Abstract
The use of antiretroviral medications in HIV-negative individuals as pre-exposure prophylaxis (PrEP) is a promising approach to prevent HIV infection. Tenofovir disoproxil fumarate (TDF) and emtricitabine exhibit desirable properties for PrEP including: favourable pharmacokinetics that support infrequent dosing; few major drug-drug or drug-food interactions; an excellent clinical safety record; and pre-clinical evidence for efficacy. Several large, randomized, controlled clinical trials are evaluating the safety and efficacy of TDF and emtricitabine for this new indication. A thorough understanding of variability in drug response will help determine future investigations in the field and/or implementation into clinical care. Because tenofovir and emtricitabine are nucleos(t)ide analogues, the HIV prevention and toxicity effects depend on the triphosphate analogue formed intracellularly. This review identifies important cellular pharmacology considerations for tenofovir and emtricitabine, which include drug penetration into relevant tissues and cell types, race/ethnicity/pharmacogenetics, gender, cellular activation state and appropriate episodic or alternative dosing strategies based on pharmacokinetic principles. The current state of knowledge in these areas is summarized and the future utility of intracellular pharmacokinetics/pharmacodynamics for the PrEP field is discussed.
Keywords: clinical pharmacology, intracellular pharmacology, pharmacokinetics, pharmacodynamics, pharmacogenomics, nucleoside analogue pharmacology
Introduction
Although HIV prevention strategies such as promotion of safe sex practices and clean needle exchange programmes are effective, additional approaches are needed to help reduce the number of new HIV infections, which are estimated at 2.7 million cases annually.1,2 The ideal prevention tool would be a protective vaccine, but the feasibility and timelines for a highly effective HIV vaccine remain unclear.3
A new hope for a powerful HIV prevention tool has emerged in chemoprophylaxis with antiretroviral medications, referred to as pre-exposure prophylaxis (PrEP).4–6 PrEP is the use of antiretroviral medications in HIV-negative persons to prevent HIV infection should HIV exposure take place. Antiretroviral drugs are currently used for HIV prevention in other settings, including after high-risk HIV exposures such as needlesticks [post-exposure prophylaxis (PEP)], rape or high-risk sexual exposures [non-occupational PEP (nPEP)] or for prevention of mother to child transmission (PMTCT).7–9
One of the differences between the prevention uses listed above and PrEP is that the target population for PrEP may not have the same imminent risk of HIV exposure and may be young adults in prime health.10 For these reasons, PrEP will require a very low toxicity profile along with proven benefit for an acceptable risk–benefit balance. Until recently there were few antiretroviral agents that provided an adequate safety or tolerability profile for an acceptable risk–benefit ratio. This situation is changing, however, as newer agents with more favourable safety profiles become available.11
In addition to excellent safety and tolerability, prototypical PrEP agents should exhibit high cost-effectiveness, a relatively benign HIV resistance profile should PrEP fail and pharmacokinetic properties that support once-daily or less frequent dosing. Ideally the pharmacokinetics should be resilient to drug interactions and food effects, and should be effective and safe during periods of missed doses (‘pharmacokinetically forgiving’).12–14 Drug distribution into tissues and cells susceptible to HIV infection would be another important requirement.
Tenofovir and emtricitabine, two nucleos(t)ide analogue reverse transcriptase inhibitors (NRTIs), have emerged as viable PrEP agents based upon the favourable pharmacological characteristics described above, considerable safety experience in humans and compelling pre-clinical data. A number of large, randomized, controlled trials in humans are under way or have been completed to evaluate the safety and efficacy of tenofovir or tenofovir disoproxil fumarate (TDF) with and without emtricitabine as PrEP (Table 1).6,10 The trials are taking place in diverse regions of the world and include broad patient populations such as at-risk heterosexual women, men who have sex with men, injection drug users and serodiscordant couples.
Table 1.
Major clinical trials involving tenofovir or TDF ± emtricitabine (adapted from reference 6)
| Location | Sponsor | Population | PrEP strategy(ies) being tested | Status/expected completion |
|---|---|---|---|---|
| USA (CDC 4323) | CDC | 400 MSM | daily TDF | completed/2009; final analysis/2010 |
| Thailand (CDC 4370) | CDC | 2400 injecting drug users | daily TDF | enrolling/2010 |
| S. Africa (CAPRISA 004) | CAPRISA, FHI, CONRAD, USAID, LIFElab | 1200 women | coital-dependent topical TFV gel | completed/2009; final analysis/2010 |
| Botswana (CDC 4940) | CDC | 1200 heterosexual men and women | daily TDF/FTC (switched from TDF early 2007) | decreased scope to focus on safety and not efficacy/2010 |
| Brazil, Ecuador, Peru, S. Africa, Thailand, USA (iPrEX) | NIH, BMGF | 3000 MSM | daily TDF/FTC | fully enrolled/2010 |
| Kenya, Uganda (Partners PrEP) | BMGF | 3900 serodiscordant heterosexual couples | daily TDF or daily TDF/FTC | enrolling/2013 |
| Kenya, Malawi, S. Africa, Tanzania, Zambia (FEM-PrEP) | FHI, USAID, BMGF | 3900 women | daily TDF/FTC | enrolling/2013 |
| S. Africa, Uganda, Zambia, Zimbabwe (VOICE MTN 003) | MTN, NIH | 5000 women | daily TDF or daily TDF/FTC or daily topical TFV gel | enrolling/2013 |
| Kenya, Uganda (IAVI E001 & E002) | IAVI | 150 serodiscordant couples or at risk men and women | daily TDF/FTC or twice weekly with coital dosing TDF/FTC | fully enrolled/2010 |
| USA (PCS 082) | NICHD | 99 young MSM | daily TDF/FTC | enrolling/2011 |
BMGF, Bill & Melinda Gates Foundation; CAPRISA, Centre for the AIDS Programme of Research in South Africa; FHI, Family Health International; FTC, emtricitabine; IAVI, International AIDS Vaccine Initiative; MSM, men who have sex with men; MTN, Microbicide Trials Network; TFV, tenofovir; USAID, United States Agency for International Development.
The global implications of the impending trial results (Table 1) necessitate significant reflection to help guide implementation, if warranted. While several perspectives have already been published regarding cost-effectiveness models and other considerations for PrEP implementation, little has been written about the clinical and cellular pharmacology of tenofovir and emtricitabine in this new setting and patient population.15,16 The purpose of this review is to identify important clinical and cellular pharmacology considerations for tenofovir ± emtricitabine as it relates to the use of these agents to prevent HIV infection and to summarize the current state of knowledge in these areas. This review focuses mostly on oral TDF ± emtricitabine; readers are directed to other important papers for review of topical microbicides or other aspects of HIV prevention.2,4,5,14,17–19
Clinical pharmacology for PrEP
PrEP efficacy trials such as those in Table 1 use acquisition of HIV as a primary outcome and are consequently large and test relatively few regimens and dosing strategies. Unlike therapy research, in which plasma viral RNA levels are used to optimize dosing in small studies, there are no surrogate markers for prophylactic effects that can similarly guide smaller studies to optimize dose, dosing interval or route of dosing. As a result, a risk–benefit assessment of study results becomes increasingly subjective with increasing variability in drug effectiveness or toxicity. It is in the context of variable drug response that the perspectives and role of clinical pharmacology become most important. The goals of clinical pharmacology are to elucidate the causes of variable drug response by examining pharmacokinetic, pharmacodynamic and pharmacogenetic differences among individuals.
Cellular pharmacology of tenofovir and emtricitabine
The clinical pharmacology of tenofovir and emtricitabine depends upon their cellular pharmacology, as these medications are sequentially phosphorylated in cells, by host enzymes, to the pharmacologically active triphosphate (TP) anabolites.20,21 Tenofovir is a monophosphate analogue and thus requires diphosphorylation to become a TP analogue [i.e. tenofovir-diphosphate (DP)].20 Tenofovir is polar and ionized and therefore has poor oral bioavailability, thus the disoproxil di-ester prodrug TDF was created to mask the two ionic regions of the phosphonic acid to enhance penetration of gut membranes.22 TDF is thought to be rapidly converted by a carboxylesterase to a monoester upon absorption across the intestinal wall and then to tenofovir by a phosphodiesterase upon first pass through the liver, such that tenofovir is the predominant circulating species.23,24
Once formed in the cell, NRTI-TPs compete with their endogenous 2′-deoxynucleotide counterparts for incorporation by HIV reverse transcriptase into the growing HIV DNA chain.25,26 For example, tenofovir-DP is a 2′-deoxyadenosine-TP (dATP) analogue and thus competes with dATP for utilization by HIV reverse transcriptase. If the drug is incorporated, HIV DNA chain elongation terminates.22
Intracellular NRTI-TPs have also been implicated in clinical toxicities, many of which have been attributed to inhibition of mitochondrial DNA polymerase gamma by the drug-TP, in the same manner as inhibition of HIV DNA replication described above.27 This potential non-specificity leads to reduced mitochondrial DNA and lower RNA and protein output, resulting in mitochondrial dysfunction with resultant anaerobic respiration, lactic acid production, oxidative damage and subsequent clinical symptoms.28–30 In general, neither tenofovir nor emtricitabine effectively inhibit DNA polymerase gamma in comparison with other NRTIs based upon in vitro cellular and enzymatic assays.31–33 However, the main toxicity concern for tenofovir is worsening or new renal insufficiency due to proximal tubule damage, which is thought to be a mitochondrial toxicity arising from over-accumulation of tenofovir (and presumably tenofovir-DP) mediated by organic anion transporter (OAT)-1 and OAT-3 uptake of tenofovir into those cells.34–36 Subclinical proximal renal tubulopathy, observed as decreased re-absorption of glucose, amino acids, uric acid or phosphate, has been associated with tenofovir use in clinical cohorts.35 This condition may alter phosphate metabolism and contribute to changes in bone mineral density.37 Renal and bone changes will be important endpoints in PrEP trials, because a stringent risk–benefit balance will be needed in this population. Serious renal toxicity for tenofovir is rare, especially in those with no predisposition to renal impairment (<1%), and other clinical adverse effects attributable to mitochondrial toxicity are not common for either tenofovir or emtricitabine.38–40
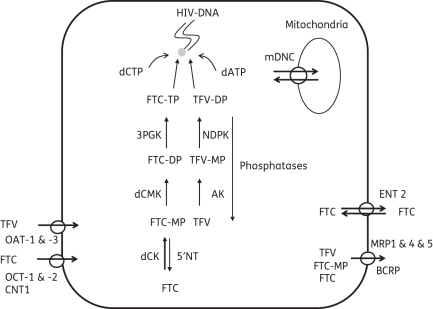
The dependence of the pharmacological activity of tenofovir and emtricitabine on intracellular TP is a unique feature of the NRTI class and is fundamental to understanding how these drugs work in all treatment scenarios, including PrEP. The cellular pharmacology of tenofovir and emtricitabine, including the enzymes and transporters thought to govern the intracellular profiles, is shown in Figure 1.20,22,34,35,41–47 Enzymes and transporters that influence lamivudine were included where there was limited information available for emtricitabine, as the cellular pharmacology of emtricitabine and lamivudine are thought to be similar.48,49 ABCC10 (MRP7) and ABCC11 (MRP8) efflux adefovir (a close analogue of tenofovir) in vitro, which suggests that additional transporters may influence tenofovir disposition.50,51 Transporter and enzyme expression may be tissue specific (e.g. OAT-1 and OAT-3 specifically expressed in the kidney), which suggests that different tissues/cell types may exhibit different cellular pharmacology.52 It should be noted that most information regarding the enzymes and transporters in Figure 1 arose from in vitro experiments and the extent of applicability to the in vivo setting is not well understood.
Figure 1.
Summary of the intracellular disposition of tenofovir and emtricitabine including the transporters and enzymes responsible for the production of tenofovir-DP and emtricitabine-TP. mDNC, mitochondrial deoxynucleotide carrier; 3PGK, 3′-phosphoglycerate kinase; NDPK, nucleotide diphosphate kinase; 5′NT, cytosolic 5′-nucleotidase; dCK, deoxycytidine kinase; dCMK, uridylate-cytidylate kinase; OAT-1 & -3, organic anion transporter 1 & 3; OCT-1 & -2, organic cation transporter 1 & 2; CNT 1, concentrative nucleoside transporter 1; ENT 2, equilibrative nucleoside transporter 2; MRP 1 & 4 & 5, multidrug resistance protein 1 & 4 & 5; BCRP, breast cancer resistance protein; AK, adenylate kinase; dATP, 2′-deoxyadenosine-triphosphate; dCTP, 2′-deoxycytidine-triphosphate; TFV, tenofovir; FTC, emtricitabine; MP, monophosphate; DP, diphosphate; TP, triphosphate.
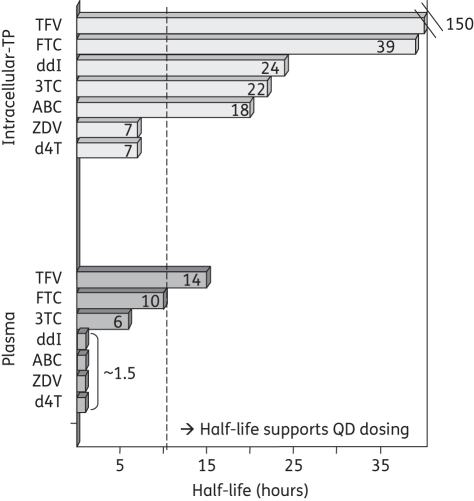
NRTI-phosphates are ionized and trapped within cells and therefore persist with half-lives that are much longer than the parent drug in plasma, generally by about 2- to >10-fold (Figure 2).13,21,47,53–58 This trapping and persistence of the active NRTI-TP at the intracellular site of action, even when the parent drug is cleared from plasma, is an elegant pharmacological characteristic. In fact, the longer intracellular NRTI-TP half-life enables the decreased dosing frequencies used clinically for many NRTIs (Figure 2), emphasizing the clinical importance of NRTI cellular pharmacology. The half-lives of intracellular tenofovir-DP (∼150 h or 6.25 days) and emtricitabine-TP (∼39 h or 1.6 days) are the longest for the NRTI class, a potentially favourable pharmacological characteristic for PrEP, but also a potential concern.21,56,58
Figure 2.
Half-lives for intracellular triphosphates (TPs) (top bars) and plasma (bottom bars) for tenofovir (TFV), emtricitabine (FTC), didanosine (ddI), lamivudine (3TC), abacavir (ABC), zidovudine (ZDV) and stavudine (d4T). Note, the intracellular triphosphate for didanosine is dideoxyadenosine-TP and that for abacavir is carbovir-TP. QD, once daily.
General pharmacokinetic considerations
Long half-life drugs are often viewed favourably as they may provide ‘pharmacokinetic forgiveness’ in times of suboptimal adherence or may better withstand minor drug-drug or drug-food interactions.12,13 Importantly, the concept of ‘pharmacokinetic forgiveness’ is most relevant once concentrations are at steady state. An underappreciated consideration that has importance for the PrEP field is the time to reach steady-state concentrations. Steady state is reached in about 4 half-lives of continuous dosing. When no loading dose is administered, the difference between concentrations after the first dose and steady state (the accumulation factor) is also a function of half-life and this first dose steady-state difference increases in size the longer the half-life. For example, using a 150 h (6.25 day) tenofovir-DP half-life, a steady-state tenofovir-DP concentration of 80 fmol/106 cells and once-daily TDF dosing, the estimated tenofovir-DP concentrations after 1, 2, 4, 6 and 8 doses are 8, 15, 28, 38 and 46 fmol/106 cells {using Ct = Css × [1 − e−(0.693/half-life)×t], where Ct is the concentration at time t and Css is 80 fmol/106 cells}.56,59 The steady-state concentration of 80 fmol/106 cells will be achieved in about 4 half-lives (∼25 days of daily dosing). It should be noted that emtricitabine-TP, with a shorter 39 h (1.6 day) half-life, would be predicted to accumulate to steady state more rapidly than tenofovir-DP (∼6 days) and would exhibit a smaller difference between first dose and steady state (∼3-fold).
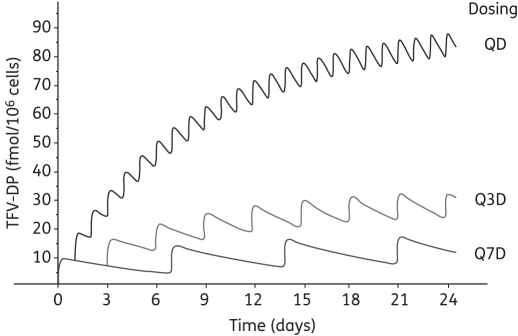
Figure 3 depicts the estimated accumulation profile for tenofovir-DP and includes a simulation of alternative dosing strategies (once-weekly and every three days dosing). The parameters for Figure 3 were estimates from HIV-infected persons and include concentrations at steady state and uses the half-life determined after TDF was discontinued.56,59 The accumulation part of this profile has not yet been elucidated. Tenofovir-DP pharmacokinetics specific to HIV-negative persons have not been characterized. These specific parameters are needed to create an accurate Figure 3 profile for the PrEP setting. The utility of such a profile will be in predicting the concentrations achieved with different dosing regimens relevant for various PrEP scenarios. For example, if a target concentration of 30 fmol/106 cells is optimal (the actual threshold has not yet been elucidated), then the concentrations achieved with weekly and every three day dosing would be insufficient. For daily dosing, the threshold concentration would not be achieved until the third to fourth day of dosing, a prediction relevant for coital-dependent dosing or episodic uses. The model could then be used to predict concentrations achieved after a loading dose or other alternative dosing strategies.
Figure 3.
Predicted tenofovir (TFV)-DP accumulation to steady state in humans with three different dosing strategies: daily TDF (QD); TDF every three days (Q3D); and weekly TDF (Q7D). See text for parameters used for simulation.
The concentration–effect relationship
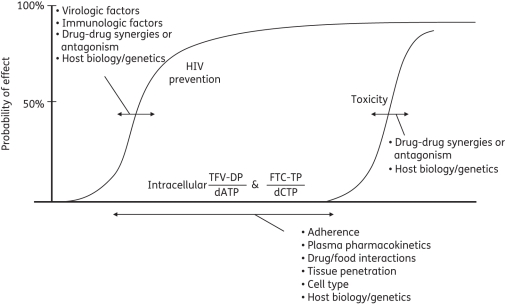
A central piece of information needed to put Figure 3 into proper context is the intracellular concentrations associated with the probabilities of HIV prevention and toxicity. A comprehensive pharmacokinetic–pharmacodynamic relationship will also contain important sources of variability. A depiction of the theoretical concentration-effect profile for PrEP, including potential sources of variability, is shown in Figure 4. The theoretical pharmacokinetic-pharmacodynamic profile suggests that the most accurate drug exposure metric for tenofovir and emtricitabine will be the drug-TP:endogenous-TP ratio in cells and tissues relevant to HIV transmission (see also Figure 1). TDF studies in macaques and chronically HIV-infected humans have demonstrated that antiviral effects are related, in part, to endogenous dATP concentrations: higher dATP was associated with a reduced ratio and lower antiviral activity.60,61 Whether tenofovir-DP and emtricitabine-TP pharmacokinetics will suffice for concentration-effect relationships for PrEP (as opposed to the ratio) is a question yet to be addressed.
Figure 4.
Theoretical concentration-effect profile for tenofovir (TFV) and emtricitabine (FTC) for HIV prevention, including potential sources of pharmacokinetic–pharmacodynamic variability. The sigmoid curves define the main drug effects of HIV prevention and toxicity. The horizontal arrows represent factors that influence the positions of the curves and the horizontal arrow on the x-axis represents factors that influence the intracellular drug and endogenous nucleotides. Identified sources of variability are discussed in the text.
The profile in Figure 4 identifies potential sources of pharmacodynamic variability (shown by arrows on the HIV prevention and toxicity curves). For instance, pharmacodynamics will be influenced by immunological and host characteristics such as the presence of the CCR5 Δ32 null allele or concomitant ulcerative sexually transmitted diseases, as well as the resistance profile of the virus.5,62–64 The implications of reduced PrEP efficacy with HIV resistance and/or the potential increase in the spread of resistant HIV as a result of PrEP are important considerations for the prevention field.65 A mathematical model that used some resistance characteristics for tenofovir/emtricitabine encountered in pre-clinical studies (M184V and/or K65R) suggests that PrEP with TDF/emtricitabine may reduce overall transmission by resistant virus, although the proportion of new infections from resistant virus may actually increase.66 As resistance and transmission data from clinical trials become available, model updates may refine these predictions.
Drug-drug synergies can also, in effect, shift the HIV prevention curve. For example, in vitro studies demonstrate more than additive antiviral activity when tenofovir and emtricitabine are combined.67,68 This provides a therapeutic advantage for the combination if toxicities are not additive or overlapping. Evidence to date does not support additive toxicities for tenofovir plus emtricitabine, although ongoing PrEP trial results will provide better information for this population (Table 1).38–40 Theoretically the same principles would also apply to the toxicity curve. For instance, individuals taking multiple nephrotoxic medications may experience a higher probability of nephrotoxicity at lower tenofovir exposures, in effect shifting the toxicity curve to the left.39
Factors that influence the intracellular drug profile (pharmacokinetics) will also affect the probability of response (arrow along the x-axis). The intracellular drug profile is part of a pharmacological continuum for TDF and emtricitabine that includes the dose, adherence, plasma pharmacokinetics, tissue penetration (e.g. rectal or cervical) and TP-analogue accumulation and kinetics in relevant cells (as well as the endogenous TP profile). All parts of the continuum are important. For instance, suboptimal adherence to PrEP is the most intuitive example of a factor that could substantially lower drug exposures, thereby decreasing the probability of HIV prevention. Factors that slow tenofovir clearance and raise tenofovir concentrations are likely to increase the risk of renal toxicity (such as elevated baseline serum creatinine, low body weight and advanced age).37,39
Tissue penetration of extracellular tenofovir and emtricitabine after oral TDF/emtricitabine dosing has been characterized in cervicovaginal fluids and semen. In semen, tenofovir accumulates with an AUC ratio of more than 4 times that of plasma.69 In cervicovaginal fluid, tenofovir and emtricitabine are detectable within 2 h after the first dose and concentrations are maintained above plasma concentrations for much of the dose interval.70 The median AUC ratios for cervicovaginal to plasma exposures are greater than 1 for both tenofovir and emtricitabine.4,70 These data suggest that extracellular tenofovir and emtricitabine distribute to tissues relevant for HIV transmission.
The importance of the pharmacological continuum has been demonstrated in pre-clinical studies of tenofovir or TDF ± emtricitabine to prevent simian immunodeficiency virus (SIV/SHIV) infection in macaques or HIV prevention in humanized mice. Prevention rates in these studies generally ranged from 25% to 100% and important pharmacological predictors of response (aside from virological and immunological considerations) included the dose, tenofovir plasma concentrations, the timing of therapy relative to the virus exposure(s) and the duration of therapy after the exposure(s).5,37,71–76 Recent studies have demonstrated efficacy with doses scaled to match human exposures and initial intracellular results have now been published (described below).76
As intracellular data and study results from the PrEP field become available, the need to understand the pharmacological continuum will increase in importance (Figure 4) because variable drug response in humans is likely. For instance, the recently published CAPRISA study evaluated 1% tenofovir gel versus placebo gel applied vaginally and demonstrated a 39% reduction in HIV transmission.77 Adherence, as measured by returned applicators, was an important predictor of response; nearly twice the rate of transmission was observed in those who were <50% adherent versus those who were >80% adherent. A pharmacology sub-study extended these adherence observations by showing that low levels of tenofovir in the blood and vaginal tenofovir were measurable twice as often and at higher median concentrations in women who remained HIV negative versus those who became HIV infected among the active drug arm.78 Tenofovir concentrations in the vaginal tract ranged from 0.1 to >106 ng/mL, and higher concentrations were associated with lower HIV transmission. Furthermore, tenofovir gel was associated with a reduction in HSV-2 infections and vaginal tenofovir concentrations above 10 000 ng/mL were predictive of this effect. The vaginal levels of tenofovir were several orders of magnitude higher than those achieved with oral dosing, suggesting that vaginal tenofovir levels in this study are specific to topical dosing.70 A correlation was identified between tenofovir and tenofovir-DP in vaginal and cervical tissue, but a relationship was not available for tenofovir-DP concentrations with antiviral effects.
Taken together, the intracellular pharmacokinetic profile is a first step in guiding general dosing predictions to achieve certain average concentrations (e.g. Figure 3). A more sophisticated pharmacokinetic-pharmacodynamic model will also account for sources of pharmacokinetic-pharmacodynamic variability such that potential subpopulations might be identified who may require tailored dosing strategies. Such a pharmacokinetic-pharmacodynamic model may be used to interpret variability in response from clinical trials, as was demonstrated in CAPRISA, and to inform the design of smaller pharmacokinetic studies to establish target concentrations—perhaps to include specific subpopulations—prior to launching larger efficacy trials. The following sections review the current state of knowledge for intracellular tenofovir and emtricitabine and illustrate potential sources of variability based on that knowledge.
Intracellular tenofovir-DP and emtricitabine-TP in vivo
Of about eight studies that have evaluated intracellular tenofovir-DP, mostly in small numbers of HIV-infected adults, the average steady-state tenofovir-DP values range from about 80 to 160 fmol/106 cells with significant inter-individual variability (coefficient of variation of about 50%).56,58,59,61,69,79–81 In studies that stopped TDF and followed tenofovir-DP over time, the median tenofovir-DP half-life was about 6.25 days (range 2.5 to 7.5 days).56,58 Fewer studies have evaluated emtricitabine-TP in human volunteers, with typical concentrations ranging from about 1000 to 4000 fmol/106 cells and a terminal half-life of about 39 h (1.6 days).21,82 Recent PrEP studies with TDF and emtricitabine in macaque models, where dosing was designed to match human exposures, showed intracellular tenofovir-DP and emtricitabine-TP of about 50 to 400 and 270 to 3500 fmol/106 cells, respectively.37,60,73,83 A brief summary of intracellular tenofovir-DP and emtricitabine-TP pharmacokinetics in humans and macaques is provided in Table 2.21,37,53,56,58–61,69,73,79–84 In general, while intracellular exposures are similar in primates and humans, the primate studies were limited by few animals and sampling after single doses in some cases.37,76 Human studies were in HIV-infected subjects and mostly once at steady state with no pharmacokinetics from first dose to steady state. Very few studies have included endogenous nucleotides to define the drug:endogenous ratio.60,61 Thus, while limited intracellular data have been generated to provide the initial estimates for the intracellular profiles, additional intensive studies are needed to more fully characterize the intracellular pharmacokinetics and comparability between primates and humans.
Table 2.
Cellular pharmacology of tenofovir and emtricitabine in macaques and HIV-infected or uninfected humans; macaque doses were designed to match humans
| Humans[ref(s)] | Macaques[ref(s)] | |
|---|---|---|
| TFV | ||
| TFV-DP: fmol/106 cells | 80 to 16056,58,59,61,69,79–81 | 50 to 400a,b37,60,73,76,83,84 |
| TFV-DP half-life: days; range | 6.25; 2.5 to 7.556,58 | 4.8; 1 to 7a,c37,60,76 |
| TFV plasma: µg·h/mL | 2 to 453,80 | 1.4 to 837,76 |
| TFV plasma half-life: days | 0.5 to 0.653 | 0.676,84 |
| FTC | ||
| FTC-TP: fmol/106 cells | 1000 to 400021,82 | 270 to 3500b60,73,76,83 |
| FTC-TP half-life: days; range | 1.621 | 1; 0.4 to 260,76,83 |
| FTC plasma: µg·h/mL | 8 to 1121 | 10.5 to 1373,76 |
| FTC plasma half-life: days | 0.3 to 0.421 | 0.276 |
TFV, tenofovir; FTC, emtricitabine.
aSmall numbers of animals.
bSingle doses.
cOne study showed 2-fold accumulation from first dose to steady state, which suggests 24 h half-life.
Cell type
All the intracellular concentrations described in Table 2 were measured in peripheral blood mononuclear cells (PBMCs), which has always been the reference tissue for NRTI studies in vivo.85 This is due, in part, to the easy access and high cell numbers, but also because of the clinical relevance of PBMCs, which contain the main HIV target, CD4+ T lymphocytes. As intracellular analytical methodology becomes more sophisticated, studies should also evaluate whether PBMCs reflect tenofovir-DP and emtricitabine-TP concentrations in cells relevant for HIV prevention such as CD4 purified cells, lymph node lymphocytes, rectal or cervical mucosal lymphocytes, dendritic cells, etc. However, a first step to moving in this direction is to rigorously evaluate the methods used to isolate and harvest cells from such samples, as well as the analytical procedures.
Current evidence suggests that the deoxycytidine analogue lamivudine-TP exhibits relative uniformity across many cell types. One study found similar lamivudine-TP in CD4 purified cells (99% CD4 T cells) versus PBMCs in HIV-negative volunteers and another study in HIV-infected male volunteers found similar lamivudine-TP in seminal leucocytes (cell types not reported) versus PBMCs.86,87 In vitro studies show similar lamivudine-TP across several different mononuclear cell types.88 By extension, these findings may also apply to emtricitabine-TP given the presumed similar cellular pharmacology.48,49
However, TP uniformity across cell types may not be the case for other NRTIs such as zidovudine and tenofovir. For example, zidovudine-TP was approximately 3-fold lower in purified CD4 cells and seminal leucocytes versus PBMCs in the same studies described above for lamivudine-TP.86,87 Similarly, both zidovudine-TP and tenofovir-DP varied by about 5- to 20-fold across various mononuclear cell types in the same in vitro study described above for lamivudine-TP.88
Another study in HIV-infected volunteers found about a 10-fold higher tenofovir-DP concentration in seminal leucocytes (cell types not reported) versus PBMCs.69 A small pilot study measured tenofovir-DP in stored PBMCs and mononuclear cells that were extracted from inguinal lymph nodes (cell types not reported) from seven HIV-infected volunteers. The median lymph node cell:PBMC ratio was 0.55 (n = 7, range 0.35 to 1.19).89 In macaques who were given a single TDF + emtricitabine dose (or the tenofovir prodrug GS7340), tenofovir-DP concentration appeared to be about the same in purified CD4 cells from blood, but lower or similar in lymph node lymphocytes and higher in rectal tissue cells compared with PBMCs.60,76 Emtricitabine-TP was found to be degraded (unstable) in the tissue samples.76 A summary of potential tenofovir-DP and emtricitabine-TP differences by cell type is shown in Table 3 based on information from humans and macaques.60,69,76,86,87,89
Table 3.
Potential differences of tenofovir-DP and lamivudine-TP (or emtricitabine-TP) intracellular concentrations by cell type compared with PBMCs60,69,76,79,86,87,89
Taken together, these data suggest that tenofovir-DP accumulates in tissues relevant for HIV prevention and that concentrations according to cell/tissue type may be different relative to the reference PBMC compartment. As the pharmacology field for prevention evolves, the methodology for cell/tissue measurement should be rigorously evaluated and studies in PBMCs should be augmented with cells from tissues where transmission takes place. Additional biological factors that may influence intracellular tenofovir-DP and emtricitabine-TP in vivo are described below.
Cell activation
The cellular activation state significantly influences intracellular pharmacology of NRTIs and antiviral activity, based upon in vitro studies.26,90 For instance, tenofovir-DP accumulated 2- to 3-fold higher in resting versus phytohaemaggutinin (PHA)-activated PBMCs in vitro.20 The tenofovir-DP half-life was 3 times longer in resting than activated cells in the same study. Another in vitro study showed that tenofovir-DP reached several-fold higher concentrations in resting compared with activated cells (PHA/interleukin-2) and showed time-dependent differences in accumulation between 6 and 24 h.88 A third study found 3-fold higher tenofovir in resting versus PHA-activated PBMCs, although tenofovir-DP was not greatly different in this study.91 Activation state may also increase the concentrations of dATP, which could significantly lower the tenofovir-DP:dATP ratio in activated cells, resulting in lower virological activity.26,60,61
In contrast, lamivudine (and, by extension, emtricitabine) exhibits equal or higher accumulation of TP in PHA-activated versus resting cells based on in vitro evidence.26 In studies of HIV-infected subjects, prior to becoming aviraemic (at week 2 of therapy), there was a direct correlation between HIV RNA and lamivudine-TP.55 This later finding may be associated with the presence of active HIV replication, which significantly increases cellular activation.92 Thus studies are needed to compare tenofovir-DP and emtricitabine-TP in HIV-negative versus HIV-positive persons given that these drugs may be phosphorylated differently in resting versus activated cells.20,26 Measurement of endogenous nucleotides to determine the NRTI-TP:endogenous ratio will also be important. Finally, other states in HIV-negative persons that increase cellular activation (e.g. co-infections, local inflammation) may similarly impact tenofovir and emtricitabine cellular pharmacology. Given the potential role of both resting and activated CD4 cells in HIV transmission, these considerations have relevance to understand drug effects for PrEP.
The disoproxil prodrug
Another important factor for tenofovir cellular pharmacology is the use of the disoproxil prodrug (TDF), which enables much greater cell penetration and higher tenofovir-DP concentrations. For example, a study in macaques showed that dosing with TDF produced 8-fold higher tenofovir-DP in PBMCs compared with subcutaneous tenofovir dosing despite the same tenofovir plasma exposure for the two groups.84 Studies in dogs showed a similar effect.84 Consistent with these observations, studies of intravenous tenofovir and oral TDF in HIV-infected humans showed larger HIV RNA reductions with oral TDF for the same plasma tenofovir exposure.93 These in vivo findings are consistent with in vitro studies that demonstrate 500- to 1000-fold higher tenofovir-DP and more potent antiviral activity with the disoproxil prodrug compared with the parent drug.20,84
These considerations may be relevant for tenofovir as a microbicide. Tenofovir is used in gel formulations due to better stability compared with TDF. As mentioned previously, the CAPRISA study of vaginal tenofovir gel demonstrated variability in HIV protection. Tenofovir exposures in the vagina were several orders of magnitude higher than after oral dosing and were predictive of HIV transmission.70,77,78 If future TDF formulations could improve upon stability, TDF use in microbicides might further enhance cell penetration and tenofovir-DP accumulation in cells and tissues relevant for HIV transmission.
Gender differences
A few studies with small subject numbers suggest gender differences in intracellular NRTI pharmacology.94 One such study collected an average of 10 samples in each of 29 men and 4 women receiving indinavir, zidovudine and lamivudine, and measured lamivudine-TP concentrations that were 4523 fmol/106 cells (∼1.6-fold) higher in women compared with men (P < 0.01).55 Another small study of TDF plus lamivudine with either nevirapine or lopinavir/ritonavir observed lamivudine-TP concentrations that were 6012 fmol/106 cells (∼1.6-fold) higher in women compared with men (P = 0.08).81 These lamivudine findings may be relevant for emtricitabine.48,49 The later study also found tenofovir-DP concentrations that were about 94 fmol/106 cells (1.45- to 1.8-fold) higher in women compared with men (P < 0.05).81 In both these studies, plasma concentrations of parent lamivudine and tenofovir were not different between the genders.55,81 Once potential gender differences are fully characterized, the data may be relevant for interpreting PrEP findings and designing alternative dosing strategies in different populations such as men who have sex with men (MSM) and women at high risk for heterosexual transmission.
Race and pharmacogenetics
NRTI cellular pharmacology may be influenced by the expression and/or function of influx/efflux transporters or other enzymes that govern intracellular disposition (shown in Figure 1). Some of these enzymes, such as ABCC4 (MRP4) and deoxycytidine kinase, show genetic differences in activity or expression according to race.95,96 One retrospective pharmacogenetic sub-study in 27 HIV-infected individuals compared tenofovir-DP according to single nucleotide polymorphisms (SNPs) in the genes encoding ABCC2 (MRP2) (C-24T and G1249A) and ABCC4 (MRP4) (A3463G and T4131G).59 ABCC4 (MRP4) 3463G variant carriers had tenofovir-DP concentrations that were, on average, 29 fmol/106 cells (1.35-fold) higher than wild type after adjusting for race, concomitant protease inhibitor use and glomerular filtration rate (P = 0.04).59
These SNPs were also studied in relation to lamivudine-TP in subjects taking indinavir, zidovudine and lamivudine.97 After adjusting for gender, the lamivudine-TP concentrations were, on average, 1479 fmol/106 higher (1.2-fold) in ABCC4 (MRP4) T4131G variant carriers compared with wild type (P = 0.004). These findings are consistent with reduced MRP4 function or expression with the 3463G and 4131G variant alleles. Finally, two studies have reported relationships between ABCC2 (MRP2) genotypes or haplotypes and tenofovir-induced renal dysfunction. The hypothesis for these findings is that the genotypes represent lower ABCC2 (MRP2) expression or function resulting in over-accumulation of tenofovir (and tenofovir-DP) in proximal tubules with consequential renal toxicity.35,98
Table 4 summarizes potential sources of variability in the intracellular profiles of tenofovir and emtricitabine in humans and/or macaques, separate from other aspects of the pharmacological continuum such as dose, adherence and plasma pharmacokinetics.
Table 4.
Factors that may specifically influence tenofovir-DP and lamivudine-TP (or emtricitabine-TP) in cells20,26,35,55,59,81,84,88,97,98
| Effect on intracellular concentration |
||
|---|---|---|
| tenofovir-DP | lamivudine-TP or emtricitabine-TP | |
| PHA-activated cells (relative to non-PHA activated)20,26,88 | ↓ | ↔ |
| Use of disoproxil prodrug84 | ↑ | NA |
| Female gender (relative to males)55,81 | ↑ | ↑ |
| Polymorphism in MRP459,97 | ↑ | ↑ |
| Polymorphism in MRP235,97,98 | ↑ | ↔ |
NA, not applicable.
Summary
Several factors will impact the probabilities of HIV prevention and toxicity for TDF and emtricitabine in PrEP trials, including virological and immunological considerations. Just as importantly, pharmacological factors will also drive the probabilities of drug response. This review identified potential sources of variability in the pharmacological continuum for PrEP, including adherence, tenofovir-DP and emtricitabine-TP accumulation between the first dose and steady state, drug penetration into relevant tissues and cells types, race/pharmacogenetics, gender and states of altered cellular activation (e.g. co-infections, inflammation). Many of these areas require additional information to adequately characterize the pharmacokinetic-pharmacodynamic profile for HIV prevention. The future utility and promise offered by understanding this pharmacokinetic-pharmacodynamic profile is to assist in the interpretation of emerging PrEP study results, to provide guidance regarding regimen optimization in future studies and to inform the safest most efficacious implementation into practice.
Funding
This work was supported by grants from the National Institute of Allergy and Infectious Diseases of the NIH (grant numbers AI064029 and AI084735).
Transparency declarations
P. L. A. and R. M. G. are investigators on PrEP trials funded by the NIH (and, for R. M. G., the CDC and Bill and Melinda Gates Foundation). Gilead Sciences donates study drug but no funding for this research. All other authors: none to declare.
References
- 1.UNAIDS. 09 AIDS Epidemic Update. November 2009. http://data.unaids.org/pub/Report/2009/2009_epidemic_update_en.pdf. (15 March 2010, date last accessed) [Google Scholar]
- 2.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143–67. doi: 10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolin R. HIV vaccine trial results—an opening for further research. N Engl J Med. 2009;361:2279–80. doi: 10.1056/NEJMe0909972. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Gay C, Kashuba AD, et al. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lerma JG, Paxton L, Kilmarx PH, et al. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol Sci. 2010;31:74–81. doi: 10.1016/j.tips.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.AVAC Global Advocacy for HIV Prevention. Ongoing PrEP Trials. http://www.avac.org/ht/d/sp/i/3507/pid/3507. (15 March 2010, date last accessed) [Google Scholar]
- 7.Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1–17. [PubMed] [Google Scholar]
- 8.Public Health Services Task Force. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States—Living Document. http://www.AIDSinfo.NIH.gov. (15 March 2010, date last accessed) [PubMed] [Google Scholar]
- 9.Smith DK, Grohskopf LA, Black RJ, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54(RR-2):1–20. [PubMed] [Google Scholar]
- 10.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2:e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calmy A, Hirschel B, Cooper DA, et al. A new era of antiretroviral drug toxicity. Antivir Ther. 2009;14:165–79. doi: 10.1177/135965350901400203. [DOI] [PubMed] [Google Scholar]
- 12.Anderson PL. Pharmacologic perspectives for once-daily antiretroviral therapy. Ann Pharmacother. 2004;38:1924–34. doi: 10.1345/aph.1E036. [DOI] [PubMed] [Google Scholar]
- 13.Back DJ, Burger DM, Flexner CW, et al. The pharmacology of antiretroviral nucleoside and nucleotide reverse transcriptase inhibitors: implications for once-daily dosing. J Acquir Immune Defic Syndr. 2005;39(Suppl 1):S1–23. doi: 10.1097/01.qai.0000168882.67942.3f. quiz S24–5. [DOI] [PubMed] [Google Scholar]
- 14.Derdelinckx I, Wainberg MA, Lange JMA, et al. Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection. PLoS Med. 2006;3:e454. doi: 10.1371/journal.pmed.0030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS ONE. 2007;2:e875. doi: 10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu AY, Grant RM, Buchbinder SP. Preexposure prophylaxis for HIV: unproven promise and potential pitfalls. JAMA. 2006;296:863–5. doi: 10.1001/jama.296.7.863. [DOI] [PubMed] [Google Scholar]
- 18.Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol Toxicol. 2009;49:349–75. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- 19.Guest G, Shattuck D, Johnson L, et al. Acceptability of PrEP for HIV prevention among women at high risk for HIV. J Womens Health. 2010;19:791–8. doi: 10.1089/jwh.2009.1576. [DOI] [PubMed] [Google Scholar]
- 20.Robbins BL, Srinivas RV, Kim C, et al. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–7. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LH, Begley J, St Claire RL, III, et al. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20:1173–82. doi: 10.1089/aid.2004.20.1173. [DOI] [PubMed] [Google Scholar]
- 22.Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010;85:39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 24.Naesens L, Bischofberger N, Augustijns P, et al. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob Agents Chemother. 1998;42:1568–73. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furman PA, Fyfe JA, St Clair MH, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–7. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao WY, Agbaria R, Driscoll JS, et al. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem. 1994;269:12633–8. [PubMed] [Google Scholar]
- 27.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 28.Maagaard A, Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J Antimicrob Chemother. 2009;64:901–9. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]
- 29.Mallon PW, Unemori P, Sedwell R, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–96. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 30.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen. 2007;48:166–72. doi: 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- 31.Birkus G, Hitchcock MJ, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–23. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AA, Ray AS, Hanes J, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–57. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- 33.Feng JY, Murakami E, Zorca SM, et al. Relationship between antiviral activity and host toxicity: comparison of the incorporation efficiencies of 2′,3′-dideoxy-5-fluoro-3′-thiacytidine-triphosphate analogs by human immunodeficiency virus type 1 reverse transcriptase and human mitochondrial DNA polymerase. Antimicrob Agents Chemother. 2004;48:1300–6. doi: 10.1128/AAC.48.4.1300-1306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50:3297–304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Nóvoa S, Labarga P, Soriano V, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48:e108–16. doi: 10.1086/598507. [DOI] [PubMed] [Google Scholar]
- 36.Lebrecht D, Venhoff AC, Kirschner J, et al. Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J Acquir Immune Defic Syndr. 2009;51:258–63. doi: 10.1097/qai.0b013e3181a666eb. [DOI] [PubMed] [Google Scholar]
- 37.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144–60. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arribas JR, Pozniak AL, Gallant JE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47:74–8. doi: 10.1097/QAI.0b013e31815acab8. [DOI] [PubMed] [Google Scholar]
- 39.Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–81. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 40.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. December 1, 2009; 1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. (15 March 2010, date last accessed) [Google Scholar]
- 41.Robbins B, Greenhaw J, Connelly M, et al. Metabolic pathways for activation of the antiviral agent 9-(2-phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob Agents Chemother. 1995;39:2304–8. doi: 10.1128/aac.39.10.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunsucker SA, Mitchell BS, Spychala J. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107:1–30. doi: 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Podgorska M, Kocbuch K, Pawelczyk T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim Pol. 2005;52:749–58. [PubMed] [Google Scholar]
- 44.Jung N, Lehmann C, Rubbert A, et al. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008;36:1616–23. doi: 10.1124/dmd.108.020826. [DOI] [PubMed] [Google Scholar]
- 45.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci USA. 2000;97:7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kis O, Robillard K, Chan GNY, et al. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Bazzoli C, Jullien V, Le Tiec C, et al. Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin Pharmacokinet. 2010;49:17–45. doi: 10.2165/11318110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Bethell R, De Muys J, Lippens J, et al. In vitro interactions between apricitabine and other deoxycytidine analogues. Antimicrob Agents Chemother. 2007;51:2948–53. doi: 10.1128/AAC.01204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hazen R, Lanier ER. Relative anti-HIV-1 efficacy of lamivudine and emtricitabine in vitro is dependent on cell type. J Acquir Immune Defic Syndr. 2003;32:255–8. doi: 10.1097/00126334-200303010-00003. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y, Kotova E, Chen Z-S, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278:29509–14. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 51.Hopper-Borge E, Xu X, Shen T, et al. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69:178–84. doi: 10.1158/0008-5472.CAN-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cihlar T, Ho ES, Lin DC, et al. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20:641–8. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]
- 53.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–9. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson PL, Kakuda TN, Lichtenstein KA. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin Infect Dis. 2004;38:743–53. doi: 10.1086/381678. [DOI] [PubMed] [Google Scholar]
- 55.Anderson PL, Kakuda TN, Kawle S, et al. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17:2159–68. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 56.Hawkins T, Veikley W, St Claire RL, III, et al. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr. 2005;39:406–11. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 57.Becher F, Landman R, Mboup S, et al. Monitoring of didanosine and stavudine intracellular triphosphorylated anabolite concentrations in HIV-infected patients. AIDS. 2004;18:181–7. doi: 10.1097/00002030-200401230-00006. [DOI] [PubMed] [Google Scholar]
- 58.Pruvost A, Negredo E, Benech H, et al. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2005;49:1907–14. doi: 10.1128/AAC.49.5.1907-1914.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiser JJ, Aquilante CL, Anderson PL, et al. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:298–303. doi: 10.1097/qai.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Lerma G, Cong M, Zheng Q, et al. Efficacy of intermittent prophylaxis with tenofovir and emtricitabine against rectal SHIV transmission in macaques and relationship to systemic and mucosal drug levels. Abstracts of the Seventeenth Conference on Retroviruses and Opportunistic Infections, San Francisco, 2010; Alexandria, VA, USA: Foundation for Retrovirology and Human Health; Abstract 83. [Google Scholar]
- 61.Goicoechea M, Jain S, Bi L, et al. Abacavir and tenofovir disoproxil fumarate co-administration results in a nonadditive antiviral effect in HIV-1-infected patients. AIDS. 2010;24:707–16. doi: 10.1097/QAD.0b013e32833676eb. [DOI] [PubMed] [Google Scholar]
- 62.Van Rompay KK. Antiretroviral drug studies in nonhuman primates: a valid animal model for innovative drug efficacy and pathogenesis experiments. AIDS Rev. 2005;7:67–83. [PubMed] [Google Scholar]
- 63.Philpott S, Weiser B, Tarwater P, et al. CC chemokine receptor 5 genotype and susceptibility to transmission of human immunodeficiency virus type 1 in women. J Infect Dis. 2003;187:569–75. doi: 10.1086/367995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirbod T, Broliden K. Mucosal immune responses in the genital tract of HIV-1-exposed uninfected women. J Intern Med. 2007;262:44–58. doi: 10.1111/j.1365-2796.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- 65.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 66.Supervie V, Garcia-Lerma JG, Heneine W, et al. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci USA. 2010;107:12381–6. doi: 10.1073/pnas.1006061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borroto-Esoda K, Vela JE, Myrick F, et al. In vitro evaluation of the anti-HIV activity and metabolic interactions of tenofovir and emtricitabine. Antivir Ther. 2006;11:377–84. [PubMed] [Google Scholar]
- 68.Bousquet L, Pruvost A, Guyot A-C, et al. Combination of tenofovir and emtricitabine plus efavirenz: in vitro modulation of abc transporter and intracellular drug accumulation. Antimicrob Agents Chemother. 2009;53:896–902. doi: 10.1128/AAC.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vourvahis M, Tappouni HL, Patterson KB, et al. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr. 2008;47:329–33. doi: 10.1097/QAI.0b013e3181632cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5:e157. doi: 10.1371/journal.pmed.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subbarao S, Ramos A, Kim C, et al. Direct stringency comparison of two macaque models (single-high vs. repeat-low) for mucosal HIV transmission using an identical anti-HIV chemoprophylaxis intervention. J Med Primatol. 2007;36:238–43. doi: 10.1111/j.1600-0684.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 73.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsai C-C, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–73. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Rompay KK, Kearney BP, Sexton JJ, et al. Evaluation of oral tenofovir disoproxil fumarate and topical tenofovir GS-7340 to protect infant macaques against repeated oral challenges with virulent simian immunodeficiency virus. J Acquir Immune Defic Syndr. 2006;43:6–14. doi: 10.1097/01.qai.0000224972.60339.7c. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Lerma JG, Cong ME, Mitchell J, et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2:14ra4. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 77.Karim QA, Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kashuba A, Abdool Karim E, Kraft E, et al. Do systemic and genital tract tenofovir concentrations predict HIV seroconversion in the CAPRISA 004 tenofovir gel trial?. Abstracts of the Eighteenth International AIDS Conference, Vienna, Austria, 2010; Abstract TUSS0503. [Google Scholar]
- 79.Durand-Gasselin L, Da Silva D, Benech H, et al. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob Agents Chemother. 2007;51:2105–11. doi: 10.1128/AAC.00831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiser JJ, Fletcher CV, Flynn PM, et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2008;52:631–7. doi: 10.1128/AAC.00761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pruvost A, Negredo E, Theodoro F, et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother. 2009;53:1937–43. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darque A, Valette G, Rousseau F, et al. Quantitation of intracellular triphosphate of emtricitabine in peripheral blood mononuclear cells from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1999;43:2245–50. doi: 10.1128/aac.43.9.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuklenyik Z, Martin A, Pau C-P, et al. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J Chromatogr B. 2009;877:3659–66. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Durand-Gasselin L, Van Rompay KKA, Vela JE, et al. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol Pharm. 2009;6:1145–51. doi: 10.1021/mp900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stein DS, Moore KH. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy. 2001;21:11–34. doi: 10.1592/phco.21.1.11.34439. [DOI] [PubMed] [Google Scholar]
- 86.Anderson PL, Zheng JH, King T, et al. Concentrations of zidovudine- and lamivudine-triphosphate according to cell type in HIV-seronegative adults. AIDS. 2007;21:1849–54. doi: 10.1097/QAD.0b013e3282741feb. [DOI] [PubMed] [Google Scholar]
- 87.Dumond JB, Reddy YS, Troiani L, et al. Differential extracellular and intracellular concentrations of zidovudine and lamivudine in semen and plasma of HIV-1-infected men. J Acquir Immune Defic Syndr. 2008;48:156–62. doi: 10.1097/QAI.0b013e31816de21e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balzarini J, Van Herrewege Y, Vanham G. Metabolic activation of nucleoside and nucleotide reverse transcriptase inhibitors in dendritic and Langerhans cells. AIDS. 2002;16:2159–63. doi: 10.1097/00002030-200211080-00008. [DOI] [PubMed] [Google Scholar]
- 89.Fletcher CV, King T, Bushman LR, et al. Compartmental kinetics of intracellular tenofovir. Abstracts of the Fifteenth Conference on Retroviruses and Opportunistic Infections, Boston, 2008; Alexandria, VA, USA: Foundation for Retrovirology and Human Health; Abstract 754. [Google Scholar]
- 90.Saavedra-Lozano J, McCoig CC, Cao Y, et al. Zidovudine, lamivudine, and abacavir have different effects on resting cells infected with human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 2004;48:2825–30. doi: 10.1128/AAC.48.8.2825-2830.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robbins BL, Wilcox CK, Fridland A, et al. metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy. 2003;23:695–701. doi: 10.1592/phco.23.6.695.32189. [DOI] [PubMed] [Google Scholar]
- 92.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee WA, Martin JC. Perspectives on the development of acyclic nucleotide analogs as antiviral drugs. Antiviral Res. 2006;71:254–9. doi: 10.1016/j.antiviral.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 94.Anderson PL. Recent developments in the clinical pharmacology of anti-HIV nucleoside analogs. Curr Opin HIV AIDS. 2008;3:258–65. doi: 10.1097/COH.0b013e3282f85dc1. [DOI] [PubMed] [Google Scholar]
- 95.Abla N, Chinn LW, Nakamura T, et al. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325:859–68. doi: 10.1124/jpet.108.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamba JK, Crews K, Pounds S, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther. 2007;323:935–45. doi: 10.1124/jpet.107.128595. [DOI] [PubMed] [Google Scholar]
- 97.Anderson PL, Lamba J, Aquilante CL, et al. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441–9. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- 98.Izzedine H, Hulot JS, Villard E, et al. Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis. 2006;194:1481–91. doi: 10.1086/508546. [DOI] [PubMed] [Google Scholar]