Abstract
This study evaluated the effects of atomoxetine on the symptoms of attention deficit/hyperactivity disorder (ADHD) and marijuana use in marijuana-dependent adults. In conjunction with motivational interviewing, participants received either atomoxetine (n=19) or matching placebo (n=19) for 12 weeks. Participants randomized to atomoxetine had greater improvement in ADHD on the Clinical Global Impression-Improvement scale than participants treated with placebo. No treatment group differences in self-rated ADHD symptoms, overall Wender-Reimherr Adult Attention Deficit Disorder Scale scores, or marijuana use outcomes were noted. These results suggest that atomoxetine may improve some ADHD symptoms but does not reduce marijuana use in this population.
INTRODUCTION
Marijuana is the most commonly used illicit drug in the United States. The 2006 National Survey on Drug Use and Health indicates that 97.8 million (39.8%) of Americans 12 years of age or older have tried marijuana at least once in their lifetime and 25.4 million (10.3%) have used marijuana in the past year.1 Lifetime prevalence rates of marijuana dependence have been estimated at 4% of the population.2
Marijuana has also been reported in several studies to be the most commonly used drug by adults with attention deficit hyperactivity disorder (ADHD).3,4,5 Attention deficit hyperactivity disorder (ADHD) is now recognized not just as a disease of childhood as symptoms may persist into adulthood in as many as 65% of cases.6 The prevalence rate of current adult ADHD is estimated at 4.4%.7 Adults with ADHD have an approximately two-fold higher lifetime risk of developing a substance use disorder (SUD) compared to adults without ADHD.8 Individuals with ADHD may use substances for a variety of reasons including impulsivity, impaired social and occupational functioning, and self-medication.9 Of clinical importance, individuals with ADHD have been noted to have a shorter transition time from drug abuse to dependence, a longer duration of SUD, and a slower rate of remission.10,11,12 In adolescents with ADHD, treatment with stimulant therapy has been shown to decrease risk for later substance abuse.13,14,15 However, trials evaluating the efficacy of stimulant treatment in adults with substance use disorders and ADHD have had primarily negative results.16,17
Atomoxetine is a highly selective inhibitor of the presynaptic norepinephrine transporter which has been shown to be efficacious in reducing ADHD symptoms in adults.18 By inhibiting reuptake of norepinephrine, atomoxetine increases levels of both dopamine and norepinephrine in the prefrontal cortex but does not appear to increase dopamine in subcortical areas where there are few noradrenergic nerve terminals.19 As a result, atomoxetine appears to improve cognition with little risk of abuse potential. An evaluation of atomoxetine treatment on ADHD symptoms and drinking outcomes in recently abstinent individuals with alcohol use disorders found improvements in ADHD measures and a reduction in heavy drinking days.20 The purpose of this study was to explore the safety and efficacy of atomoxetine for reducing substance use and improving ADHD symptoms in marijuana dependent adults. The primary hypothesis was that atomoxetine treatment would decrease marijuana use and craving as compared to a placebo-treated group, and the secondary hypothesis was that atomoxetine treatment would improve ADHD symptoms as compared to a placebo-treated group.
METHODS
The study was a 12-week, double-blind, placebo-controlled trial of a flexible dose of atomoxetine (up to 100 mg/day) in marijuana-dependent individuals with ADHD, conducted between November 2005 and June 2008. Patients were recruited primarily through media advertisements and fliers. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki and received approval from the Medical University of South Carolina Institutional Review Board. All participants gave written, informed consent prior to study participation.
To be eligible for participation, subjects had to be between 18 and 65 years of age and meet DSM-IV criteria for marijuana dependence. Participants also had to meet DSM-IV criteria for Attention-Deficit Hyperactivity Disorder with the exception of the criterion that the age of onset of symptoms had to be prior to seven years of age. This adjustment was made based on the DSM-IV field trial which found that the use of the seven years of age criterion diminished the reliability of clinical diagnosis.21 Further, data suggests that late-onset ADHD (in which individuals meet all DSM-IV ADHD criteria with the exception of age of onset), especially when the age of onset is no later than age 12, have similar patterns of functional impairment and psychiatric comorbidity as individuals meeting all criteria for childhood-onset ADHD.22,23 Participants were therefore included if symptoms of ADHD were present prior to the age of 12. Exclusion criteria included dependence on any other substance (with the exception of caffeine or nicotine); history of psychotic disorder; current major depression or eating disorder; current treatment with a psychoactive medication; major medical illnesses; cognitive impairment; and pregnancy, nursing, or inadequate birth control. All potential subjects received a medical history and an evaluation for medical exclusions. The medical work-up included a routine physical exam, blood chemistries, test of liver function, pregnancy test as indicated, and urine drug screen.
The Structured Clinical Interview for DSM-IV (SCID-IV)24 was used to assess for psychiatric exclusions, and the Mini Mental Status Exam25 was administered to assess absence of cognitive impairment. The Conners’ Adult ADHD Diagnostic Interview (CAADID; 26) was used to determine ADHD diagnosis. ADHD symptom ratings were also obtained from someone who knew the subject well, such as a spouse/partner, parent, sibling, or close friend, through completion of the Conners’ Adult ADHD Rating Scale-Observer (CAARS-O;26). Marijuana use for the ninety days prior to study entry was estimated using the Time-Line Follow-Back (TLFB),27 and TLFB data were collected weekly throughout the study. Weekly assessments included the Marijuana Craving Questionnaire (MCQ;28) and the Clinical Global Impression of Severity and Improvement Scales (CGI-I and CGI-S;29) to rate ADHD symptoms and marijuana use. The Hamilton Anxiety Scale (HAM-A;30), Hamilton Depression Scale (HAM-D;31), and Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADS;32) were administered at baseline and weeks 1, 2, 4, 6, 8, and 12. The CAARS-Self was completed by the participant at baseline and weeks 2, 4, 6, 8, and 12. Semi-quantitative urine drug screens for marijuana were performed at baseline and at each weekly visit.
The motivational interviewing intervention was modeled after the Drinker’s Check-Up33 and involved three sessions. The first session occurred prior to medication initiation, and a second session occurred approximately one week later. These sessions incorporated use of a personalized feedback report summarizing the participant’s frequency of marijuana use, problems related to use, reasons for quitting, and high-risk situations for use. A third session occurred at week 4, and was used to follow-up on action plans (if any) and formation of short-and long-term goals.
Simple randomization was used to assign treatments to participants using a 1:1 allocation ratio. Matching atomoxetine and placebo capsules were provided by the manufacturer of atomoxetine (Eli Lilly and Company). Medication dosage was started at 25 mg atomoxetine or placebo daily. The dose was increased to 40 mg atomoxetine or matching placebo daily during the second week, and to 80 mg atomoxetine or matching placebo during the third week as tolerated. During the fourth week, the dose could be increased to 100 mg atomoxetine or matching placebo daily. Medication side effects were evaluated weekly using a standard medication side effects checklist. Compliance was assessed by patient report and pill count. Subjects received nominal monetary reimbursement for completion of study assessments.
STATISTICAL CONSIDERATIONS
Sample Size Estimation
This study assumed there would be a 33% reduction in self-reported use in the placebo arm. This estimate was based on the 25% reduction observed in the delayed treatment control (DTC) arm of a previous report34 and an assumption that the motivational interviewing would provide an additional 10% reduction in use. Based on preliminary data,35 it was estimated that a 77% reduction in use would occur in participants receiving atomoxetine and motivational interviewing. Using summaries of the number of times marijuana was used per day,34 these estimated percent changes produced an effect size of approximately 1.0, with this effect size based on an estimated treatment difference of 1.092 times used per day with a common standard deviation of 1.1. Sample size formula for a two-tailed t-test (90% power; alpha=0.05) indicated 23 subjects per arm were necessary. An attrition rate up to 30% was anticipated, so the inflation factor of 1/(1-d), where d represented the anticipated dropout rate, was used to adjust the sample size for attrition.36 Thus, a total of 35 subjects per arm were determined to be sufficient, but enrollment was increased because there was a higher than anticipated attrition rate.
Outcome Measures
The primary efficacy outcome for this study was the mean self reported use during Week 12 of the study. Secondary efficacy endpoints included longitudinal self-reported use, weekly semi-quantitative urine drug screens, and ADHD symptoms as measured by the WRAADS and CGI improvement scale.
Primary Statistical Analysis
The protocol specified an analysis of covariance (ANCOVA) model examining the effects of treatment on the Week 12 average self-reported marijuana use while controlling for the 90 days pre-treatment average marijuana use. Last observation carried forward (LOCF) was used to impute the Week 12 value when the subject did not complete the trial (n=22). The variance of the Week 12 results was proportional to the amount of use at baseline (i.e., heteroscedasticity, a violation of the constant variance assumption), so weighted least squares was used.37. The weights for each observation were set at the reciprocal of the baseline use value. To address the inherent limitations of the LOCF imputation method,38 sensitivity analyses on the weekly self-reported marijuana use were conducted using mixed effects modeling and generalized estimating equations. Longitudinal trajectories of the weekly self-reported use were modeled using generalized linear model (normal distribution, identity link).39 A GLM, as the name implies, is a robust modeling framework that allows for non-normally distributed dependent variables and alternative ways of “linking” the dependent variable to the independent variables of interest. For this model, the distribution was specified as normal and the identity link was used; however, the normality assumption (of the residuals) was questionable, so the robust (sandwich) variance estimator was used to correct for the potentially misspecified model.40
The between group difference in percentage of positive UDS results (i.e., risk difference) was also estimated using a generalized linear model (GLM) framework.39 To estimate the risk difference directly, the GLM was configured with the identity link, a binomial distribution for the dependent variable, and a systematic component of an intercept and an indicator variable for the main effect of the randomized treatment group. Configured in this manner, the beta-coefficient associated with the main effect of the treatment group was the primary parameter of interest. That is, a test of this beta-coefficient equal to zero is a test of the null hypothesis that there was no difference in the percentage of positive UDS results between the two groups. Generalized estimating equations40 were used to account for the clustering of UDS results within a participant.
The last reported CGI measurements were compared between groups using the t approximation of the Wilcoxon Rank Sum Test. Final improvement ratings and changes from baseline in the severity rating were analyzed separately for substance abuse and ADHD symptoms and were also tested using the Wilcoxon Rank Sum Test. Safety data were summarized using standard categorical data techniques. The denominator for the analyses was the number of subjects in the modified ITT sample. Participants that experienced the same event more than once were only counted as one case. Relative risk, as a measure of association, was used to quantify the differences in the proportion of participants experiencing the adverse events between treatment groups. In the event no adverse events were observed in a particular group, the relative risk was not computed.
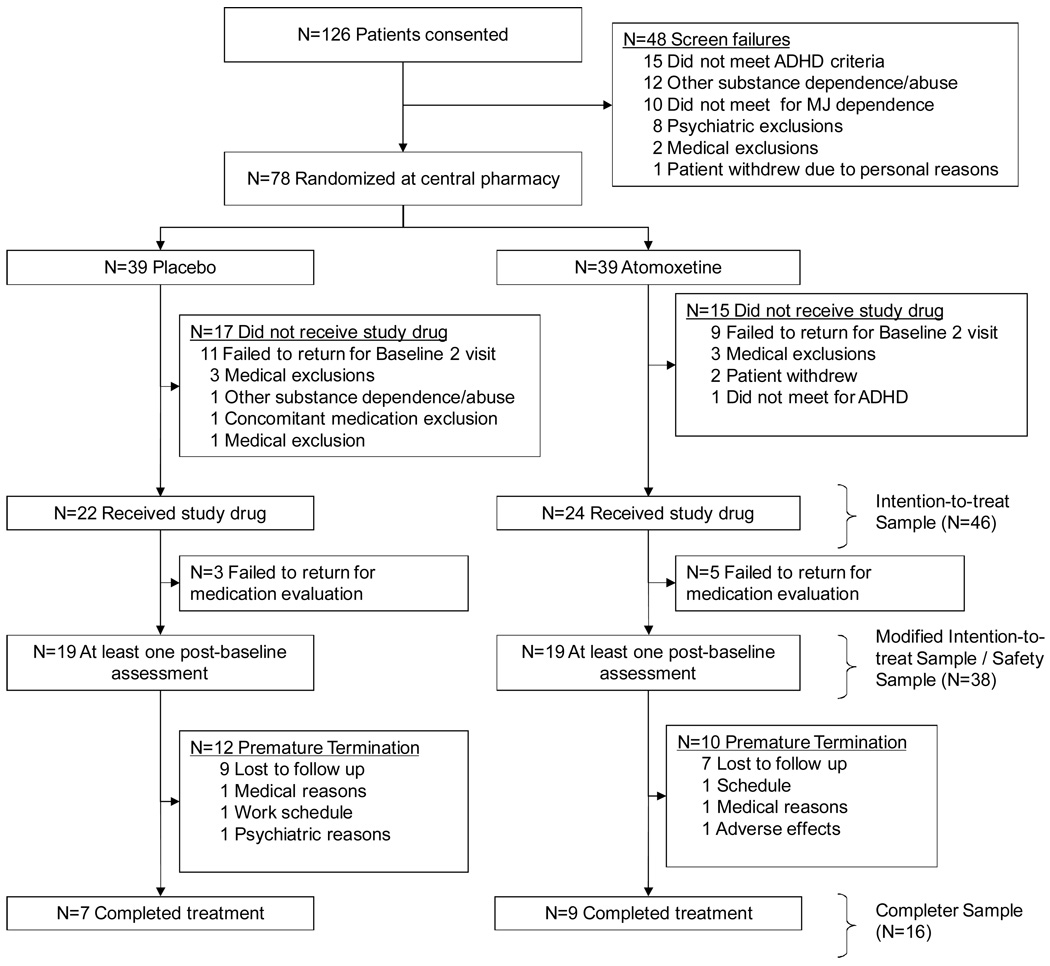
All primary analyses were conducted on the modified intention-to-treat (ITT) sample. This analysis set included all participants that were randomized and provided at least one post-randomization assessment. For comparative purposes, demographic summaries of the full ITT sample (Figure 1, n=46) were computed. All analyses were conducted using the SAS System (version 9.1.3, Cary, NC). The type I error rate was established a priori at 0.05, and no corrections for multiple comparisons have been applied to reported p-values.
FIGURE 1.
Progress of patients in the study
RESULTS
A total of 126 patients were consented. After initial eligibility was confirmed, 78 (n=39 placebo, n=39 atomoxetine) participants were randomized at the central pharmacy; however only 46 (n=22 placebo, n=24 atomoxetine) received study medication. The majority (63%) of the randomized participants who did not receive study medication failed to return to the second baseline examination, but 10 of the remaining 12 randomized participants did not pass the comprehensive medical examination at the second baseline visit and were not continued in the study. The remaining two randomized participants not included in the full ITT sample withdrew consent prior to administration of study medication. The modified ITT sample included the 38 participants (n=19 placebo, n=19 atomoxetine) who returned for at least one post-baseline assessment. Sixteen participants (42% of the modified ITT sample) completed the 12 week intervention. Participant flow through the study is summarized in Figure 1.
The majority of the modified ITT sample was male (76%) and Caucasian (92%) (Table 1). The mean (SD) age of the participants was 29.9 (11.5) years, and the majority (71%) had onset of ADHD before the age of 7. The mean baseline ADHD severity rating based on the CGI-S was 4.7 (SD=0.7). The use profile during the 90 days prior to baseline indicated that marijuana was used, on average, on 87% of the previous days. The mean times used per using day in this sample was 4.1 (SD=2.7). No statistically significant differences in baseline characteristics were found between treatment groups, but there was a trend that the placebo subjects had higher self-reported CAARS scores (p=0.06).
TABLE 1.
Sample description
| Modified Intention to Treat Sample (ITT, n=38) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full ITT Sample (n=46) | Modified ITT | Atomoxetine (n=19) | Placebo (n=19) | |||||||
| Variable | Mean | SD | Mean | SD | p-value {a} | Mean | SD | Mean | SD | p-value {b} |
| Demographics | ||||||||||
| Age | 29.9 | 10.9 | 29.9 | 11.5 | 0.48 | 29.4 | 10.0 | 30.4 | 13.0 | 0.76 |
| Sex, Male N, % | 37 | 80% | 29 | 76% | 0.12 | 16 | 84% | 13 | 68% | 0.25 |
| Race, Caucasian N, % | 42 | 91% | 35 | 92% | 0.67 | 17 | 89% | 18 | 95% | 0.55 |
| ADHD Onset Before Age 7 | 32 | 70% | 27 | 71% | 0.63 | 12 | 63% | 15 | 79% | 0.28 |
| ADHD Measures | ||||||||||
| WRAADS Total Score | 29.9 | 6.4 | 29.5 | 6.3 | 0.26 | 28.8 | 7.4 | 30.2 | 5.0 | 0.62 |
| Self Reported CAARS | 44.3 | 10.6 | 43.7 | 9.8 | 0.61 | 40.1 | 8.5 | 46.9 | 10.0 | 0.06 |
| CGI-S, ADHD Symptoms | 4.7 | 0.7 | 4.7 | 0.7 | 0.41 | 4.6 | 0.6 | 4.8 | 0.9 | 0.25 |
| Substance Use Measures | ||||||||||
| Marijuana Problem Scale | 10.3 | 6.4 | 9.9 | 6.3 | 0.50 | 9.4 | 6.8 | 10.4 | 5.9 | 0.54 |
| Marijuana Craving Questionnaire | 41.9 | 13.5 | 40.8 | 13.4 | 0.33 | 38.9 | 14.5 | 42.6 | 12.3 | 0.30 |
| CGI-S, Substance Abuse Symptoms | 5.0 | 0.8 | 5.0 | 0.8 | 0.12 | 4.8 | 0.9 | 5.2 | 0.7 | 0.18 |
| % Days of TLFB with reported use | 85.9% | 23.0% | 86.8% | 22.8% | 0.14 | 91.6% | 13.3% | 82.0% | 29.1% | 0.67 |
| Amount Using Per Using Day, Prior 90 days | 4.0 | 2.8 | 4.1 | 2.7 | 0.31 | 4.1 | 3.3 | 4.0 | 2.1 | 0.64 |
| General Psychiatric Measures | ||||||||||
| Hamilton Depression Rating Scale | 6.2 | 3.9 | 6.1 | 3.4 | 0.89 | 5.4 | 3.1 | 6.6 | 3.7 | 0.26 |
| Hamilton Anxiety Rating Scale | 8.1 | 5.3 | 8.3 | 5.2 | 0.62 | 7.4 | 4.5 | 9.1 | 5.9 | 0.37 |
For a comparison for differences in the n=8 participants included in the full ITT sample that were not included in the modified ITT sample using the Wilcoxon Rank Sum test (continuous measures) or Pearson Chi Square (categorical measures).
For a comparison of differences between treatment groups in the modified ITT sample using the same tests used in {a}
Primary Substance Abuse Efficacy Results
In weighted least squares analysis, the main effect of the randomization group was not significant (β̂ =−0.34, SE=0.44; p=0.44) at predicting the Week 12 mean self-reported use after adjusting for the mean use over the 90 days prior to randomization (Table 2). As expected, higher baseline use was associated with higher Week 12 mean self-reported use (β̂ =0.51, SE=0.12; p<0.001), and this variable explained 36% of the residual variation in the Week 12 mean self-report use after controlling for the randomized treatment assignment. In contrast, the randomized treatment assigned only explained 1.7% of the residual variance after adjustment for baseline use.
TABLE 2.
Outcome summary by treatment group
| Variable | Atomoxetine | Placebo | Test | p-value | ||
|---|---|---|---|---|---|---|
| Marijuana Dependence | ||||||
| Primary Outcome | Mean | SE | Mean | SE | ||
| Estimated (LSMean) Week 12 Self-Reported Use{b} | 2.17 | 0.34 | 1.84 | 0.34 | F Test (1, 35) | 0.44 |
| Secondary Efficacy Outcomes | ||||||
| Self-reported | Mean | SD | Mean | SD | ||
| % Days reporting use{a} | 60.1% | 31.5% | 68.1% | 31.3% | W ilcoxon Rank Sum | 0.46 |
| N | % | N | % | |||
| Reduced days using relative to baseline | 16 | 84.2% | 13 | 68.4% | Fisher's Exact | 0.45 |
| Reduced amount using per using day relative to baseline | 14 | 73.7% | 16 | 84.2% | Fisher's Exact | 0.69 |
| Urine Drug Screens | ||||||
| N | % | N | % | |||
| % of Samples Positive, as observed{c} | 125 | 85.6% | 105 | 83.3% | N/A | N/A |
| GEE Model-based estimate, % (SE) | 87.3% | 6.5% | 85.3% | 6.3% | GEE Wald Test | 0.83 |
| Clinician Global Impression | Mean | SD | Mean | SD | ||
| Improvement Rating, LOCF | 2.84 | 1.12 | 2.95 | 1.08 | W ilcoxon Rank Sum | 0.65 |
| Change in severity rating (last obs. - baseline) | −1.28 | 1.23 | −1.33 | 1.46 | W ilcoxon Rank Sum | 1.00 |
| Mean | SD | Mean | SD | |||
| Marijuana Craving Questionnaire, change from baseline | −13.39 | 13.28 | −17.05 | 15.97 | W ilcoxon Rank Sum | 0.56 |
| ADHD | Mean | SD | Mean | SD | ||
| WRAADS, change from baseline & longitudinal | −15.05 | 10.96 | −11.05 | 7.59 | W ilcoxon Rank Sum | 0.23 |
| CAARS-SELF, change from baseline & longitudinal | −12.65 | 7.60 | −10.16 | 7.73 | W ilcoxon Rank Sum | 0.34 |
| CGI Improvement, LOCF | 2.63 | 0.68 | 3.26 | 0.93 | W ilcoxon Rank Sum | 0.02 |
| CGI Change in severity rating (last obs. - baseline) | −1.22 | 0.94 | −0.89 | 1.28 | W ilcoxon Rank Sum | 0.21 |
For measures modeled longitudinally, the beta coefficient for the treatment by time interaction is reported.
Estimated mean is for the amount using per using day over the last seven days of self-reported use (last observation carried forward) from an ANCOVA model that adjusted for amount using per using day during the 90 days prior to the study.
A total of 272 UDS were collected, n=146 for the atomoxetine group, n=126
In unadjusted longitudinal analyses (only the main effect of study week or `time’), no temporal trends were detected in self-reported use when visit week was treated as a factor (degrees of freedom=11, p=0.23) or a continuous variable (df=1, p=0.14). Further, when the main effect of the randomized treatment assignment and its interaction with the continuous time were added to the model, neither parameter estimate reached statistical significance (p=0.73, p=0.19; respectively). Similar findings were observed using the semi-quantitative UDS results. In particular, the main effect of treatment, when modeled as the sole predictor in the GLM/GEE framework, was not significant (p=.83). In the more traditional longitudinal setting, the treatment assignment by study week assignment was also non significant (p=0.77; the log-odds, or logit, link was used for this analysis). Change in absolute rating and improvement rating on the CGI for substance abuse did not differ between groups (p=1.0, p=0.65; respectively).
ADHD Efficacy Results
Participants randomized to atomoxetine had greater improvement on the CGI-I scale than those participants treated with placebo (Atomoxetine M(SD): 2.6 (0.7), Placebo: 3.3 (0.9); p=0.022) (Table 2). The change in absolute severity ratings from baseline, while not statistically different, supported these findings (Atomoxetine: −1.22 (0.9), Placebo: −0.89 (1.3); p=0.21). The longitudinal linear trajectories of the WRAADS and CAARS-SELF did not differ between groups (p=0.80, p=0.73; respectively). There was some indication of separation between the two treatment groups early in the study for the WRAADS; however, this finding may not be clinically significant due to the delayed onset of atomoxetine’s therapeutic effect. Specifically, the atomoxetine participants had a greater rate of decline in ADHD symptoms measured by the WRAADS over the first 4 weeks (p=0.023), but the treatment by time interaction was not significant after week 4 (p=0.38).
In post hoc analysis, growth curve models of the WRAADS and CAARS-Self measures over the course of the study were examined using a random intercept and slope mixed model. For these exploratory models, only the main effect of study week was included (i.e., treatment and the treatment by time interaction were not included). Both models suggested that ADHD symptoms decreased significantly over the course of the treatment (p<0.001 for both dependent variables); however as was reported previously, no significant treatment effects on the rates of decline were found in these measures over the course of the entire treatment period.
Exploratory Analyses
In exploratory analyses, the rates of heavy use while on study were examined. Heavy use was defined post hoc as use beyond the 75th percentile of baseline use, and for this study, six standard marijuana units was determined to constitute heavy use. The median percentage of study days reported with heavy use for the atomoxetine group was 0% of study days (interquartile range-IQR: 0% to 1.2%) and 2.1% (IQR: 0% to 6.0%) for the placebo group (p=0.46). Further, the percentages of subjects with no heavy use days while on study were 68% (13/19) and 47% (9/19) for atomoxetine and placebo treated participants, respectively (p=0.32).
An exploratory analysis evaluating the association of improvement in WRAADS score with a reduction in marijuana use was conducted. Participants were categorized as either reducing the percent days in which they had a positive cannabinoid UDS during the study from the 90 days prior to entering (n=29) or not reducing their percent days positive (n=9). Participants who reduced use had a mean reduction (SD) in WRAADS score of 14.5 (9.9) points compared to a reduction of 8.6 (6.6) points observed in participants who did not reduce their marijuana use during the study. Although these findings are suggestive of a relationship between a reduction in marijuana use and improvement in ADHD symptomatology, the findings were not statistically significant (p=0.11).
Safety and Tolerability
The majority of adverse events were mild to moderate in severity. Two subjects (one subject receiving placebo and one subject receiving atomoxetine) were removed from the study for medical reasons unrelated to study participation. All (19 of 19) of the atomoxetine-treated participants experienced at least one adverse event compared to 84% of placebo-treated participants (Table 3). Due to the small sample size, all of the confidence intervals for the relative risk were wide and none supported the conclusion that the adverse event profile differed between the two treatment groups; however, there were several known adverse effects of atomoxetine that were unobserved in the placebo group rendering the calculation of relative risk as undetermined. Notably, 26% of atomoxetine-treated participants experienced sexual dysfunction compared to 0% in the placebo arm. Gastrointestinal side effects have been commonly reported in patients receiving atomoxetine; this study suggests that the risk of such side effects were 2.25 times higher for subjects treated with atomoxetine relative to placebo (95% CI for Relative Risk: 0.83 to 6.06; p>0.05).
TABLE 3.
Adverse event summary for the Safety Sample
| Atomoxetine (n=19) | Placebo (n=19) | Relative Risk {a} | ||||
|---|---|---|---|---|---|---|
| Adverse Event | Freq. | Percent | Freq. | Percent | Estimate | 95% CI |
| At least one AE | 19 | 100% | 16 | 84% | 1.19 | [0.98, 1.44] |
| Anxiety/depression | 3 | 16% | 2 | 11% | 1.50 | [0.28, 7.99] |
| Dizziness/lightheaded | 6 | 32% | 3 | 16% | 2.00 | [0.58, 6.85] |
| Drowsiness | 3 | 16% | 3 | 16% | 1.00 | [0.23, 4.34] |
| Dry mouth | 3 | 16% | 3 | 16% | 1.00 | [0.23, 4.34] |
| Gastrointestinal | 9 | 47% | 4 | 21% | 2.25 | [0.83, 6.06] |
| Headache | 7 | 37% | 5 | 26% | 1.40 | [0.54, 3.64] |
| Hot/cold flashes | 3 | 16% | 0 | 0% | -- | -- |
| Increased Urination | 0 | 0% | 2 | 11% | -- | -- |
| Insomnia | 0 | 0% | 4 | 21% | -- | -- |
| Irritability | 2 | 11% | 1 | 5% | 2.00 | [0.20, 20.24] |
| Musculoskeletal | 6 | 32% | 5 | 26% | 1.20 | [0.44, 3.27] |
| Respiratory | 0 | 0% | 1 | 5% | -- | -- |
| Sexual dysfunction | 5 | 26% | 0 | 0% | -- | -- |
| Sinus/allergies/flu-like symptoms | 7 | 37% | 9 | 47% | 0.78 | [0.37, 1.66] |
| Other | 5 | 26% | 3 | 16% | 1.67 | [0.46, 6.01] |
Relative risk, which is the ratio of the Atomoxetine percentage to the Placebo percentage, is not reported if one of the two percentages is zero.
DISCUSSION
The results of this study suggest atomoxetine may have some utility in improving ADHD symptomatology in marijuana-dependent adults. In particular, significant differences were observed in the clinician rated level of ADHD improvement with atomoxetine treatment. However, no statistically significant between group differences in self-reported ADHD symptoms or overall WRAADS scores were observed, and there was no statistically significant improvement in marijuana use outcomes.
Consistent with the caution by Kraemer et al,41 the effect sizes of this initial investigation across multiple potential efficacy measures were highly variable. In particular, the ADHD effect sizes based on the pooled standard deviation ranged from as high as 0.80 for the CGI Improvement rating to as low as 0.31 for the change in CGI severity ratings over the course of the study. The median ADHD effect size was 0.39, which is similar to the effect size reported in studies of atomoxetine in adults with ADHD without comorbid substance use disorders (0.35 and 0.40).42 Thus, the improvements in ADHD symptomatology seen in this sample of marijuana-dependent adults are comparable to improvements in ADHD symptomatology among non-substance dependent adults; however, this study was not designed to detect an effect size of this magnitude.
The lack of improvement in several of the ADHD rating scales may be reflective of the effects of marijuana on attention and cognitive processing. Laboratory experiments where controlled doses of marijuana were administered have shown acute effects of marijuana on attention processes,43 free recall, and other memory functions.44 Several studies have also examined neuropsychological functioning in groups with varying levels of marijuana use after some period of abstinence. Pope and Yurgelun-Todd45 reported that after one day of abstinence from marijuana, participants with a history of heavy marijuana use showed deficits in memory and mental flexibility when compared with infrequent marijuana smokers. A follow-up study found that individuals with heavy marijuana use exhibited cognitive deficits at least seven days following use; however, by day 28 of abstinence, there were no significant differences on test performance among groups of current heavy users, former heavy users, and a control group.46 These studies suggest that the neuropsychological impairments associated with marijuana use may be more reflective of residual rather than long-term effects. In the current investigation, the majority of the subjects did not achieve abstinence from marijuana; hence, it is possible that the therapeutic benefit of atomoxetine on attention was overshadowed by the acute effects of marijuana use.
There was no improvement in either self-reported marijuana use or urine drug screen outcomes among participants receiving atomoxetine compared to those receiving placebo, with an effect size based on the observed Week 12 self reported use of <0.1. A lack of improvement in marijuana use measures is congruent with a recent open-label study of atomoxetine in marijuana dependent adults without ADHD.47 Further, a recent open-label trial of atomoxetine in cocaine-dependent individuals with ADHD also failed to find improvements in substance use outcomes.48 Wilens and colleagues reported a reduction in heavy drinking days in atomoxetine-treated individuals with comorbid alcohol use disorders; however, no differences in mean drinks per day, drinks per drinking day, or proportion of drinking days was observed.20 To note, in the study by Wilens and colleagues, a brief period of abstinence was required of participants, which may suggest that atomoxetine is more effective when initiated when individuals are not actively using. Thus, evidence across multiple studies and multiple drugs of abuse suggests that atomoxetine, while beneficial to non-drug outcomes, has minimal effect on drug use behavior.
This study has several limitations. The sample size was small, and a significant number of participants did not complete the 12-week trial period. Poor retention has also been reported in an open-label study of atomoxetine in cocaine-dependent individuals with ADHD.48 A limitation of the study’s efficacy findings stems from the modified ITT definition. The modification came from including only those subjects with at least one post-randomization assessment instead of including all randomized subjects. Screening was completed over two baseline study visits; however, randomization was completed prior to the second baseline visit. As Figure 1 illustrates, 32 randomized participants did not receive study drug at the second baseline visit. An additional eight participants did return to the second baseline visit to receive study medication but failed to return to any subsequent visits. Under ITT, these participants should be included in the analysis, but these participants did not contribute to the preliminary description of safety and efficacy in this population. As such, these participants were excluded from this preliminary investigation (i.e., their data were not imputed for the primary analysis).
The analysis of this data also included numerous comparisons of the group effect. In a confirmatory setting, these analyses are widely known to inflate the Type I error rate; however, in the current pilot study setting, these exploratory analyses were important to better understand whether atomoxetine should be further tested in this comorbid population. While most of the results were not statistically significant (i.e., the conclusions reached would not change with the addition of multiple testing correction factors), caution is still warranted when interpreting the findings. The overall impression is that while atomoxetine treatment provided some improvement in ADHD symptoms, its efficacy in treating marijuana dependence was far less pronounced. As marijuana dependence and ADHD commonly co-occur and present treatment challenges, additional research on this topic is needed.
Acknowledgment
This work was supported by grants R21DA18221 (Dr. McRae-Clark), K23DA15440 (Dr. McRae-Clark), K23DA020482 (Dr. Carpenter), and K24DA00435 (Dr. Brady) from the National Institute on Drug Abuse, Bethesda, MD. Medication for this project was provided by Eli Lilly & Co.
Dr. Brady has received previous grant support and honoraria from Eli Lilly & Co. Dr. McRae-Clark has received previous grant support from Shire Pharmaceuticals.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. NSDUH Series H-34, DHHS Publication No. SMA 08-4343. [Google Scholar]
- 2.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exper Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- 3.Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys: educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50:565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 4.Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone S. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- 5.Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: comorbidities and adaptive impairments. Compr Psychiatry. 1996;37:393–401. doi: 10.1016/s0010-440x(96)90022-x. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 3rd ed. New York, NY: Guilford Press; 2006. ADHD in Adults; pp. 248–296. [Google Scholar]
- 7.Kessler RC, Adler LA, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric co-morbidity, cognition and psychosocial functioning in adults with attention deficit disorder. Am J Psychiatry. 1993;150:1792–1798. doi: 10.1176/ajp.150.12.1792. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann N Y Acad Sci. 2001;931:251–270. doi: 10.1111/j.1749-6632.2001.tb05783.x. [DOI] [PubMed] [Google Scholar]
- 10.Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- 11.Wilens TE, Biederman J, Mick E. Does ADHD affect the course of substance abuse? Findings from a sample of adults with and without ADHD. Am J Addict. 1998;7:156–163. [PubMed] [Google Scholar]
- 12.Schubiner H. Substance abuse in patients with attention-deficit hyperactivity disorder: Therapeutic implications. CNS Drugs. 2005;19:643–655. doi: 10.2165/00023210-200519080-00001. [DOI] [PubMed] [Google Scholar]
- 13.Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64 Suppl 11:3–8. [PubMed] [Google Scholar]
- 14.Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry. 2007;68 Suppl 11:15–22. [PubMed] [Google Scholar]
- 15.Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for Attention-Deficit/Hyperactivity Disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008;162:916–921. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schubiner H, Saules KK, Arfken CL, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- 17.Levin FR, Evans SE, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: Double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155:693–695. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- 19.Stahl SM. Selective NRIs are smart drugs: exploiting regionally selective actions on both dopamine and norepinephrine to enhance cognition. J Clin Psychiatry. 2003;64:110–111. doi: 10.4088/jcp.v64n0201. [DOI] [PubMed] [Google Scholar]
- 20.Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. 2008;96:145–154. doi: 10.1016/j.drugalcdep.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Applegate B, Lahey BB, Hart EL, et al. Validity of the age-of-onset criterion for ADHD: a report from the DSM-IV field trials. J Am Acad Child Adolesc Psychiatry. 1997;36:1211–1221. [PubMed] [Google Scholar]
- 22.Faraone SV, Biederman J, Doyle A, Murray K, Petty C, Adamson JJ, Seidman L. Neuropsychological studies of late onset and subthreshold diagnoses of adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60:1081–1087. doi: 10.1016/j.biopsych.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163:1720–1729. doi: 10.1176/ajp.2006.163.10.1720. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders, Patient Edition (SCID-I/P, Version 2.0) New York, NY: Biometrics Research Department; 1994. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS) North Tonawanda, NY: Multi-Health Systems; 1999. [Google Scholar]
- 27.Sobell MB, Sobell LC. Behavioral treatment of alcohol problems. New York, NY: Plenum Press; 1978. [Google Scholar]
- 28.Heishman SJ, Singleton EG, Ligouri A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- 29.Guy W. ECDEU assessment manual for psychopharmacology, revised 1976. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch; 1976. pp. 217–222.pp. 313–331. [Google Scholar]
- 30.Hamilton M. The assessment of anxiety states by rating. Br J Psychiatry. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wender PH, Reimherr FW, Wood D, Ward M. A controlled study of methylphenidate in the treatment of attention-deficit disorder in adults. Am J Psychiatry. 1985;142:547–552. doi: 10.1176/ajp.142.5.547. [DOI] [PubMed] [Google Scholar]
- 33.Miller WR, Benefield GS, Tonigan JS. Enhancing motivation for change in problem drinking: A controlled comparison of two therapist styles. J Consult Clin Psychol. 1993;61:455–461. doi: 10.1037//0022-006x.61.3.455. [DOI] [PubMed] [Google Scholar]
- 34.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- 35.McRae AL, Brady KT, Carter RE. Buspirone for treatment of marijuana dependence: a pilot study. Am J Addict. 2006;15:404. doi: 10.1080/10550490600860635. [DOI] [PubMed] [Google Scholar]
- 36.Meinert CL. Clinical Trials Design, Conduct, and Analysis. New York: Oxford University Press; 1986. [Google Scholar]
- 37.Mendenhall W, Sinich T. A second course in statistics: regression analysis. 5th ed. Upper Saddle River, NJ: Prentice Hall, Inc.; 1996. [Google Scholar]
- 38.Hedden SL, Woolson RF, Carter RE, Palesch Y, Upadhyaya HP, Malcolm RJ. The impact of loss to follow-up on hypothesis tests of the treatment effect for several statistical methods in substance abuse clinical trials. J Substance Abuse Treat. 2009;37:54–63. doi: 10.1016/j.jsat.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- 40.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 41.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- 42.Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–120. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- 43.Solowij N, Mitchie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry. 1995;37:731–739. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- 44.Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory and performance. Pharmacol Biochem Behav. 1996;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- 45.Pope HG, Yurgelun-Todd D. The residual effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- 46.Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 47.Tirado CF, Goldman M, Lynch K, Kampman KM, Obrien CP. Atomoxetine for treatment of marijuana dependence: A report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend. 2008;94:254–257. doi: 10.1016/j.drugalcdep.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levin FR, Mariani JJ, Secora A, et al. Atomoxetine treatment for cocaine abuse and adult attention deficit/hyperactivity disorder (ADHD): A preliminary open trial. J Dual Diagn. 2009;5:41–56. doi: 10.1080/15504260802628767. [DOI] [PMC free article] [PubMed] [Google Scholar]