Abstract
Spasticity is a common disorder following spinal cord injury that can impair function and quality of life. While a number of mechanisms are thought to play a role in spasticity, the role of motoneuron persistent inward currents (PICs) is emerging as pivotal. The presence of PICs can be evidenced by temporal summation or wind-up of reflex responses to brief afferent inputs. In this study, a combined neurophysiological and novel biomechanical approach was used to assess the effects of passive exercise and modafinil administration on hyper-reflexia and spasticity following complete T-10 transection in the rat. Animals were divided into 3 groups (n=8) and provided daily passive cycling exercise, oral modafinil, or no intervention. After 6 weeks, animals were tested for wind-up of the stretch reflex (SR) during repeated dorsiflexion stretches of the ankle. H-reflexes were tested in a subset of animals. Both torque and gastrocnemius electromyography showed evidence of SR wind-up in the transection only group that was significantly different from both treatment groups (p<0.05). H-reflex frequency dependent depression was also restored to normal levels in both treatment groups. The results provide support for the use of passive cycling exercise and modafinil in the treatment of spasticity and provide insight into the possible contribution of PICs.
Keywords: Exercise, H-reflex, modafinil, spasticity, spinal cord injury, stretch reflex
INTRODUCTION
Spasticity is a common disorder following spinal cord injury (SCI) that can impair function and quality of life. While there has been progress in understanding the spinal pathophysiology involved in the plastic changes that occur post injury, the exact mechanisms remain unknown (Hultborn, 2006, Nielsen, et al., 2007) In addition, quantifying spasticity continues to be a challenge. Voerman et al. reviewed a number of neurophysiological methods for spasticity assessment citing their advantages and limitations. Because there is no gold standard in spasticity measurement, these authors concluded that a combined neurophysiological and biomechanical approach might be used to provide a more complete assessment (Voerman, et al., 2005).
One measurement that has been used extensively for quantifying hyper-reflexia is the electrical analogue of the classic stretch reflex, referred to as the Hoffman or H-reflex. This technique has been used to document reflex changes following SCI as well as the influence of passive exercise on hyper-reflexia in both humans and animal models of SCI (Grey, et al., 2008, Kiser, et al., 2005, Schindler-Ivens and Shields, 2000, Skinner, et al., 1996, Yates, et al., 2008a, 2008b). Specifically, frequency dependent depression (FDD) or post activation depression of the H-reflex has been used to assess the monosynaptic reflex arc involved in the stretch reflex (SR) (Thompson, et al., 1998, 1992). However, a number of differences in the H-reflex and mechanical SR have been documented (Morita, et al., 1998), including fundamental differences in spinal motoneuron EPSPs that are evoked by stretch versus a single synchronous discharge that produces the H-reflex (Enriquez-Denton, et al., 2002). Additionally, the timecourse of the delayed onset differs with hyper-reflexia firmly established by 14–21 days post transection while SR temporal summation is delayed until 49 days post SCI (Yates, et al., 2010). Finally, polysynaptic reflexes composed of group II afferents have been suggested to play a major role in spasticity following SCI (Jankowska and Hammar, 2002), and these contributions would not be fully assessed by the H-reflex.
Biomechanical measures of the SR, including measurement of EMG and torque response to a movement perturbation, have been reported in humans (Hornby, et al., 2006, Schmit and Benz, 2002, Schmit, et al., 2002, 2000, 1999). Similar techniques have been used in animal models to quantify the SR in rats, including documentation of the velocity dependent response in normal rats (Thompson, et al., 1996), and in rats with a contusion injury of the spinal cord (Bose, et al., 2002). Furthermore, Thompson documented the time course of the SR response post injury. However, the SR response in a transection injury has not been reported, and evidence from human studies indicates that responses differ based on completeness of injury (Calancie, et al., 2002, Nakazawa, et al., 2006). From a methodological standpoint, single session quantification of the EMG response to imposed stretch is possible, but longitudinal comparison of EMG responses in rodents is problematic because of the inability to normalize the EMG signal. Validity of non-normalized comparisons can be compromised because measured differences could be due to altered electrode location or spacing rather than a true treatment effect (Soderberg and Knutson, 2000). Altered passive tissue properties post SCI (Olsson, et al., 2006) also potentially confound observed torque responses to imposed stretches. One paradigm that overcomes this limitation is the study of the SR wind-up of repeated stretches. In a SR wind-up protocol, the EMG and torque response to the first stretch is used to normalize subsequent responses, thus enabling longitudinal comparisons.
SR wind-up behavior is characterized by temporal facilitation that results in increased amplitude and duration of the reflex responses. It has been suggested that the mechanism for this prolonged reflex response is due to alterations in intrinsic motoneuron properties, namely persistent inward currents (PICs) (Bennett, et al., 2004). These voltage gated Na and Ca currents are linked to the presence of endogenous monoamines (Harvey, et al., 2006, Li, et al., 2007, Li and Bennett, 2003). In reduced preparations, PICs demonstrate the ability to amplify and prolong the response to brief inputs (Crone, et al., 1988, Hounsgaard, et al., 1988). These PICs disappear in acute spinal preparations but their reemergence is linked to the onset of hyperreflexia in chronic spinal rats (Bennett, et al., 1999, Bennett, et al., 2001). Indirect evidence of PICs has also been presented in humans post SCI (Norton, et al., 2008). Furthermore, temporal summation has been demonstrated for flexor reflexes (Hornby, et al., 2003) and stretch reflexes post SCI (Hornby, et al., 2006).
While a number of pharmacological interventions such as Baclofen and Tizanadine are commonly prescribed to decrease symptoms of spasticity, the side effects can limit their use in some patients (Zafonte, et al., 2004). Two interventions, passive exercise and oral modafinil, have demonstrated the ability to decrease hyper-reflexia and show promise for the human patient with a SCI (Kiser, et al., 2005, Yates, et al., 2009, 2008b). While a decrease in FDD of the H-reflex has been shown in rats with complete transection, these interventions have not been assessed by a direct SR measure.
The purpose of this investigation was to utilize a SR wind-up protocol to provide a measure of spasticity in animals undergoing 1) spinal cord transection, 2) spinal cord transection plus passive exercise, or 3) spinal cord transection plus pharmacological treatment with modafinil. We expected to find evidence of SR wind-up in the chronic spinal rat and that this wind-up would be diminished through interventions of passive exercise and modafinil. Preliminary results have been previously reported in abstract form (Garrison, et al., 2008).
MATERIALS AND METHODS
Surgery
Adult female Sprague-Dawley rats (Harlan, 250–290 g, n = 24) underwent a lower thoracic laminectomy under ketamine (60 mg · kg−1, i.m.) and xylazine (10 mg · kg−1, i.m.) anesthesia. A complete transection (Tx) of the spinal cord was made by aspiration and the transected ends of the cord retracted, producing a 2–3 mm cavity. Surgery and postsurgical care was performed as previously described (Reese, et al., 2006). All procedures were approved by the Institutional Animal Care and Use Committee. We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
Group assignment
Animals were randomly assigned to one of 3 groups. All animals were housed in individual cages and were free to roam. The Tx only group (n=8) received no intervention other than daily bladder expression and preventive care. The exercise (Ex) group (n=8) was initiated on a passive cycling exercise regimen one week after Tx. Exercise consisted of daily 1 hr sessions performed 5 days per week (see (Reese, et al., 2006) for details). The modafinil group (MOD) (n=8) was given orally administered doses of modafinil at 4 mg · kg−1. Both exercise and modafinil were given following assessment procedures to avoid possible short term effects on the SR measures.
Stretch Reflex Testing
After surgery, testing of the SR was performed every two weeks beginning on post-operative day 8. Bladders of the animals were manually expressed before testing. A thermoplastic splint with a soft cover was manufactured to help stabilize the animals and minimize movement. The left hind paw was taped to the footplate such that the ankle was at a 100° angle (slight plantarflexion) and the axis of the ankle rotation aligned with the actuator shaft. Electromyography (EMG) of the gastrocnemius was recorded using percutaneous fine wire electrodes (Nylon coated stainless steel, 0.05mm dia) inserted via a 27 g needle. A clamp electrode applied to the skin of the tail was used as the reference. EMG recordings were made using amplifier (Grass P511) filter setting of 3 Hz to 1 kHz with the 60 Hz notch filter in use. All data were sampled at 2000 Hz using a National Instruments (Austin, TX) USB6216.
The SR wind-up protocol consisted of 10 sequential stretches (17 degrees at 500 deg · s−1) at intervals of 1 sec, 0.4 sec or 0.25 sec. Trials were randomly presented with 1 min of rest between each trial. Three trials were collected for each stretch interval and the average was used for analysis. Trials with spontaneous EMG activity occurring before the first stretch were discarded. The actuator control and data collection were synchronized by custom written LabVIEW® software.
Terminal H-Reflex testing
At the conclusion of the experiment, a subset of the animals were tested for FDD of the H-reflex. Animals were anesthetized with ketamine (60 mg · kg−1, i.m.) and xylazine (10 mg/kg, i.m.) and maintained with 10% doses as needed such that vibrissal and pinna pinch reflexes were absent. A bipolar cuff electrode was placed on the tibial nerve for stimulation (0.1 msec pulses). Exposed tissue was covered with mineral oil to prevent drying. The plantar surface of the foot was thoroughly cleaned with alcohol and a surface electrode was placed over the digital interosseous muscles between the fourth and fifth metatarsals for EMG recording and referenced to a second surface electrode applied to the skin on the heel. Recordings were made using amplifier (Grass P511) filter settings of 3 Hz to 3 kHz with the 60 Hz notch filter in use. Responses to the stimulus were digitized and averaged using a GW Instruments (Somerville, MA) digitizer module and Super-Scope® software.
Measurement and statistics
Variables of interest included peak plantarflexion (PF) torque and integrated EMG of the gastrocnemius. Reaction torque was obtained from a Lebow 1701 rotary torque transducer (Troy, MI, USA). After low pass filtering the torque signal at 200 Hz using Matlab®, the peak torque was identified during each PF stretch. Inertial artifact and passive tension was effectively removed by normalizing all repetitions to the first stretch. Gastrocnemius EMG burst activity occurred reflexively beginning at approximately 12 msec following the stretch and often continued throughout the plantarflexion and dorsiflexion movements. Matlab® was used to integrate the filtered and rectified EMG signal over the 250 msec period after the initiation of stretch. Similar to torque, the integrated EMG values for each stretch repetition were normalized to the first stretch. For H-reflex analysis, peak to peak measures were taken for each frequency of stimulation and data were normalized to the 0.2 Hz trial. For comparison of data across groups, univariate analyses were conducted with a one way ANOVA. When significant differences were found, post hoc testing (least significant difference- LSD) was performed to determine difference between groups. Differences were considered significant at p<0.05 for all tests.
RESULTS
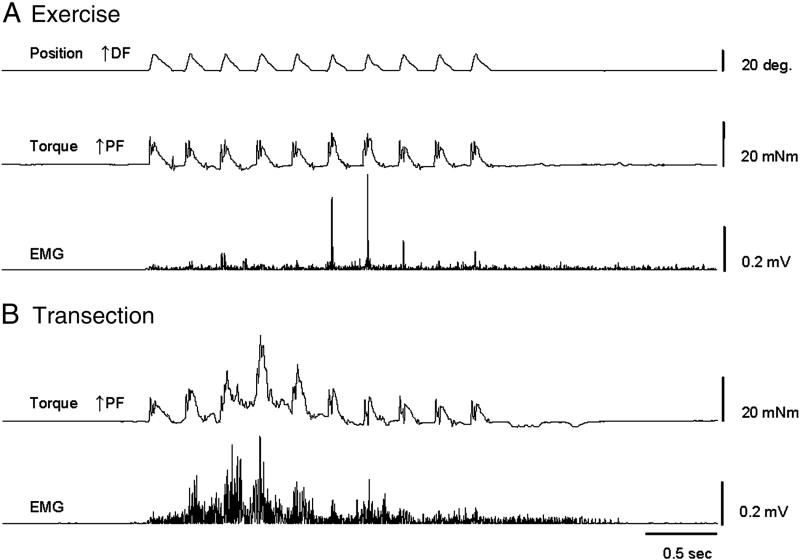
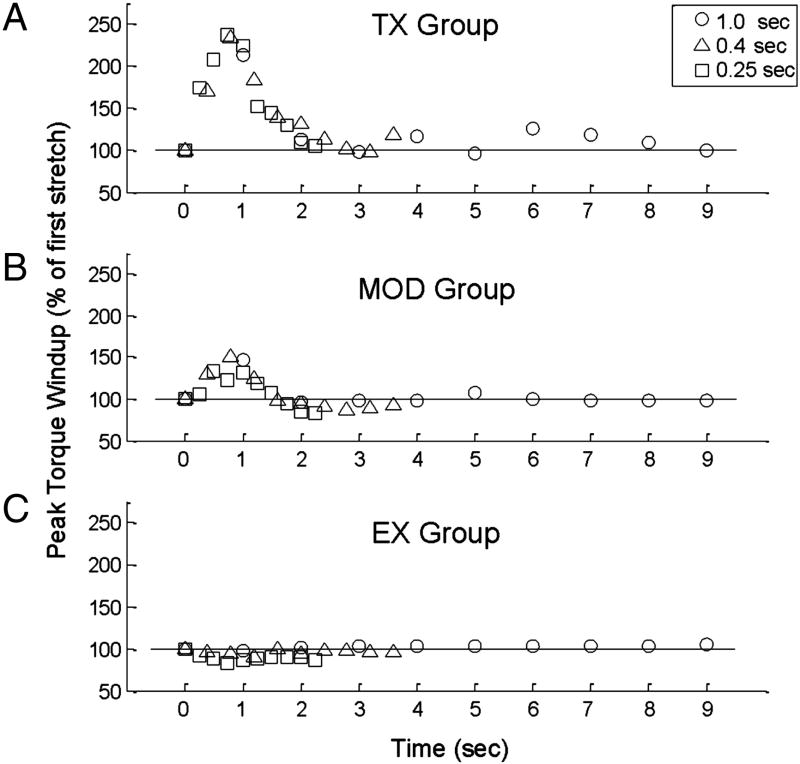
All data presented here were collected at 49 days post Tx. Typical data from the SR wind-up protocol is shown in figure 1 for a Tx only animal and an animal in the Ex group. Ankle position, torque, and rectified gastrocnemius EMG are plotted for single trials with stretch intervals of 0.25 sec. The time course of the SR wind-up at the various stretch intervals can be seen in figure 2. Group averages of all ten repetitions were plotted over time. While MOD and Ex groups failed to show clear SR wind-up at any stretch interval, the response of the Tx only animals showed robust SR wind-up of the torque (figure 2), and EMG response (data not shown). Responses peaked by 1 sec following the first stretch and a rapid habituation followed by 2 sec. While EMG and PF torque responses continued to be present in the later repetitions (see figure 1), they were typically at or below levels of the first stretch.
Figure 1. Stretch reflex recordings showing the SR wind-up protocol at a 0.25 sec interval.
Representative data is shown at 49 days post spinal cord transection (Tx) for a Tx only animal and an animal in the Ex group. Ankle position, ankle torque and rectified gastrocnemius EMG are plotted across time. A) After 6 weeks of daily exercise, animals demonstrated SR activity (see EMG) but responses did not wind up with repeated stretches. B) Tx animals showed a strong SR wind-up of both the EMG and torque responses to repeated stretches with a peak during the 4th repetition. DF – dorsiflexion, PF – plantar flexion.
Figure 2. Time course of SR wind-up and habituation at various stretch intervals.
All values are normalized to the first stretch and group means are shown for peak plantarflexion torque at 49 days post Tx. Similar results were noted for gastrocnemius EMG. A) Regardless of stretch interval, Tx only animals (n=7) showed marked SR wind-up with a peak response occurring during stretch 2, 3, 4 for stretch intervals of 1 sec, 0.4 sec and 0.25 sec, respectively. Habituation of the response occurred rapidly with no evidence of SR wind-up by 2 sec. B) Modafinil treated animals (n=8) showed a slight increase in torque but values were not significantly increased compared to the first stretch. C) Exercise animals (n=8) failed to show evidence of SR wind-up at any stretch interval.
EMG
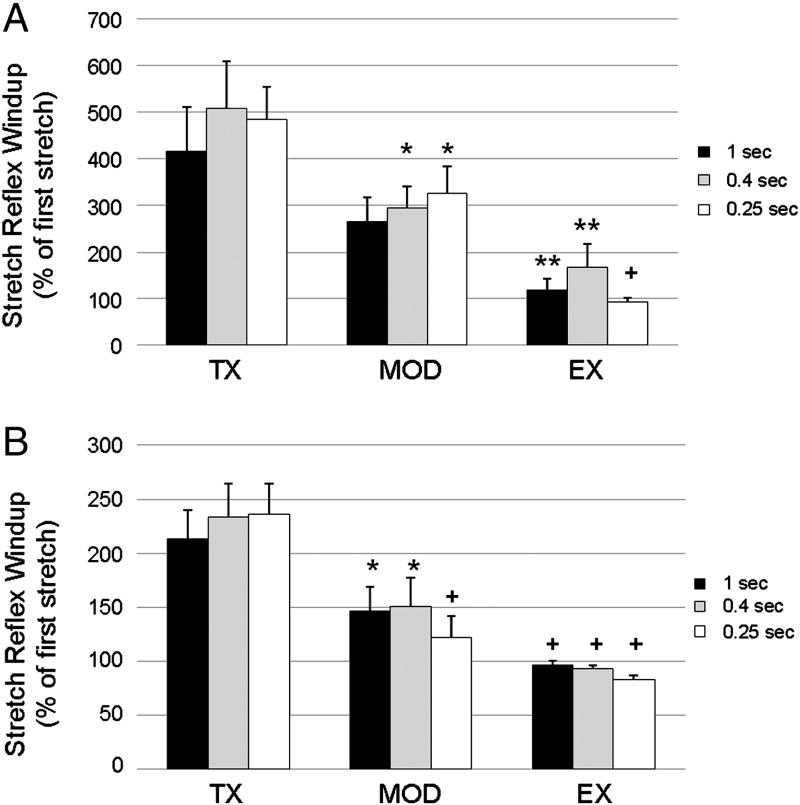
The ANOVA showed significant differences for EMG at intervals of 1 sec (df=2, F=5.84, p=0.01), 0.4 sec (df=2, F=6.38, p<0.01), and 0.25 sec (df=2, F=14.40, p<0.001). Post hoc analysis using least significant differences (LSD) testing revealed that the Ex group was significantly different than the Tx group at 1 sec and 0.4 sec (p<0.01), and 0.25 sec (p<0.001). The MOD group showed mixed results for EMG with significance difference from Tx at 0.4 sec and 0.25 sec (p<0.05), but not at 1 sec (p=0.1). Figure 3A shows a comparison of means across groups for each stretch interval.
Figure 3. Comparison of SR wind-up of integrated EMG and peak torque at 49 days post Tx.
Peak wind-up is shown as a percentage of the first stretch with group means (±SE) plotted for various stretch intervals. A) Peak plantarflexion torque, and B) integrated gastrocnemius EMG showed similar patterns across groups. Tx (n=7) animals showed marked SR wind-up at all frequencies of stretch. Ex (n=8) and Mod (n=8) animals were prevented from developing SR wind-up. Significant differences from the Tx group are noted (*= p<0.05, ** = p<0.01, + = p<0.001).
Torque
Similar results were found for peak plantarflexion torque across groups and stretch intervals. Analysis of variance showed significant differences at 1 sec (df=2, F=8.61, p<0.01), 0.4 sec (df=2, F=9.06, p<0.01), and 0.25 sec (df=2, F=16.21, p<0.001). Post hoc analysis revealed the Ex group was significantly different from the Tx group at 1 sec, 0.4 sec, and 0.25 sec (p<0.001). The MOD group showed consistent results with significant differences from Tx at 1 sec, 0.4 sec (p<0.05), and 0.25 sec (p<0.001). Figure 3B shows a comparison of means across groups for each stretch interval.
H-reflex
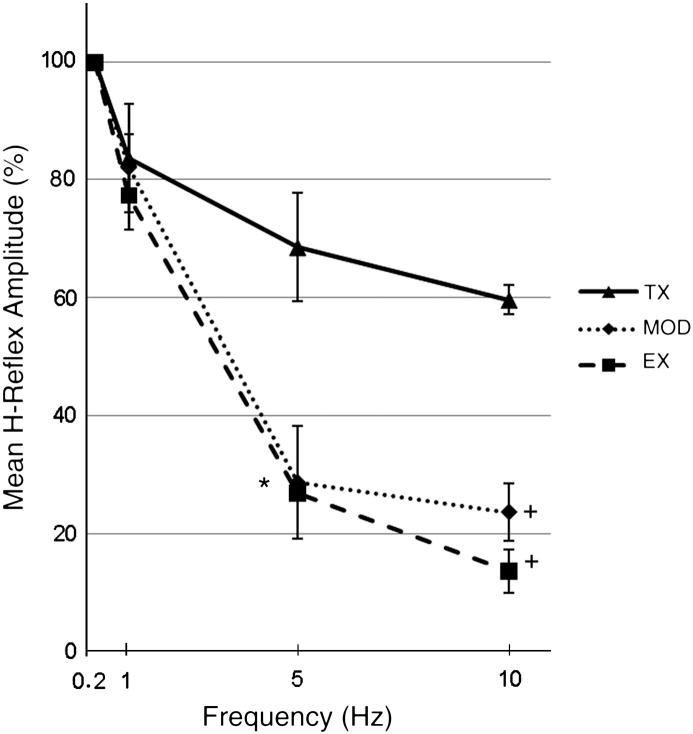
The results of terminal H-reflex testing of a randomly chosen subset of animals from each group are presented in figure 4. These results are consistent with previous studies on the effects of Ex and MOD on the FDD of the H-reflex (Yates, et al., 2009). ANOVA showed significant differences at 5 Hz (df=2, F=7.49, p<0.05), and 10 Hz (df=2, F=28.20, p<0.001). Post hoc analysis revealed significant differences from Tx only animals for both Ex and MOD animals at 5 Hz (p<0.05) and 10 Hz (p<0.001).
Figure 4. Frequency dependent depression of the H-Reflex.
Mean H-reflex amplitude at 0.2, 1, 5 and 10 Hz for transection (Tx, n=3), Exercise (Ex, n=4) and Modafinil treated animals (MOD, n=4) at 49 days post SCI. Data are normalized to 0.2 Hz values. Ex and MOD animals underwent 6 weeks of treatment. The Tx group failed to demonstrate normal habituation at higher frequencies. Ex and MOD groups both showed habituation that was significantly different than the Tx group at 5Hz (*p<0.05) and 10Hz (+p<0.001).
DISCUSSION
The current study demonstrates a minimally invasive biomechanical measure of spasticity based upon the temporal summation or wind-up of the SR in the rat post complete SCI. Furthermore, the prevention of SR wind-up following the interventions of passive exercise (Ex) and modafinil (MOD) are shown along with normalization of the electrophysiological H-reflex FDD. While there are multiple sources/circuits contributing to spasticity (Nielsen, et al., 2007), these combined measures, and the responses to the interventions, provide insight and direction for further study of the mechanisms involved in spasticity.
Effect of Exercise
The effect of passive exercise on preserving the H-reflex FDD has been shown to be dependent on afferent feedback from group I and II fibers (Ollivier-Lanvin, et al., 2009). While this requisite afferent input could arise from the stretch of the gastrocnemius, the tibialis anterior muscle is also stretched during the cycling exercise which mimics the locomotor cycle. Reciprocal disynaptic inhibition is present in the ankle dorsiflexors and plantarflexors in normal subjects (Nielsen, et al., 1995), and has been shown to be reduced in patients with SCI (Crone, et al., 2003). It is possible that passive exercise provides afferent input to these dorsiflexor pathways resulting in restoration of this normal inhibition on gastrocnemius motoneurons with an end result of diminishing the SR wind-up.
Exercise has been shown to increase levels of neurotrophins, including BDNF (Vaynman and Gomez-Pinilla, 2005), and its role in recovery following contusion injury has been documented (Macias, et al., 2007, Ying, et al., 2008). Recently, BDNF has been shown to upregulate KCC2 and restore FDD in SCI rats (Boulenguez, et al., 2010), consistent with the demonstrated effect of passive exercise on the H-reflex in the current study. Boulenguez et al. additionally suggested the downregulation of the potassium chloride cotransporter, KCC2, and the upregulation of PICs in chronic SCI, may work synergistically in the manifestation of spasticity. While the exact mechanism of the beneficial effects of exercise is unknown, both the SR and H-reflex are robustly affected by this intervention, which shows promise for the treatment of spasticity following SCI.
Effect of modafinil
Modafinil is a wakefulness promoting agent that has been used for a number of off label indications (Ballon and Feifel, 2006). Several studies have examined the benefits of modafinil for the treatment of spasticity in children with cerebral palsy with mixed results (Hurst, et al., 2004, Murphy, et al., 2008). More recently, restoration of H-reflex FDD has been shown in SCI rats treated with modafinil (Yates, et al., 2009). The proposed mechanism of action of modafinil in spasticity is based upon the promotion of connexin-36 (Cx-36) gap junction proteins (see Yates, et al., 2010 for a review). Further molecular studies have demonstrated that an exercise regimen fails to increase Cx-36 mRNA levels in the ventral horn, while animals treated with modafinil show a return to control levels (Yates, et al., 2010).
A second possible mechanism of action of modafinil on the SR and FDD is through modulation of monoamine transporters and receptors (see Minzenberg and Carter, 2008 for a review). Cyproheptadine, a 5-HT2 receptor antagonist, has been used clinically to alleviate spasticity (Nance, 1994), and a positive correlation between the H/M ratio and serotonin levels has been shown in a contusion model of spinal cord injury (Lee, et al., 2005). However, the effects of modafinil in a transection model is less clear since little to no serotonin is present when descending tracts are lost. Moreover, the effects of modafinil on monoamine transporters takes place at concentrations higher than those used herein, so that we are uncertain if there was a significant effect in our case.
In the current study, normalized peak torque and EMG levels in the MOD group were 50–100% higher than in the Ex group for all stretch intervals. This was consistent with H-reflex recordings that showed less pronounced FDD in the MOD group. These data suggest different mechanisms for the two interventions, and future studies could explore the possibility of additive effects as has been shown for passive exercise and L-dopa (A. Arfaj, 2009).
In animals, motoneuron PICs have been shown to be responsible for the prolonged excitatory output following a brief afferent input (Heckmann, et al., 2005). The occurrence of PICs is strongly dependent on the presence of monoamines in the spinal cord (Harvey, et al., 2006, Li, et al., 2007). While the majority of monoamines are lost following a complete injury (Newton and Hamill, 1988), there is a spontaneous return of PICs that coincides with the onset of spasms (Li and Bennett, 2003). Supersensitivity of receptors to remaining levels of serotonin has been proposed (Rank, et al., 2007), but recent findings (Murray, et al., 2010), show constitutive activity of serotonin receptors that enables PIC activity in the absence of serotonin. Quantification of spasms in these studies has been through in vitro dorsal root or in vivo cutaneous tail stimulation with changes seen in the long latency flexion withdrawal response. However, differences in flexor reflex and SR wind-up have been shown in humans (Hornby, et al., 2006, 2003). The plateau behavior characterized by SR wind-up in rats adds to the body of knowledge regarding the possible role of PICs in spasticity, in addition to the more established association with flexor spasms.
The time course of the wind-up to repeated stretches was found to be very short with a habituation of the response by 2 sec (figure 2) regardless of the stretch interval. The rate dependency is similar to the findings in humans with SR wind-up occurring at intervals of less than 0.5 sec. However, a rapid habituation of the SR wind-up was not seen in the human responses as EMG and torque values continued to increase over the six imposed stretches (Hornby, et al., 2006). A possible reason for this decrease in reflex output may be from the habituation of Ia afferents resulting in a diminished input to the motoneurons (Haftel, et al., 2004), presumably to a level that is sub-threshold for activation of PICs.
One of the limitations of the current study is the lack of SR wind-up testing in control animals. In pilot testing, an attempt was made to test spinal intact animals but the repeated perturbations produced a dominating voluntary EMG and force activity that prevented assessment of the reflex component. However, spinal intact individuals fail to demonstrate temporal summation in response to repeated stretches (Hornby, et al., 2006), and a clear depression is demonstrated at slower frequencies (Grey, et al., 2008). Based on these consistent human studies, the authors would expect similar findings in spinally intact rats.
The inherent variability of spasticity that occurs day-to-day and even hour-to-hour makes quantifying this impairment a challenge. The irregularity is evident in the current variables with much greater variance noted in the EMG than torque measures. More modest variance in torque may be due to the inherent low pass filtering of the mechanical response. The torque measure also perhaps demonstrates a more valid measure as it is the combined output of all the ankle plantarflexor muscles rather than a single head or even muscle bundle, as is the case with fine wire EMG recordings.
The authors acknowledge that changes in musculotendinous properties likely contributed to differences in the absolute PF torque for the groups. The relative immobilization of the Tx group would be expected to result in increased stiffness (Dietz, et al., 1981, Gracies, 2005, Sinkjaer, et al., 1993). This could account for some of the difference when compared to the Ex group but would not account for observed differences between the Tx and MOD groups which had similar cage activity. No attempt was made to subtract the torque due to passive tissue properties from the active/reflex component. The normalization process was used to minimize this effect along with the large inertial components of the torque response.
The EMG data does provide some insight that the H-reflex and torque responses fail to reveal. While EMG of the gastrocnemius does not wind-up in the Ex group, there remains a prolonged long latency EMG response to the imposed stretches (figure 1). This suggests that, while the interventions reduced spasticity and hyper-reflexia, spasticity was not completely eliminated. This is consistent with observations during manual expression of the bladder, when the rats often demonstrate multi-joint spastic reflexes (Schmit and Benz, 2002) characterized by extension at the knees bilaterally with ankle PF.
As the search for optimal therapies for spasticity continues, it should be kept in mind that complete suppression of all motor activity should not be the goal. Although spasticity is a commonly reported negative sequela of SCI, patients do report benefits of spasticity (Cook, et al., 2007, Little, et al., 1989). The current research provides additional evidence to a growing body of work that support the use of passive exercise (Kiser, et al., 2005, Phadke, et al., 2009), and modafinil (Yates, et al., 2010, 2009) for the treatment of spasticity and hyper-reflexia. The SR wind-up also provides an objective, non-invasive measure of spasticity that can be used to monitor the effects of experimental interventions in both animals and humans with SCI.
Acknowledgments
Supported by USPHS Grants RR020146, RR016460, and NS062363
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arfaj A, RDS, Yates C, Garrison K, Reese NB, Garcia-Rill E. Neurosci Abstr. Las Vegas, NV: 2009. Reversal of H-reflex Hyperactivity by L-dopa and Exercise in Alert, Chronically Spinalized Rats. [Google Scholar]
- 2.Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol. 2001;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic Long-Lasting Reflexes in the Awake Rat After Sacral Spinal Cord Injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bose P, Parmer R, Thompson FJ. Velocity-dependent ankle torque in rats after contusion injury of the midthoracic spinal cord: time course. J Neurotrauma. 2002;19:1231–1249. doi: 10.1089/08977150260338029. [DOI] [PubMed] [Google Scholar]
- 7.Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium–chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 8.Calancie B, Molano MR, Broton JG. Interlimb reflexes and synaptic plasticity become evident months after human spinal cord injury. Brain. 2002;125:1150–1161. doi: 10.1093/brain/awf114. [DOI] [PubMed] [Google Scholar]
- 9.Cook KF, Teal CR, Engebretson JC, Hart KA, Mahoney JS, Robinson-Whelen S, Sherwood AM. Development and validation of Patient Reported Impact of Spasticity Measure (PRISM) J Rehabil Res Dev. 2007;44:363–371. doi: 10.1682/jrrd.2006.04.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- 12.Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- 13.Enriquez-Denton M, Morita H, Christensen LO, Petersen N, Sinkjaer T, Nielsen JB. Interaction between peripheral afferent activity and presynaptic inhibition of ia afferents in the cat. J Neurophysiol. 2002;88:1664–1674. doi: 10.1152/jn.2002.88.4.1664. [DOI] [PubMed] [Google Scholar]
- 14.Garrison MK, Yates C, Ishida K, Yuen B, Skinner RD, Garcia-Rill E. Neurosci Abstr. Washington, DC: 2008. Passive exercise alters stretch reflex properties in rats with spinal cord transection. [Google Scholar]
- 15.Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve. 2005;31:535–551. doi: 10.1002/mus.20284. [DOI] [PubMed] [Google Scholar]
- 16.Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sorensen F, Ravnborg M, Nielsen JB. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185:189–197. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- 17.Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol. 2004;91:2164–2171. doi: 10.1152/jn.01147.2003. [DOI] [PubMed] [Google Scholar]
- 18.Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- 20.Hornby TG, Kahn JH, Wu M, Schmit BD. Temporal facilitation of spastic stretch reflexes following human spinal cord injury. J Physiol. 2006;571:593–604. doi: 10.1113/jphysiol.2005.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of Flexion Reflexes in Chronic Human Spinal Cord Injury: A Marker for Neuronal Plateau Potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- 22.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hultborn H. Spinal reflexes, mechanisms and concepts: from Eccles to Lundberg and beyond. Prog Neurobiol. 2006;78:215–232. doi: 10.1016/j.pneurobio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Hurst DL, Lajara-Nanson WA, Dinakar P, Schiffer RB. Retrospective review of modafinil use for cerebral palsy. J Child Neurol. 2004;19:948–951. doi: 10.1177/08830738040190120701. [DOI] [PubMed] [Google Scholar]
- 25.Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Research Brain Research Reviews. 2002;40:19–28. doi: 10.1016/s0165-0173(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 26.Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, Pait TG, Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Emch GS, Johnson CS, Wrathall JR. Effect of spinal cord injury severity on alterations of the H-reflex. Exp Neurol. 2005;196:430–440. doi: 10.1016/j.expneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2007;97:1236–1246. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- 30.Little JW, Micklesen P, Umlauf R, Britell C. Lower extremity manifestations of spasticity in chronic spinal cord injury. American Journal of Physical Medicine & Rehabilitation. 1989;68:32–36. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Macias M, Dwornik A, Ziemlinska E, Fehr S, Schachner M, Czarkowska-Bauch J, Skup M. Locomotor exercise alters expression of pro-brain-derived neurotrophic factor, brain-derived neurotrophic factor and its receptor TrkB in the spinal cord of adult rats. Eur J Neurosci. 2007;25:2425–2444. doi: 10.1111/j.1460-9568.2007.05498.x. [DOI] [PubMed] [Google Scholar]
- 32.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 33.Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol. 1998;80:610–620. doi: 10.1152/jn.1998.80.2.610. [DOI] [PubMed] [Google Scholar]
- 34.Murphy AM, Milo-Manson G, Best A, Campbell KA, Fehlings D. Impact of modafinil on spasticity reduction and quality of life in children with CP. Dev Med Child Neurol. 2008;50:510–514. doi: 10.1111/j.1469-8749.2008.03019.x. [DOI] [PubMed] [Google Scholar]
- 35.Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakazawa K, Kawashima N, Akai M. Enhanced stretch reflex excitability of the soleus muscle in persons with incomplete rather than complete chronic spinal cord injury. Arch Phys Med Rehabil. 2006;87:71–75. doi: 10.1016/j.apmr.2005.08.122. [DOI] [PubMed] [Google Scholar]
- 37.Newton BW, Hamill RW. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Research Bulletin. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen J, Crone C, Sinkjaer T, Toft E, Hultborn H. Central control of reciprocal inhibition during fictive dorsiflexion in man. Exp Brain Res. 1995;104:99–106. doi: 10.1007/BF00229859. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity--from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 40.Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain. 2008;131:1478–1491. doi: 10.1093/brain/awn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ollivier-Lanvin K, Keeler BE, Siegfried R, Houle JD, Lemay MA. Proprioceptive neuropathy affects normalization of the H-reflex by exercise after spinal cord injury. Exp Neurol. 2009;221:198–205. doi: 10.1016/j.expneurol.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsson MC, Kruger M, Meyer LH, Ahnlund L, Gransberg L, Linke WA, Larsson L. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol. 2006;577:339–352. doi: 10.1113/jphysiol.2006.116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phadke CP, Flynn SM, Thompson FJ, Behrman AL, Trimble MH, Kukulka CG. Comparison of single bout effects of bicycle training versus locomotor training on paired reflex depression of the soleus H-reflex after motor incomplete spinal cord injury. Arch Phys Med Rehabil. 2009;90:1218–1228. doi: 10.1016/j.apmr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol. 2007;97:3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- 46.Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmit BD, Benz EN. Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp Brain Res. 2002;145:520–527. doi: 10.1007/s00221-002-1134-5. [DOI] [PubMed] [Google Scholar]
- 48.Schmit BD, Benz EN, Rymer WZ. Afferent mechanisms for the reflex response to imposed ankle movement in chronic spinal cord injury. Exp Brain Res. 2002;145:40–49. doi: 10.1007/s00221-002-1080-2. [DOI] [PubMed] [Google Scholar]
- 49.Schmit BD, Dewald JP, Rymer WZ. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil. 2000;81:269–278. doi: 10.1016/s0003-9993(00)90070-4. [DOI] [PubMed] [Google Scholar]
- 50.Schmit BD, Dhaher Y, Dewald JP, Rymer WZ. Reflex torque response to movement of the spastic elbow: theoretical analyses and implications for quantification of spasticity. Ann Biomed Eng. 1999;27:815–829. doi: 10.1114/1.234. [DOI] [PubMed] [Google Scholar]
- 51.Sinkjaer T, Toft E, Larsen K, Andreassen S, Hansen HJ. Non-reflex and reflex mediated ankle joint stiffness in multiple sclerosis patients with spasticity. Muscle Nerve. 1993;16:69–76. doi: 10.1002/mus.880160112. [DOI] [PubMed] [Google Scholar]
- 52.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- 53.Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Phys Ther. 2000;80:485–498. [PubMed] [Google Scholar]
- 54.Thompson FJ, Browd CR, Carvalho PM, Hsiao J. Velocity-dependent ankle torque in the normal rat. Neuroreport. 1996;7:2273–2276. [PubMed] [Google Scholar]
- 55.Thompson FJ, Parmer R, Reier PJ. Alteration in rate modulation of reflexes to lumbar motoneurons after midthoracic spinal cord injury in the rat. I. Contusion injury. J Neurotrauma. 1998;15:495–508. doi: 10.1089/neu.1998.15.495. [DOI] [PubMed] [Google Scholar]
- 56.Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- 57.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 58.Voerman GE, Gregoric M, Hermens HJ. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil. 2005;27:33–68. doi: 10.1080/09638280400014600. [DOI] [PubMed] [Google Scholar]
- 59.Yates C, Charlesworth A, Allen SR, Reese NB, Skinner RD, Garcia-Rill E. The onset of hyperreflexia in the rat following complete spinal cord transection. Spinal Cord. 2008a doi: 10.1038/sc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yates C, Garrison M, Reese N, Charlesworth A, Garcia-Rill E. Novel mechanism for hyper-reflexia and spasticity. Prog Brain Res. 2010;187 doi: 10.1016/B978-0-444-53825-3.00016-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yates C, Garrison MK, Reese NB, Garcia-Rill E. Therapeutic approaches for spinal cord Injury induced spasticity. Translational Neurosci. 2010;1:160–169. [Google Scholar]
- 62.Yates CC, Charlesworth A, Reese NB, Ishida K, Skinner RD, Garcia-Rill E. Modafinil normalized hyperreflexia after spinal transection in adult rats. Spinal Cord. 2009;47:481–485. doi: 10.1038/sc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yates CC, Charlesworth A, Reese NB, Skinner RD, Garcia-Rill E. The effects of passive exercise therapy initiated prior to or after the development of hyperreflexia following spinal transection. Exp Neurol. 2008b;213:405–409. doi: 10.1016/j.expneurol.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zafonte R, Lombard L, Elovic E. Antispasticity medications: uses and limitations of enteral therapy. Am J Phys Med Rehabil. 2004;83:S50–58. doi: 10.1097/01.phm.0000141132.48673.fa. [DOI] [PubMed] [Google Scholar]