality screening and interpretation.
IVUS measurements were performed in accordance with current standards.16 Briefly, using the National Institutes of Health Image (version 1.62; National Institutes of Health public domain software, Bethesda, MD), the operator performeda calibration by measuring 1-mm grid marks encoded in the image. Manual planimetry was used to trace the leading edges of the luminal and external elastic membrane (EEM) borders.
Definitions and Calculations
For each woman, we measured vessel volume (the EEM), lumen volume, atheroma volume (the difference between vessel and lumen volume), percent atheroma volume, percent vessel involvement (percentage of segments analyzed that contain atherosclerosis), and atherosclerosis (defined as plaque thickness >0.5mm). Normal reference points with plaque thickness <0.5mm that were adjacent to areas of atherosclerosis were used to determine vessel tapering and calculate an expected vessel cross-sectional area (CSA) for each 1-mm diseased segment. For each segment with atherosclerosis, CSA >1.05 times the expected CSA defined positive remodeling, a CSA <0.95 times the expected CSA defined negative remodeling and a CSA within the range of 0.95 to 1.05 times the expected CSA defined no remodeling.
Coronary artery remodeling was characterized by the relationship between arterial dimensions and size of atherosclerotic plaque focusing on actual lumen diameter and compensatory enlargement of the external elastic lamina. Positive remodeling refers to relative preservation of lumen in relation to plaque size. The vessel volumes and lumen volumes were calculated as the sum of their respective CSAs across all segments evaluated. Similarly, total atheroma volume was calculated as the sum of the differences between the EEM and lumen areas also across all segments analyzed. Percent atheroma volume was calculated as atheroma volume/vessel volume times 100. For each segment, presence of atherosclerosis was defined as a maximum plaque thickness ≥ 0.5 mm by IVUS. Percent vessel involvement was defined as the number of CSAs meeting criteria for atherosclerosis divided by the total number of CSAs analyzed.
Using the standard WISE demographic data forms completed at baseline, the number of risk factors was derived, incorporating clinical characteristics of age (≤45 years=0, 46–55 years=1, ≥ 56 years=2), diabetes (no=0, yes=1), family history of CAD (no=0, yes=1), hypertension (no=0, yes=1), dyslipidemia (no=0, yes=1), absence of oral contraceptive use (no=1, yes=0), and hormone replacement therapy use (no=0, yes=1). These risk factors (range 0–8) were then compared with atheroma volume and vessel involvement indices. Correlation coefficients were calculated for percent atheroma volume and the individual relationships to the number of risk factors and age.
Statistics
The study was designed to have adequate power (0.80 or greater) to detect correlations of 0.30 or greater between IVUS measurements and continuous risk factors. For categorical risk factors, there was adequate power to detect differences in mean IVUS values in the range of 0.57 to 0.79 s.d. units, depending on the distribution of the risk factors. Summary statistics and graphical methods were used to characterize the data. Pearson correlation coefficients were used to examine the relationship between age, number of risk factors, percent atheroma volume, and percent vessel involvement. Grouped comparisons were made using two-sample t-tests. A p value <0.05 was considered statistically significant. As these were considered exploratory analyses, no adjustments were made for multiple comparisons.
Results
From August 7, 2000, to January 11, 2005, 154 women were consented for the IVUS substudy and 100 were enrolled at the University of Florida, University of Pittsburgh, and Rhode Island Hospital. Fifty-four patients (35%) had CAD leading to their exclusion. Of the remaining 100 patients, 92 had IVUS images that were satisfactory for core lab analysis and form the basis of this analysis. The primary reason for not being able to obtain a satisfactory recording was that the left anterior descending and circumflex branches originated at acute angles prohibiting guidewire placement, or the vessels were too small to safely manipulate the IVUS transducer. Based on the angiographic core lab analysis there were 64 (69.6%) patients without and 28 (30.4%) with minimal CAD. The mean age of these 92 women was 55 years, and pertinent clinical characteristics are summarized in Table 1. About one third had histories of hypertension and dyslipidemia, and only 16% were diabetic. Most were postmenopausal with 53% having used oral contraceptives and approximately half with a history of hormone replacement therapy. More than half of patients had never smoked while only 14% were current smokers.
Table 1.
Characteristics of Study Participants
| Variable | Mean ±Standard Deviation (range) |
|---|---|
| Age (years) (n=92) | 55.1±9.6 (31.1–75.8) |
| Body Mass Index (n=88) | 32.2±8.8 (19.0–58.7) |
| Variable | Percent |
| Hypertension (n=91) | 35.2% |
| Diabetes (n=92) | 16.3% |
| Dyslipidemia (n=92) | 42.4% |
| Family with CAD (n=92) | 44.6% |
| Postmenopausal (n=77) | 81.8% |
| Oral contraceptive use (n=90) | 53.3% |
| Hormone replacement therapy (n=91) | 48.4% |
| Statin use (n=24) | 33.0% |
| Smoking: (n=91) | |
| Never | 56.0% |
| Former | 29.7% |
| Current | 14.3% |
The IVUS results of women with (plaque thickness ≥ 0.5 mm) and without atherosclerosis are summarized in Table 2. Over a sample pullback length (mean 36±16 mm), 19 women (21%) had no atherosclerosis (eg, plaque thickness <0.5 mm by IVUS). For the remaining 73 women (79%), the percent atheroma volume was 27±8% and the mean maximum plaque thickness was 0.53±0.22 mm. As expected, the atheroma volume was higher in women with atherosclerosis than in women without atherosclerosis (3.5±1.5 vs. 1.9+1.2 mm3) despite equal lumen volumes (mean 9.5 mm3). The average percent vessel involvement by IVUS was 40%. There were 38/73 subjects (52%) who had ≥ 30% atherosclerotic vessel involvement and 23 women (34%) who had >50% vessel involvement. The largest mean maximum plaque thickness in any patient was 1.27mm. Examples of IVUS and coronary angiography performed immediately before the IVUS exam from one patient are shown (Figure 1).
Table 2.
Intravascular Ultrasound Characteristics of the Coronary Artery
| Women with atherosclerosis | Women without atherosclerosis | |||||
|---|---|---|---|---|---|---|
| N=73 | N=19 | |||||
| Variable | Mean | SD | Range | Mean | SD | Range |
| Pullback length (mm) | 37.8 | 15.3 | 7.0 – 72.0 | 28.7 | 15.0 | 3.0 – 49.0 |
| Atheroma volume (mm3) | 3.5 | 1.5 | 0.8 – 7.6 | 1.9 | 1.2 | 0.4 – 6.1 |
| Lumen volume (mm3) | 9.5 | 3.1 | 4.2 – 19.8 | 9.5 | 4.2 | 4.5 – 21.8 |
| Vessel volume (mm3) | 13.0 | 3.8 | 6.2 – 27.2 | 11.4 | 5.2 | 4.9 – 27.9 |
| Percent atheroma volume | 27.2 | 8.4 | 6.8 – 48.9 | 16.1 | 5.8 | 3.8 – 26.5 |
| Mean maximal plaque thickness (mm) | 0.53 | 0.22 | 0.21 – 1.27 | 0.26 | 0.07 | 0.11 – 0.38 |
| Percent vessel involvement | 40.1 | 29.5 | 2.1 – 100.0 | 0.0 | - | - |
| Variable | N | % | ||||
| Vessel involvement | ||||||
| <30% | 34 | 47% | ||||
| 30%–50% | 15 | 19% | ||||
| >50% | 23 | 34% | ||||
SD indicates standard deviation.
Figure 1.
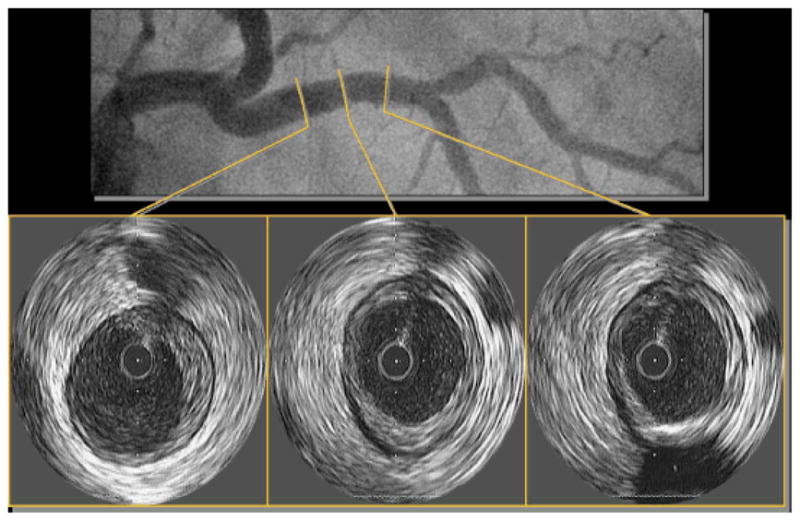
Left coronary angiogram and left anterior descending IVUS images from a 65-year-old woman with diabetes, hypertension, dyslipidemia, and hormone replacement therapy use. Note plaque in proximal left anterior descending with maintenance of lumen area.
Of the 73 women with IVUS-defined atherosclerosis, 5 women (7%) had atherosclerosis so diffuse that remodeling could not be assessed because no reference points were available. Nine other women could not be classified because they lacked one reference segment. Remodeling was assessed in the remaining 59/73 women (81%), over 662 diseased coronary segments. Remodeling was found to be positive in 223 segments (34%), negative in 177 segments (27%), and not present in 262 (40%) segments. Overall, 11 women (19%) had lesions consistent with expected CSA (eg, no remodeling), 19 women (32%) had exclusively positive remodeling, 5 women (8%) had only negative remodeling, and 24 women (41%) had evidence of a combination of positive and negative remodeling. Thus, 43/59 women (73%) demonstrated evidence of positive remodeling. The 14 patients who could not be evaluated for remodeling were found to have a similar degree of atherosclerosis as defined by percent atheroma volume and percent vessel involvement (Table 3).
Table 3.
Percent Atheroma Volume and Vessel Involvement in Patients with and without Remodeling Compared with Those Not Evaluated
| Group | n | % Atheroma Volume mean (SD) | % Vessel Involvement mean (SD) |
|---|---|---|---|
| Atherosclerosis, no remodeling | 11 | 22.1 (10.0) | 24.8 (28.3) |
| Only positive remodeling | 19 | 23.2 ( 5.3) | 22.4 (13.6) |
| Only negative remodeling | 5 | 29.5 ( 5.3) | 39.2 (16.3) |
| Both positive and negative remodeling | 24 | 30.8 ( 7.2) | 51.0 (23.2) |
| Diffuse Disease not evaluated | 14 | 29.3 (10.0) | 58.0 (41.0) |
SD indicates standard deviation.
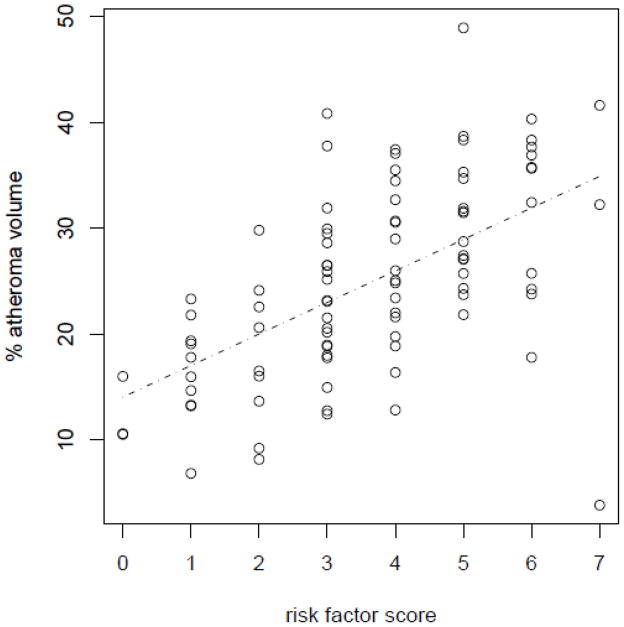
The number of risk factors based on age, hypertension, diabetes, dyslipidemia, family history of CAD, history of oral contraceptive use, and use of hormone replacement therapy strongly correlated with mean percent atheroma volume (r=0.53, p<0.0001) and mean percent vessel involvement (r=0.51, p<0.0001). Both of these measures of IVUS-based atherosclerosis increased with a higher number of risk factors (Table 4). The strongest independent correlate of atheroma volume was age (Pearson correlation 0.47, p<0.0001). Figure 2 shows the relationship between percent atheroma volume and the number of risk factors. A direct relationship was observed between increasing number of risk factors and percent atheroma volume. The presence of an individual risk factor tended to increase the percent atheroma volume and/or vessel involvement and was statistically significant for a history of hypertension, dyslipidemia, a postmenopausal state, prior use of oral contraception, and use of hormone replacement therapy (Table 5). In this cohort of women, smoking was not a risk factor likely because too few currently smoked. This is also demonstrated by the absence of a correlation between smoking status, percent atheroma volume, and percent vessel involvement in these women (Table 5).
Table 4.
Association of the Number of Risk Factors and Mean Percent Atheroma Volume and Mean Percent Vessel Involvement
| Risk Factors | N | Mean % Atheroma Volume | Mean % Vessel Involvement |
|---|---|---|---|
| 0 – 1 | 13 | 15.6 | 7.6 |
| 2 | 9 | 17.9 | 23.6 |
| 3 | 22 | 23.9 | 22.4 |
| 4 | 18 | 26.6 | 33.6 |
| 5 | 16 | 31.0 | 46.4 |
| 6 – 7 | 14 | 30.4 | 55.8 |
Risk factors: Age group: ≤ 45 years = 0; 46–55 = 1; ≥ 56 = 2; Diabetes: no = 0, yes = 1; Family history CAD: no = 0, yes = 1; Hypertension: no = 0, yes = 1; Dyslipidemia: no = 0, yes = 1; BCP use: no = 1, yes = 0; HRT use: no = 0, yes = 1.
CAD indicates coronary artery disease; BCP, birth control pills; HRT, hormone replacement therapy.
Figure 2.
The relationship between the number of risk factors and percent atheroma volume.
Table 5.
The Relationship Between the Presence of Risk Factors, Percent Atheroma Volume and Vessel Involvement
| Risk Factor | % Atheroma Volume | % Vessel Involvement | ||
|---|---|---|---|---|
| Mean (SD) | P-value | Mean (SD) | P-value | |
| History of hypertension | ||||
| No | 23.0 (9.0) | 0.0036 | 27.9 (29.2) | 0.07 |
| Yes | 28.7 (8.1) | 40.1 (32.7) | ||
| History of diabetes | ||||
| No | 24.6 (9.0) | 0.58 | 29.4 (29.3) | 0.08 |
| Yes | 26.1 (10.0) | 44.6 (36.6) | ||
| History of dyslipidemia | ||||
| No | 22.7 (8.6) | 0.0072 | 23.8 (25.1) | 0.0052 |
| Yes | 27.8 (9.1) | 42.8 (34.8) | ||
| Family history of coronary disease | ||||
| No | 23.7 (9.1) | 0.18 | 26.5 (28.2) | 0.07 |
| Yes | 26.3 (9.0) | 38.5 (32.9) | ||
| Postmenopausal | ||||
| No | 16.1 (5.8) | <.0001 | 11.4 (18.3) | 0.0004 |
| Yes | 26.5 (8.9) | 35.9 (31.0) | ||
| BCP use ever | ||||
| No | 26.8 (9.1) | 0.05 | 36.9 (31.3) | 0.011 |
| Yes | 23.1 (8.8) | 26.7 (28.7) | ||
| HRT use ever | ||||
| No | 22.3 (7.9) | 0.0044 | 25.9 (29.5) | 0.045 |
| Yes | 27.7 (9.6) | 38.9 (31.2) | ||
| Cigarette smoking | ||||
| Never | 24.7 (8.6) | 0.83 | 31.6 (29.5) | 0.94 |
| Past | 25.7 (9.8) | 33.8 (33.5) | ||
| Current | 24.0 (10.4) | 30.3 (33.6) | ||
| Correlation coefficient estimate | P-value | Correlation coefficient estimate | P-value | |
| Age | 0.47 | <.0001 | 0.36 | 0.0004 |
| BMI | 0.11 | 0.29 | 0.10 | 0.34 |
SD indicates standard deviation; BCP, birth control pills; HRT, hormone replacement therapy.
Discussion
In the United States, CAD remains the single leading cause of morbidity and mortality among women. Although women clearly present with a much higher frequency of angina-like chest pain than men, women with chest pain have a much lower prevalence of severe epicardial stenosis by angiography4, 5, 17, 18 than men. Unfortunately, a lack of stenosis has the potential to lead to the assumption that such women do not have coronary atherosclerosis. As a consequence they may not be considered for prevention treatment measures that may lower future event rates. Approximately 20% or more of women presenting with acute coronary syndromes have normal or nonobstructive CAD by angiography, yet these women have an increased risk of death or myocardial infarction at 30 days of follow-up.1 We have previously reported an unexpectedly high incidence of cardiac events in patients from WISE despite the absence of significant angiographic stenosis.3, 7, 8 In the current study, we found that most of those with suspected ischemia, but without significant angiographic disease, have evidence of atherosclerosis by IVUS. While the atherosclerosis appeared to be diffuse (the majority with ≥ 30% vessel involvement), it was only modest in terms of percent atheroma volume, mean maximal plaque thickness, and percent vessel involvement. To put this finding in context, IVUS-defined atherosclerosis was found in 51.7% of coronary arteries from presumably asymptomatic female heart transplant donors.19 The average age of those female donor coronary arteries studied was 33.4 years. In a large study of symptomatic CAD patients referred for coronary intervention, the presence of atherosclerosis was detected by IVUS in 93.2% of patients in angiographically normal coronary segments. This was a different population, however, in which patients who had previously undergone a percutaneous intervention in another coronary territory were found to have additional disease that was more extensive than the disease found in the WISE patients.13 The incremental value of the current study is that it was performed in a female specific population that did not involve presentation with an acute coronary syndrome. To our knowledge, despite the small number of women, this has not previously been reported.
Both the presence of coronary atherosclerosis as detected by IVUS in patients without obstructive CAD and the limitations of angiography in detecting atherosclerosis have been well described,13, 14, 20 but evaluation specifically related to women has not been emphasized. This WISE substudy is the first report providing IVUS information from a sample cohort of women without significant angiographic disease. To our knowledge a comparable study of men has not been published. Defining the presence, extent, and severity of epicardial stenosis by coronary angiography is relatively simple and clinically attractive, but atherosclerosis must be severe, and positive remodeling must be exhausted before a raised lesion is detected as a flow-limiting stenosis on the lumenogram.20 Despite small numbers, a majority of the women identified for this substudy had evidence of positive or adaptive coronary remodeling, which may explain why plaque is underestimated by angiography. A detailed morphometric study of ruptured plaques by Virmani and colleagues21 indicates that women with sudden death more often have evidence of erosions than rupture. If some of the plaques identified by IVUS represent the precursor lesions responsible for rupture or erosion, our findings could explain, at least in part, the disparity between the angiographic findings and subsequent events in WISE and other studies.
Coronary arterial remodeling is believed to be a focal process rather than a generalized process.22 This process results in outward displacement of the vessel wall in segments developing plaque.23–25 In the early stages of atherosclerosis, smooth muscle and adventitial thinning are associated with external elastic membrane expansion rather than plaque encroaching in the lumen (positive remodeling).21 Later in its course, compensatory enlargement is insufficient and the lumen begins to narrow. According to autopsy studies, compensatory enlargement decreases when a plaque occupies >40% of the cross-sectional area defined by the internal elastic lamina.26 Serial IVUS studies, mostly in men, suggest atheroma burden may not be the prime determinate of arterial enlargement.27 More recent IVUS studies show that compensatory enlargement continues to occur with much greater atheroma burden than suggested previously.28 Positive coronary artery remodeling leads to an angiographic underestimation of atheroma volume due to lumen preservation and has previously been associated with adverse outcomes,29–31 however these patients had severe atherosclerosis unlike women in the current study. Positive remodeling as a compensatory mechanism has been described in coronary arteries of the physically fit Masai of East Africa who consume a diet rich in dairy and meat products.32 Given the relatively high cardiovascular event rate in WISE patients experiencing chest pain without obstructive CAD, it may be that positive remodeling is not purely compensatory or protective. In these women, it may represent an early stage of disease. Persistent chest pain3 and abnormalities in endothelial as well as smooth muscle function in this population of women have been predictive not only of the development of CAD, but also of cardiovascular events in follow-up.10,33
Our IVUS finding of coronary atherosclerosis that is not recognized by coronary angiography in the WISE women would suggest that atheroma burden and its characteristics contribute to their increased risk. Although such minimal luminal lesions do not directly limit blood flow, observational studies suggest that this undetected atherosclerosis likely represents an important substrate for future coronary events.34, 35 These plaques are rich sources for upregulation of angiotensin converting enzyme, degrading bradykinin and producing angiotensin II locally.36 These substances have important local (epicardial) and downstream (microvascular) actions that contribute to plaque development and matrix degradation. Chronic reduction of nitric oxide release in experimental models has been associated with microvascular remodeling (increased wall-to-lumen ratio and perivascular fibrosis).37
It has been previously demonstrated that approximately three quarters of women with chest pain but without severe coronary stenoses have impaired coronary flow reserve indicating microvascular dysfunction, and many have endothelial-mediated vasodilator dysfunction as well. Our lab has previously published work from WISE showing endothelial dysfunction with acetylcholine to be predictive of adverse events independent of angiographic CAD severity 10. Additionally, we have observed that an abnormal coronary microvascular response to adenosine also predicts major adverse events during follow-up among these women.12 Both the mechanisms of vascular dysfunction and possible association of vascular dysfunction with undetected coronary atherosclerosis in women with normal or minimally abnormal angiograms remain to be further elucidated. It is logical to hypothesize that IVUS-documented coronary atherosclerosis, evidence for positive remodeling, and vasomotor dysfunction are likely mediated by atherosclerosis risk conditions and associated with an increased incidence of adverse cardiovascular events. We need to better understand the basis for these findings, develop improved diagnostic tools, and advance treatment options for this patient population.
Study Limitations
WISE included only a relatively small cohort of women with signs and symptoms suggestive of ischemia who were referred for clinically indicated coronary angiography, possibly resulting in a referral bias. This may make it difficult to generalize conclusions to a larger patient population. Additionally, while coronary IVUS imaging depends on a rigorous image-acquisition technique and was performed by experienced operators, the IVUS evaluation was performed over a limited portion of only one vessel per patient. This single-vessel sampling potentially underestimates the true prevalence and extent of atherosclerosis in these women. The mean pullback length was slightly longer in the group with atherosclerosis than in the group without atherosclerosis. This too may have led to an underestimation of the true prevalence of disease. There were 14 patients of the 73 with atherosclerosis whose remodeling status could not be defined due to lack of an adequate reference segment. This could have resulted in bias and an underreporting of remodeling. Lastly, the findings reported here are descriptive and do not include outcome data.
Conclusions and Clinical Implications
Women presenting with ischemic chest discomfort but without angiographic coronary artery disease have atheroma that correlates with the number of risk factors. Our finding of mild-to-moderate atherosclerosis by IVUS in about 80% of the WISE women suggests that atheroma in these patients is underestimated by angiography and might contribute to increased risk. In this small cohort, the majority of women had evidence of positive or adaptive coronary remodeling. It is hypothesis-generating only but remodeling in these women may represent an early marker of disease and a harbinger of future adverse events. The relationship between the IVUS findings in women with minimal or no CAD by angiography and the increased events in WISE and other studies remains to be fully understood.
Acknowledgments
Funding Sources
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751; a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania; and The Edythe L. Broad Women’s Heart Research Fellowship, The Women’s Guild, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, California.
Abbreviations and Acronyms
- CAD
coronary artery disease
- CSA
cross-sectional area
- EEM
external elastic membrane
- IVUS
intravascular ultrasound
- WISE
Women’s Ischemia Syndrome Evaluation
Footnotes
Disclosures
There are no conflicts of interest to disclose among any of the authors.
References
- 1.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: Bias or biology? Circulation. 2007;115:823–826. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- 2.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: Protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 3.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: Results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 4.Papanicolaou MN, Califf RM, Hlatky MA, et al. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am J Cardiol. 1986;58:1181–1187. doi: 10.1016/0002-9149(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 5.Kaski JC, Rosano GM, Collins P, et al. Cardiac syndrome X: Clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 6.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: A changing philosophy. JAMA. 2005;293:477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 7.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 8.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women’s Ischemia Syndrome Evaluation study and the St James Women Take Heart project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 10.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 11.Pepine CJ, Kerensky RA, Lambert CR, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47:S30–35. doi: 10.1016/j.jacc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results From the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mintz GS, Painter JA, Pichard AD, et al. Atherosclerosis in angiographically “normal” coronary artery reference segments: An intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995;25:1479–1485. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- 14.Nissen S. Coronary angiography and intravascular ultrasound. Am J Cardiol. 2001;87:15A–20A. doi: 10.1016/s0002-9149(01)01420-5. [DOI] [PubMed] [Google Scholar]
- 15.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937–941. A3. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 16.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 17.Davis KB, Chaitman B, Ryan T, et al. Comparison of 15-year survival for men and women after initial medical or surgical treatment for coronary artery disease: A CASS registry study. Coronary Artery Surgery Study. J Am Coll Cardiol. 1995;25:1000–1009. doi: 10.1016/0735-1097(94)00518-u. [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Chen AY, Peterson ED, et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: Results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–647. doi: 10.1016/j.ahj.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: Evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 20.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MCA, Rittersma SZH, Winter RJ, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–132. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006;47:992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Zarins CK, Weisenberg E, Kolettis G, et al. Differential enlargement of artery segments in response to enlarging atherosclerotic plaques. J Vasc Surg. 1988;7:386–394. [PubMed] [Google Scholar]
- 24.Weissman NJ, Mendelsohn FO, Palacios IF, et al. Development of coronary compensatory enlargement in vivo: Sequential assessments with intravascular ultrasound. Am Heart J. 1995;130:1283–1285. doi: 10.1016/0002-8703(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 25.Berglund H, Luo H, Nishioka T, et al. Highly localized arterial remodeling in patients with coronary atherosclerosis: An intravascular ultrasound study. Circulation. 1997;96:1470–1476. doi: 10.1161/01.cir.96.5.1470. [DOI] [PubMed] [Google Scholar]
- 26.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 27.Sipahi I, Tuzcu EM, Schoenhagen P, et al. Compensatory enlargement of human coronary arteries during progression of atherosclerosis is unrelated to atheroma burden: Serial intravascular ultrasound observations from the REVERSAL trial. Eur Heart J. 2006;27:1664–1670. doi: 10.1093/eurheartj/ehi796. [DOI] [PubMed] [Google Scholar]
- 28.Schoenhagen P, Sipahi I. Arterial remodelling: An independent pathophysiological component of atherosclerotic disease progression and regression. Insights from serial pharmacological intervention trials. Eur Heart J. 2007;28:2299–2300. doi: 10.1093/eurheartj/ehm276. [DOI] [PubMed] [Google Scholar]
- 29.Schoenhagen P, Ziada KM, Kapadia SR, et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: An intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M, Nishikawa H, Mukai S, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: An intravascular ultrasound study. J Am Coll Cardiol. 2001;37:63–69. doi: 10.1016/s0735-1097(00)01097-4. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Shen W, Zhang D. Relationship between coronary arterial remodeling and clinical presentation. Chin Med J (Engl) 2003;116:263–266. [PubMed] [Google Scholar]
- 32.Mann GV, Spoerry A, Gray M, et al. Atherosclerosis in the Masai. Am J Epidemiol. 1972;95:26–37. doi: 10.1093/oxfordjournals.aje.a121365. [DOI] [PubMed] [Google Scholar]
- 33.Bugiardini R, Manfrini O, Pizzi C, et al. Endothelial function predicts future development of coronary artery disease: A study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 34.Ge J, Erbel R, Zamorano J, et al. Coronary artery remodeling in atherosclerotic disease: An intravascular ultrasonic study in vivo. Coron Artery Dis. 1993;4:981–986. doi: 10.1097/00019501-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 36.Diet F, Pratt RE, Berry GJ, et al. Increased accumulation of tissue ace in human atherosclerotic coronary artery disease. Circulation. 1996;94:2756–2767. doi: 10.1161/01.cir.94.11.2756. [DOI] [PubMed] [Google Scholar]
- 37.Numaguchi K, Egashira K, Takemoto M, et al. Chronic inhibition of nitric oxide synthesis causes coronary microvascular remodeling in rats. Hypertension. 1995;26:957–962. doi: 10.1161/01.hyp.26.6.957. [DOI] [PubMed] [Google Scholar]