Abstract
ArgBP2 (Arg/Abl-Binding Protein) is expressed at high levels in the heart and is localized in the Z-bands of mature myofibrils. ArgBP2 is a member of a small family of proteins that also includes vinexin and CAP (c-Cbl-associated protein), all characterized by having one sorbin homology (SOHO) domain and three C-terminal SH3 domains. Antibodies directed against ArgBP2 also react with the Z-bodies of myofibril precursors: premyofibrils and nascent myofibrils. Expression in cardiomyocytes of plasmids encoding Yellow Fluorescent Protein (YFP) fused to either full length ArgBP2, the SOHO, mid-ArgBP or the SH3 domains of ArgBP2 led to Z-band targeting of the fusion proteins, whereas an N-terminal fragment lacking these domains did not target to Z-bands. Although ArgBP2 is not found in skeletal muscle cells, YFP- ArgBP2 did target to Z-bodies and Z-bands in cultured myotubes. GST-ArgBP2-SH3 bound actin, alpha-actinin and vinculin proteins in blot overlays, cosedimentation assays, and EM negative staining techniques. Over-expression of ArgBP2 and ArgBP2-SH3 domains, but not YFP alone, led to loss of myofibrils in cardiomyocytes. Fluorescence Recovery After Photobleaching was used to measure the rapid dynamics of both the full length and some truncated versions of ArgBP2. Our results indicate that ArgBP2 may play an important role in the assembly and maintenance of myofibrils in cardiomyocytes.
Keywords: Myofibrillogenesis, sarcomere, premyofibril, nascent myofibril, mature myofibril, dynamics, actin, alpha-actinin, ArgBP, vinculin
Introduction
ArgBP2 (Arg/Abl-binding protein, variant 1) is a 70kD protein that is expressed in non-muscle cells where it localizes in actin stress fibers and in the heart where it localizes in Z-bands (Wang et al., 1997; Roenty et al., 2005). There is a transcript variant of ArgBP2 (variant 2) in neural tissue, nArgBP2, which is approximately 200 kDa, but has 90% identity with the 620 amino acids that make up the ArgBP2 sequence (Kawabe et al., 1999). Based on cDNA sequencing data (Genbank, NCBI), at least eight isoforms of human ArgBP2 ranging from 492 to 1100 amino acids (52 to 117 kDa actual molecular weights) have been derived. A single gene SOR BS2 located on 4q35.1 codes these isoforms, but multiple variants result from alternative splicing.
The 75% amino acid sequence identity (82% positive) of ArgBP2 between chicken and human suggests conservation of important functions. First discovered in a yeast two-hybrid screen as a binding partner of c-Abl (Abelson non-receptor tyrosine kinase) and Arg (Abelson related protein) (Wang et al., 1997), ArgBP2 proteins are now known to be members of a small family of proteins that also includes vinexin and CAP (c-Cbl-associated protein)/ponsin (Kioka et al., 2002), all characterized by having one sorbin homology (SOHO) domain and three C-terminal SH3 domains (Figure 1). CAP/ponsin is localized in cell-cell and cell-matrix junctions in non-muscle cells (Mandai et al., 1999), in the intercalated discs and costameres in cardiomyocytes (Gehmlich et al., 2007; Zhang et al., 2007), and associates with the insulin receptor (Baumann et al., 2000). Vinexin is also localized in cell junctions and colocalizes with vinculin in non-muscle cells (Kioka et al., 2002). Both of these family members (CAP/ponsin and vinexin) are present in skeletal muscle, but their localization in myofibrils has not been reported. ArgBP2 is expressed at high levels in the heart but is absent in skeletal muscle (Wang et al., 1997).
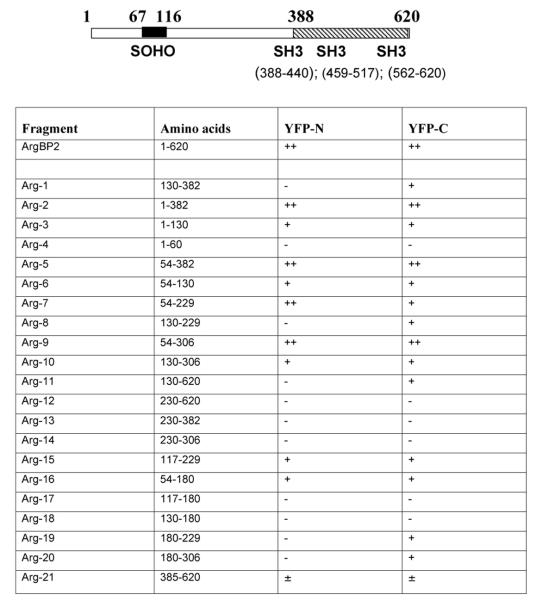
Figure 1.
Schematic diagram of the arrangement of the domains of ArgBP2. This Figure lists ArgBP2 truncations that were expressed in cardiomyocytes as YFP-N or YFP-C fusions. Z-body and Z-band localizations were scored as clear localization (++) e.g., Fig. 8 a, b; localization accompanied by diffuse unincorporated protein (+) e.g. Fig. 8 c; weak localization with high level of unincorporated protein (±); and no Z-body or Z-band localization (−)
The SH3 domains in vinexin and CAP bind Sos, a guanine exchange factor for Ras and Rac, and the SH3 domains of vinexin, CAP and nArgBP2 each bind vinculin (Kioka et al., 1999). ArgBP2 interacts with protein in the postsynaptic density fraction in brain, and the second and third SH3 domains bind vinculin and l-afadin, an actin-binding protein (Kawabe et al., 1999).
The SOHO domains of ArgBP2 (Haglund et al., 2004), CAP and vinexin α (Kimura et al., 2001) each bind flotillin, an integral membrane protein found in lipid rafts that are sites of vesicle trafficking and signal transduction (Bickel et al., 1997). The binding of CAP to flotillin is required for insulin signaling (Baumann et al., 2000). In skeletal muscle, flotillin was seen in a striated pattern suggesting localization in T-tubules or cisternae of sarcoplasmic reticulum (Voldstedlund et al., 2001). Levels of flotillin in skeletal muscle were found to be dramatically higher in hypertensive rats (James et al., 2001).
In non-muscle cells ArgBP2, like CAP, binds Cbl. When ArgBP2 is complexed with Cbl and Abl, it enhances the Cbl-induced ubiquitination and degradation of Abl (Soubeyran et al., 2003). Thus, in this scenario, ArgBP2 negatively regulates Abl kinase in neuronal PC12 cells. ArgBP2 can also simultaneously bind Cbl and Pyk2, a proline-rich tyrosine kinase, resulting in targeting of the complex to lipid rafts and stimulating lamellipodia formation possibly through a series of steps that promote binding of Cbl to effectors of cytoskeletal remodeling such as Crk and PI3K (Haglund et al., 2004). Pyk2 is present in rat cardiomyocytes at levels that are much higher in actively contracting myocytes than quiescent cells, and are reduced when cells are treated with nifedipine to block Ca transients (Bayer et al., 2001). Whether any of these associations with ArgBP2 occurs in cardiomyocytes is not known.
In this report, we demonstrate that antibodies directed against ArgBP2 react in cardiomyocytes with the Z-bodies in premyofibrils and nascent myofibrils. Expression in cardiomyocytes of plasmids encoding YFP fused to either full length ArgBP2, the SOHO, mid-ArgBP2 or the SH3 domains of ArgBP2 led to Z-band targeting of the fusion proteins, whereas an N-terminal fragment lacking these domains did not target to Z-bands. FRAP analysis exhibited rapid exchange of full length ArgBP2 and two ArgBP2 truncations in the Z-bodies and Z-bands during myofibrillogenesis. We show that purified ArgBP2 as well as the midregion and the SH3 domains of ArgBP2 bind F-actin in addition to alpha-actinin and vinculin. Over-expression of ArgBP2 and ArgBP2-SH3 domains, but not YFP alone, led to disassembly of myofibrils in cardiomyocytes. Binding of ArgBP2 to a complex of proteins containing alpha-actinin, vinculin, spectrin, paxillin, Pyk2, flotillin was demonstrated in pull-down experiments from heart extracts. In contrast to these former proteins, zyxin was not detected in the ArgBP2 pull-down experiments. Extrapolating from our results on cardiomyocytes and from the published work on ArgBP2 in non-muscle cells, we hypothesize that ArgBP2 may be involved in three major processes in cardiomyocytes: the assembly of myofibrils, the attachment of myofibrils to the cell surfaces; and a role in coordinating signaling at the Z-band level in cardiomyocytes.
Materials and Methods
Constructs
The c-DNA for ArgBP2 was isolated from a human cardiac library (Stratagene) and its sequence confirmed with the GenBank submission (Wang et al., 1997) that has been recently updated (GenBank GI: 41352708). The c-DNA was cloned into the pEYFP vector (Clontech) and used to transfect cells isolated from eight-day embryonic chick and quail hearts as well as quail myotubes. Figures 1 and 2 list the truncated constructs of YFP-ArgBP2 that were expressed in cardiomyocytes to determine the Z-band targeting domains.
Figure 2.
Localization in Z-bands of Argbp2 fragments with internal deletions
Preparation of HA and FLAG tagged ArgBP2 in a bacculovirus expression system
Full length human ArgBP2 with duel 6-His and FLAG-Tag was expressed in bacculovirus system. The Arg-BP2 cDNA was cloned in frame with sequences for 6-His and FLAG tags at N-terminal in a pFastBacHTA vector (Invitrogen) and expressed in Spodoptera frugiperda (Sf9) insect cells (Wistar Institute Protein Core Facility, Philadelphia). The 6-His tag was utilized for purification while FLAG tag used in binding studies. The transfected Sf9cells were cultured for 72 hr, harvested, and the resulting pellets were stored at −80°C. The frozen pellets were thawed, resuspended in buffer containing 1% Triton, 0.4 M NaCl, 50 mM Tris buffer, pH 8.0 and a mixture of protease inhibitors (Roche), and sonicated on ice. After 30 min. incubation at 4°C, the cell extract was centrifuged at 13,000-x g for 20 min, and the resulting supernatant (Triton fraction) was filtered through 20 um filter. The residual pellets were again sonicated on ice with 10-second pulses in 8M urea, 50 mM Tris buffer, pH 8.0. After 1 h incubation at 4°C, the suspension was cleared by centrifugation at 13,000 x g at 4°C for 20 min (Urea fraction). Both fractions were separately used to purify 6-His-ArgBP2 on Ni-resin according to manufacturer recommendations. Briefly, cleared fractions were incubated with Ni-resin for 1 h or overnight (material from 250 ml of cell culture was incubated with 1 ml of resin equilibrated in the buffer of choice, 1% Triton or 8 M urea). To eliminate non-specific binding, the resins were extensively washed with corresponding buffer containing 0.4 M NaCl and 5 mM imidazole. 6-His-FLAG ArgBP2 was eluted with buffer containing 0.5 M imidazole.
Preparation of GST tagged fragments of ArgBP2
The C-terminal SH3 fragment, ArgBP2SH3 (Arg-21, Figure 1), and the middle fragment between the SOHO and SH3 domains, midArgBP2 (Arg15, Figure 1), were expressed in bacteria as fusions with GST (Amersham Biosciences), and purified according to manufacturer recommendations. Briefly, expression constructs were transfected into E. coli BL-21 cells (Agilent Technologies). Cultures of the transfected bacterial cells were induced with IPTG (1mM final concentration) and grown at 37° C for overnight with vigorous shaking. After the cells were harvested by centrifugation at 4° C, they were lysed and the fusion protein was purified by affinity chromatography using Glutathione Sepharose 4B column following manufacturer's protocol (Amersham Biosciences). The purified protein was desalted by passing through PD-10 column, and protein concentration was determined by Bio-RAD protein assay reagent.
Antibody production for ArgBP2
Purified ArgBP2 fractions were subjected to sodium dodecyl sulfate 7.5% polyacryamide gel (SDS-PAGE) electrophoresis (Figure 3A). After electrophoresis, the bands visualized by Coomassie staining were excised from the gel and used for the subcutaneous injections into two New Zeeland rabbits. Rabbit polyclonal anti-ArgBP2 was produced by Covance. Pre-immune serum was collected prior injections of ArgBP2 antigen. Immunoglobulin fraction (IgG) was purified from a whole serum using Protein A Sepharose according to the standard manufacturer's protocol. Western blot analysis was used to test purified antibodies and whole antiserum against purified recombinant ArgBP2 protein and mouse heart tissue extracts (Figure 3B). Adult hearts were extracted using two different solutions HTE buffer (2% Triton, 150 mM NaCl, 50 mM HEPES, 2 mM EDTA in the presence of protease inhibitors, 5 mM NaF, 1 mM orthovanadate); or RIPA buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% DOC, 0.1% SDS; RIPA buffer derives its initials from the original assay for which it was developed, i.e., Radio-ImmunoPrecipitation Assay).
Figure 3.
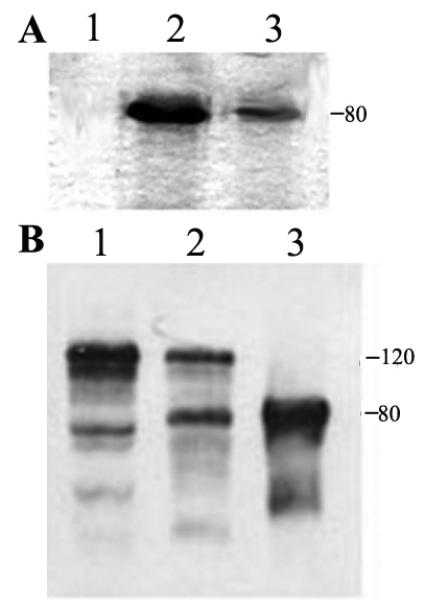
(A) ArgBP2 containing Triton X-100 extract from Sf9 cell was used directly to purify ArgBP2-Flag protein using Ni resins as described in the Materials and Methods. Lane 1 was the void volume. Lanes 2 and 3 show the eluted single band of ArgBP2. This single 80 kDa band was excised from the gel and used for immunization to produce polyclonal antiserum.
(B). Differently extracted samples of adult hearts were subjected to SDS-PAGE followed by the Western blot analysis. Lane 1- HTE- soluble fraction (Heart Triton X-100 Extract); Lane 2 – RIPA-soluble fraction; Lane 3, recombinant ArgBP2 protein was used as a standard. Note the presence of two isoforms of ArgBP2 in adult hearts (80 and 120 kDa).
Western blotting of lysate from chicken cardiac muscle with our ArgBP2 antibody reacted with a band of the appropriate molecular weight for ArgBP2 (80 kDa), an alternatively spliced isoform of ArgBp2 (120 kDa) and minor lower molecular bands (Figure 3), presumably breakdown products. To further confirm the specificity of the antibody, ArgBP2 was partially purified from chicken cardiac lysates by adapting the procedure used for CAP/ponsin purification from rat liver (Mandai et al., 1999). The two major reactive bands were excised for MALDI analysis and identified as ArgBP2.
Binding of full length ArgBP2 to muscle proteins
Immunoblot. Actin, alpha-actinin and vinculin were prepared as previously described (Sanger et al., 1984, 1986, 2000; Chowrashi et al., 2002). Purified gizzard alpha-actinin was also purchased from Sigma. To analyze binding properties of full-length ArgBp2-Flag protein, purified vinculin and alpha-actinin were allowed to interact with ArgBP2 anti-Flag resin for two hours with constant agitation. Extensive washing with binding buffer eliminated non-specific binding. ArgBP2-Flag/anti-Flag resin complex was incubated with alpha-actinin and vinculin for 12-18 h at 4° with constant rotation. After washing of unbound material, proteins were eluted with 0.1 M Glycine, pH 3.5. In control experiments, alpha-actinin and vinculin were incubated directly with anti-Flag resin without ArgBP2 protein. Eluted fractions were concentrated on Microcon concentrators (Amicon), and bound proteins were determined by Western blot analyses using specific antibodies.
Western blots. Interaction of ArgBP2 protein with cardiac muscle proteins from Triton X-100 mouse heart extract (HTE) was assessed by pull-down experiments using purified full length recombinant ArgBP2. For preparation of HTE, pulverized frozen heart tissue from C57 BL/6 mice was solubilized with Buffer A (2% Triton/150 mM NaCl/50 mM HEPES/ 2 mM EDTA in the presence of protease inhibitors, 5 mM NaF, 1 mM orthovanadate in Buffer A) and centrifuged at 100,000 × g for 1 h. The extraction was repeated twice; supernatants were combined and diluted to the final concentration of 1% Triton. For the binding assay, 50-80 mg of ArgBP2-Flag was incubated with 1.0 ml of HTE for overnight at 4°. To isolate ArgBP2-Flag-muscle proteins complex, 250 microL of anti-Flag M2 agarose beads (Sigma) were added, and the suspension was incubated for at least 2 h at 4° at constant rotation. Beads were extensively washed with binding buffer, and protein complex was eluted with 0.1 M glycine (pH 3.5). Eluted samples were concentrated and subjected to Western blot analyses. The following panel of antibodies was used for immunoblotting: anti-alpha-actinin (sarcomeric), anti-vinculin, anti-Flag (Sigma), anti-paxillin, anti-flotillin, anti-Cbl, anti-Pyk2, anti-zyxin (Transduction Laboratories), anti-a2-spectrin (Chemicon). Nitrocellulose replicas were blocked with 5% nonfat milk in TBS/0.5% Tween 20 (TTBS) and immunoblotted with appropriate antibodies. Horseradish peroxidase-conjuated (HRP) anti-mouse (dilution 1:3000) or anti-rabbit (dilution 1:6000 – 10 000) IgG were used as secondary antibodies. After extensive wash with TTBS and TBS, the immunoreactivity was detected using ECL kit (Amersham).
Binding of ArgBP2 fragments to actin
Negative staining. The C-terminal SH3 fragment, ArgBP2SH3 (Arg-21, Figure 1), and the middle fragment between the SOHO and SH3 domains, midArgBP2 (Arg15, Figure 1), were expressed as fusions with GST and purified as described above. Binding to F-actin was determined by exposing freshly prepared actin filaments (polymerized in 0.1 M KCl and 0.002 M MgCl2 from G-actin (1 mg/ml)) to purified GST-ArgBP2SH3 (0.5 mg/ml) or GST alone (0.5 mg/ml). The actin filaments were centrifuged at high speed (100,000 g for one hour) to pellet the actin filaments and any bound proteins. After the pellets were rinsed with PBS several times to remove any unbound proteins, they were resuspended in PBS and transferred in a drop to formvar-coated grids, rinsed with water, and then negatively stained with 1 % uranyl acetate. Negatively stained images of pure F-actin, F-actin plus GST protein, and F-actin bound to GST-ArgBP2SH3 were examined with a Philips 201 Electron Microscope.
In vitro binding assays. In vitro binding was assayed using the method described by Urbancikova and Hitchcock-DeGregori (1994). The reaction mixture in a total volume of 50 microl contained the following: 5 mM F-actin, 200 mM NaCl, 10 mM Tris-HCl buffer (pH 7.5), 2 mM MgCl2, 0.5 mM DTT and 5 microg of purified GST protein (GenScript USA Inc) or GST-Arg-15 affinity purified protein. In another control reaction mixture only GST-Arg-15 protein (no F-actin) was added (lanes 7 and 8). The reaction mixtures were incubated for 60 minutes in room temperature and then centrifuged for 30 minutes in an ultracentrifuge at 4°C. After centrifugation, supernatant from each tube was taken out carefully and the sediments were washed with 50 microl of reaction buffer only. The pellets and the supernatants (20 microl from each tube) were subjected to SDS-PAGE (6-12% gradient gel from Invitrogen) that was stained subsequently with Amido Black. The experiment was repeated at least 3 times. The ~45 kDa bands of GST-Arg-15 in lanes 6 and 8 for the supernatant fractions were quantified by using NIH Image-J software.
Cell culture and transfection
Cardiomyocytes were isolated from the excised hearts of 9-day old chick and quail embryos and plated on 35 mm MatTek dishes at concentrations of about 105 cells per dish according to procedures described in Dabiri et al. (1999). Myoblasts were isolated from quail embryos as described by Dabiri et al. (1999). The cells were transfected with full length or truncated GFP-ArgBP2 plasmid DNA using FuGene6 after 2 days of cell culture (Dabiri et al., 1999; Ayoob et al., 2000, 2001; Wang et al., 2007).
Immunostaining of fixed cells
Cultured cardiomyocytes for immunostaining study were grown on dishes with glass cover slips 105 cells per dish for 3 days and then fixed with buffered 3% paraformaldehyde, permeabilized, and stained with rhodamine-labeled antibodies against sarcomeric α-actinin (Clone EA-53, Sigma, St. Louis, MO, USA), ArgBP2 (generous gift of Dr. Gary Kruh, Institute for Cancer Research, Fox Chase, PA) and FITC-phalloidin (Molecular Probes, OR, USA) (Dabiri et al., 1999). A new antibody, directed against purified full length ArgBP2 was generated for us by Covance. This full-length ArgBP2 antibody as described above recognizes ArgBP2 in Western blots as well as in immunofluorescence experiments. An IgM titin antibody (also called zeugmatin; Turnacioglu et al., 1997) was obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). Fluorescently labeled secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Cultured myotubes were fixed after four days and prepared for immunostaining as described above for cardiomyocytes.
Microscopy of living cells
The cells were observed with a Nikon inverted wide field microscope with a 100X phase-contrast objective lens. The technique of Fluorescence Recovery After Photobleaching or FRAP was performed to measure the dynamics of ArgBP2 in the Z-bands of cardiac and skeletal muscle cells. The details of this procedure and analysis are described in detail in Wang et al. (2005a, 2007, 2008). The regular FRAP experiments were conducted on a Zeiss LSM 510 or a Leica SP5 confocal microscope using time points separated by 30 second intervals over about 10 to 15 minute collection period. In one set of rapid FRAP experiments for unbound YFP versus YFP-Arg-15 comparisons, the measurements were taken at 0.5 second intervals for 30 seconds. Unless otherwise noted, all FRAP data were obtained with YFP-N fusions. The transfected cells were maintained at 37 °C, 5% CO2 with humidity as previously reported (Dabiri et al, 1997). Images of living and stained cells were acquired with a Hamamatsu C47492-95 CCD camera and processed with MetaMorph image processing software and Adobe Photoshop as previously reported (Siebrands et al., 2004; Golson et al., 2004).
Results
ArgBP2 is localized in Z-bodies, Z-bands and costameres in cultured avian muscle cells
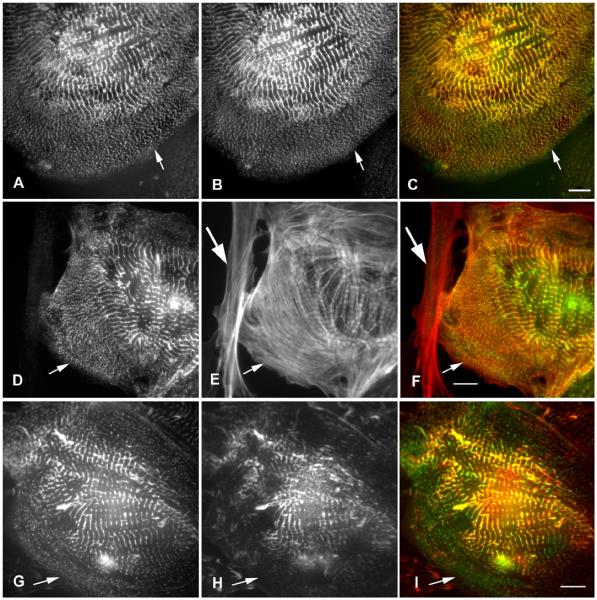
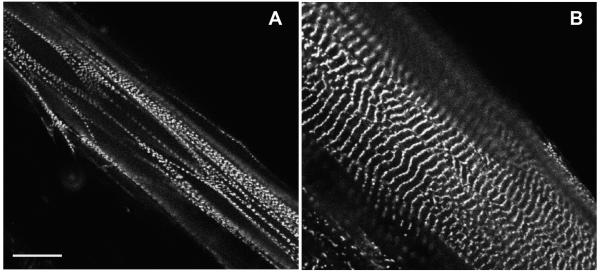
The localization of ArgBP2 in cardiac Z-bands (Wang et al., 1997) is also seen in Z-bodies, the precursors of Z-bands (Figure 4 A, D, G). Spreading cardiomyocytes stained with anti-ArgBP2 antibody and co-stained with antibodies to three other Z-band proteins. There is exact colocalization of ArgBP2 and alpha-actinin (Figure 4B) in the Z-bodies of premyofibrils and nascent myofibrils, as well as in the Z-bands of the mature myofibrils (Figure 4 A-C). In contrast to this ArgBP2 reactivity in Z-bodies in premyofibrils and nascent myofibrils, and dense bodies in stress fibers in some non-muscle cells (Wang et al., 1997), there was no reactivity in dense bodies in stress fibers in cardiac fibroblasts in the culture derived from embryonic chick hearts (Figure 4 D-F). Vinculin is a marker for the attachment of Z-bands to the cell surfaces, i.e., costameres (Prado et al., 1983; Danowski et al., 1992; Ervasti, 2003). Although vinculin binds ArgBP2 in non-muscle cells (Kawabe et al., 1999; Cestra et al., 2005), vinculin co-localized with ArgBP2 in only the subset of Z-bands near the cell membrane, and was not present with ArgBP2 in the Z-bodies of the premyofibrils and nascent myofibrils (Figure 4 G-I).
Figure 4.
(A-I) Co-staining of cardiomyocytes with anti-ArgBP2 antibody (A, D, G) and anti-sarcomeric alpha-actinin antibody (B); phalloidin (E); or anti-vincluin antibody (H). (A-C) ArgBP2 (A, green in C) co-localizes with alpha-actinin (B, red in C) in the Z-bodies (arrows) in the spreading lamella of a cardiomyocyte where myofibrils are known to assemble (Dabiri et al., 1997) and in Z-bands in the center of the cell. (D-F) ArgBP2 (D, green in F) localizes in the Z-bands in the middle of the phalloidin-stained I bands and in a beaded distribution along the unbanded phalloidin-stained actin fibers (E, red in F) in the spreading edge (small arrows) of the cardiomyocyte. ArgBP2 does not localize in actin fibers in the fibroblast on the left side of the image (E, F large arrow). (G-I) ArgBP2 (G, green in I) colocalizes with concentrations of vinculin (H, red in I). In addition ArgBP2 localizes in Z-bodies (arrows, H, I) and some Z-bands where vinculin is not present. Bars = 10 microns.
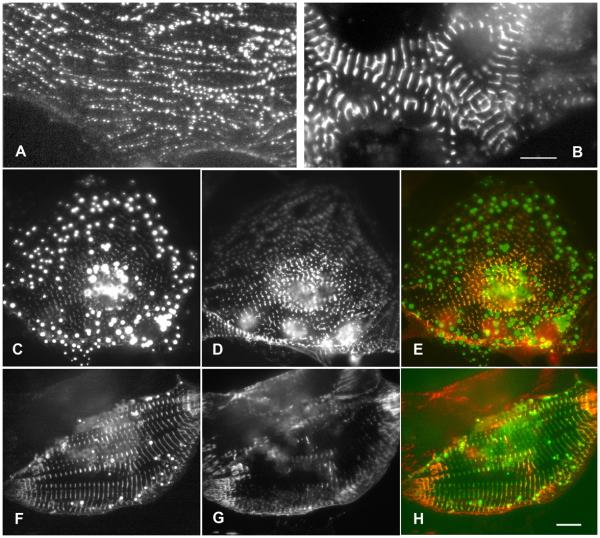
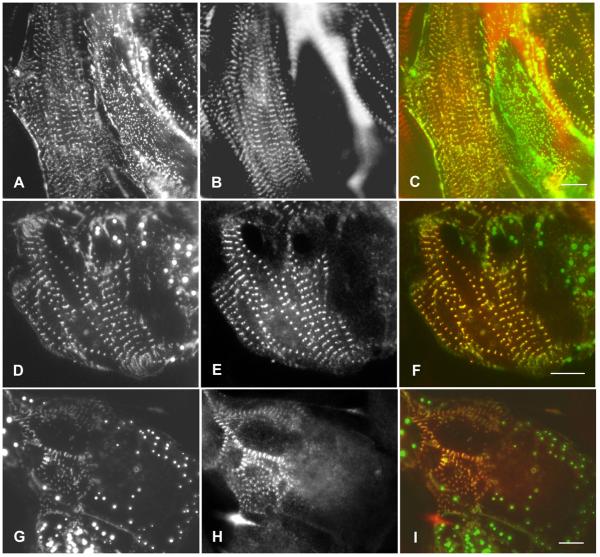
In the first day or two after transfections with the full length ArgBP2-YFP construct, ArgBP2-YFP localized in the Z-bodies of forming myofibrils (Figures 5A, 6A) and in the Z-bands of mature myofibrils (Figures 5B, 6A) in cultured cardiomyocytes. The position of YFP on the N-terminus versus the C-terminus of the full-length protein had no effect on the localization of ArgBP2 (Figure 1). All of the cardiomyocytes illustrated in Figures 5 and 6 exhibit the localization of YFP-N constructs. Transfected cardiomyocytes stained with a titin antibody revealed that titin staining was absent from the YFP-ArgBP2 containing Z-bodies but present in the Z-bands of the mature myofibrils (Figure 6 A-F). The absence of titin form the ArgBP2 containing Z-bodies was expected from our previous published studies, where we had reported that titin antibodies did not stain the alpha-actinin containing Z-bodies of the premyofibrils (Rhee et al., 1994). Starting at two days posttransfections, the presence of round aggregates of fluorescent ArgBP2 fusion proteins that did not react with antibodies to any Z-band proteins, e.g., alpha-actinin (Figure 5 C-E), vinculin (Figure 5 F-H), or titin (Figure 6 D-I) were detected, and often associated with cells with decreased numbers of myofibrils (Figure 5 C-E, Figure 6 D-I). Since these aggregates were not observed in untransfected cells (Figure 4), we assume that they are due to over expression of the fusion proteins. Overexpression effects were independent of the position of YFP on the N-terminus versus the C-terminus of ArgBP2. The over expression of full length ArgBP2-YFP in cardiomyocytes lead to the presence of premyofibrils and nascent myofibrils at the edges of the cells (contrast the control untransfected cells in Figure 4 with transfected cells over expressing full length ArgBP2-YFP in Figure 5 C-E and in Figure 6 D-I.
Figure 5.
Representative images of cardiomyocytes cultured from 10-day quail embryo and transfected with ArgBP2-YFP. (A, B, C, F, and green in E, H) ArgBP2 targets to (A) Z-bodies of pre- and nascent myofibrils and (B) Z-bands of mature myofibrils. (C-H) Overexpression of ArgBP2-YFP leads to the accumulation of spherical aggregates of ArgBP2-YFP protein that do not react with antibodies to Z-band proteins as shown for alpha-actinin (D and red in E), and vinculin (G, red in H). Bars = 5 microns (A, B) and 10 microns (C-H).
Figure 6.
ArgBP2A and titin in cardiomyocytes. Cells were transfected with GFP-ArgBP2A (A, D, G) and then fixed, permeabilized, and stained with an anti-titin (zeugmatin) antibody (B, E, H). The titin antibody colocalizes with the GFP-ArgBP2A in the Z-bands (C, F, I), but the GFP-ArgBP2A containing Z-bodies of the forming myofibrils do not stain for titin (B, C). (D-I). Overexpression of the GFP-ArgBP2A leads to the loss of myofibrils. Bars = 10 microns.
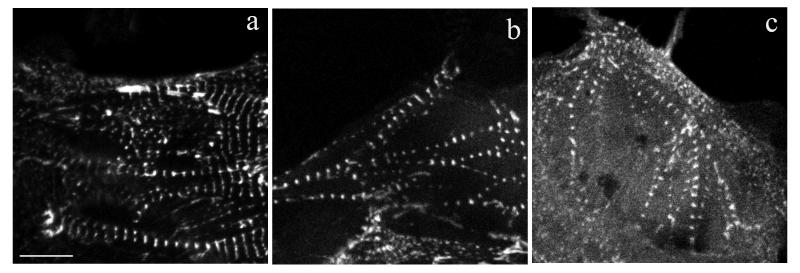
The two different anti-ArgBP2 antibodies did not stain myofibrils in myotubes cultured from embryonic chick skeletal muscle, an expected outcome since ArgBP2 is not present in skeletal muscle cells (Wang et al., 1997). When expressed in myotube cultures, however, YFP-ArgBP2 localized in both Z-bodies and in Z-bands of myotubes (Figure 7). It is of interest to note that transfected myotubes were followed for several days post-transfection, and there were no signs of round aggregates of YFP-ArgBP2 or loss of myofibrils. Transfected fibroblasts in both cardiac and in skeletal muscle cultures incorporated YFP-ArgBP2 into the dense bodies in stress fibers. Since alpha-actinin, a binding partner of ArgBP2, is in the dense bodies of stress fibers (Sanger et al., 1983), we were not surprised at this result.
Figure 7.
Skeletal muscle myotubes expressing GFP-ArgBP2 in (a) Z-body and (b) Z-band locations of the probe. Bar = 10 microns.
Targeting of ArgBP2-YFP fragments in cardiomyocytes
To determine which regions of ArgBP2 were required for targeting to Z-bands and Z-bodies, truncated sequences of ArgBP2 (Figure 1) were expressed as YFP fusions in cultured cardiomyocytes isolated from quail or chick (Figures 5, 6). Two constructs were generated for each truncation with YFP ligated to either the C- or N-terminus. Five fragments truncated just after the SOHO domain failed to target when YFP was on the N-terminus, whereas the same fragments with C-terminal YFP tags did target, e.g., Arg-1, Arg-8, Arg-11, Arg-19 and Arg-20 (Figure 1). Each of the three major regions of the protein targeted to Z-bands and Z-bodies: the N-terminus including the SOHO domain (Arg-3), the three SH3 domains at the C-terminus (Arg-21) (Figure 8), and the middle region between the SOHO and the SH3 domains (Arg-10). Targeting was best with full length ArgBP2; with a fragment that extended from the N-terminus to the start of the SH3 domain (Arg-2); and two fragments that began at the start of the SOHO domain and included 70% of the adjacent middle region (Arg-9) or entire the middle region up to the start of the SH3 domain (Arg-5) (Figure 1). Smaller fragments of ArgBP2 that included the SOHO domain and part of the middle region all targeted reliably to Z-bodies and Z-bands (Arg-3, Arg-6, Arg-7: Figure 9b, and Arg-16). The shortest fragment in the middle region that targeted was the 112 amino-acid piece that began at the end of the SOHO domain (Arg-15: Figure 6c). The N-terminal 60 amino acids (Arg-4), preceding the SOHO domain, and several fragments from the middle region (Arg-13, Arg-14, Arg-17, Arg-18) failed to target to Z-bands and Z-bodies. In cells transfected with those probes, the YFP fluorescence was diffuse, and in contrast to truncated constructs that targeted to the Z-bodies and Z-bands, expression of these non-targeting probes did not lead to the disassembly of myofibrils and the inhibition of myofibrillogenesis.
Figure 8.
(A-E) Representative images of myofibrils in cardiomyocytes transfected with the C-terminal three SH3 domains of ArgBP2 (Arg-21). (A-C) The SH3 domains (A, green in C) target to the prominent Z-bands and to Z-bodies along the right side of the cell. Evidence of some myofibril disruption is seen in the round aggregates in the YFP image in (A) and in the green balls in (C). Alpha-actinin antibody staining (B, red in merged image in C) is colocalized with the ArgBP2 fragment except in the round aggregates. (D, E) Higher levels of expression cause disruption of myofibrils and formation of more round aggregates. Alpha-actinin antibody staining colocalizes with the disassembling myofibrils, but not with the round aggregates (D). Bars = 10 microns.
Figure 9.
A comparison of the localization in living quail cardiomyocytes of the full construct of (a) full length ArgBP2; (b) Arg 7, a 175 amino acid fragment that extends from the beginning of the SOHO domain into the middle region; and (c) Arg-15, a 112 amino acid fragment that extends from the end of the SOHO domain into the middle region. Bar = 10 microns.
A construct with an internal deletion that removed only the SOHO domain targeted better than constructs with internal deletions of the middle region with or without the SOHO domain (Figure 2). Internal deletion constructs lacking the SOHO domain, mid-region or midregion plus the SOHO domain all targeted to the Z-bands equally well and resembled the targeting seen with the three SH3 domains alone (Arg-21) (Figure 8). Over expression of the Arg fragments (e.g., see Arg-21 in Figure 8 D, E) leads to the absence of premyofibrils, the first stage in myofibrillogenesis (Du et al, 2008) in the attached cardiomyocytes.
Immunoblot analyses of binding of ArgBP2-Flag to cardiac muscle proteins
We utilized sequential extraction of ArgBP2-Flag protein expressed in Sf 9 insect lysates with buffers with increased stringency to demonstrate a differential solubility and complete extraction of the protein and, more importantly, its strong ability to interact with other proteins after reconstitution. All experiments were performed in triplicates. Specific binding of ArgBP2-Flag to selected cytoskeleton proteins was demonstrated by using resin with and without bound anti-Flag antibodies (Figure 10A). We also showed that the ability to interact with other proteins was preserved in both highly insoluble fractions of ArgBP (Triton X-100 and 8M Urea extractions) after reconstitution into the binding buffer (Figure 10B).
Figure 10.

ArgBP2-Flag (full-length) binding to major cardiac proteins.
A. Cardiac muscle proteins binding to ArgBP2-Flag-M2-anti-Flag complex. Recombinant ArgBP2-Flag protein was allowed to bind to M2 mouse anti-Flag resin. HTE extract was incubated with M2 resin alone (left lane) or with the complex ArgBP2-Flag-M2-resin (right lane).
B. ArgBP2 protein purified from 8 M urea extract effectively binds to cardiac muscle proteins after reconstitution into the binding buffer.
ArgBP2-Flag was incubated with the supernatant of extracts of mouse hearts overnight at 4°, before the addition of anti-Flag M2 agarose beads. The spun-down samples were processed as described above in Materials and Methods and run on gels for analysis with different antibodies for Western blot analyses to detect the association of ArgBP2 to different sarcomeric, costameric and signaling proteins. The following panel of antibodies was used for immunoblotting: anti-sarcomeric alpha-actinin, anti-vinculin, anti-paxillin, anti-flotillin, anti-Cbl, anti-Pyk2, anti-zyxin, and anti-a2-spectrin. All of these proteins but zyxin were detected in Western blot analyses (Figure 10). The ability of ArgBp2 to bind to alpha-actinin and vinculin was confirmed by its ability to bind to purified alpha-actinin and vinculin in pull down experiments illustrated in Figure 11 A and B.
Figure 11.

(A, B) Western blots demonstrating full length ArgBP-2-Flag binding to (A) α-actinin in lane 1 and (B) vinculin in lane 2. (A, B) Note that the anti-FLAG antibodies alone do not pull down α-actinin (A, lane 2) or vinculin (B, lane 1) in the absence of ArgBP2. Lanes three in (A, B) represent the gel positions of purified (A) α-actinin and (B) vinculin. (C) Western blots with α-actinin antibody staining of the coprecipitation of purified fusion protein (lane 1) GST-midArgBP (ArG15) and (lane 2) ArgBP-SH3 (Arg-21) with α-actinin. Lane 1 demonstrates binding of ArgBP-SH3 (lane 1) and ArgBP-SH3 (lane 2) and α-actinin. Lane three indicates that free GST does not pull down α-actinin. Lane 4: purified α-actinin sample unreacted with fusion protein. (D) Similar Western blot using the same fusion proteins in (C) but testing for the binding of purified vinculin. Lane 1 demonstrates binding of ArgBP-SH3 (lane 1) and ArgBP-SH3 (lane 2) with vinculin. Lane three indicates that free GST does not pull down vinculin. Lane 4: purified vinculin sample unreacted with fusion protein.
Binding of truncated ArgBP2 proteins to α-actinin, actin and vinculin
Two of the ArgBP2 fragments (Figure 1), Arg-15 (midArgBP2) and Arg-21 (ArgBP2SH3) that localized in Z-body/Z-bands when expressed as YFP fusions in cardiomyocytes (Figures 8 and 9c), were expressed as GST fusions and tested for interactions with vinculin and α-actinin that were isolated and purified from chicken gizzards (Figure 11 C, D). Purified samples of α-actinin or vinculin were incubated separately with beads binding purified GST-Arg-15 or GST-Arg-21. The beads were collected, extracted as described in the Methods and the extract subjected to Western blot analysis using specific antibodies against either alpha-actinin or vinculin (Figure 11 C, D). Both Arg-15 and Arg 21 bound both alpha-actinin and vinculin whereas no binding of these proteins was observed with beads coupled to just GST (Figure 11 C, D). As others had previously shown for the SH3 domain of vinexin, CAP and nArgBP2 (Kawabe et al., 1999), the SH3 domain of ArgBP2 coprecipitated with vinculin. When talin was tested in similar co-precipitations, we found no evidence of interaction with ArgBP2. In addition, Arg-21 also bound to F-actin filaments in negative staining experiments in the electron microscope (Figure 12). The binding of Arg-21 to F-actin was detected by the presence of thick irregularly coated actin filaments (Figure 12 middle panel). We were disappointed to detect no regular binding patterns of Arg-21 to F-actin, similar to those reported by Huxley (1963) for the binding of proteolytic patterns of HMM or S1 to F-actin This irregular coating was not present in either the centrifuged naked actin filaments or in actin filaments centrifuged down in a solution of free GST. These experiments indicated to us that F-actin bound Arg-21, not via GST, but via the truncated part of ArgBp2 (Arg-21 in Figure 1). This interpretation was supported by an experiment to be described below that indicated that GST was not pulled down by high-speed centrifugation (Figure 13, lanes 3 and 4). The GST remained in the supernatant above the pelleted actin filaments (Figure 13 lane 4). In contrast, GST-midArgBP2 (GST-Arg-15) was shown to co-sediment with F-actin (Figure 13, lanes 5 to 8). These centrifugation experiments support the conclusion from the negative staining experiments in Figure 12 that the GST tag does not mediate the association of Arg-15 and Arg-21 with F-actin, rather it is mediated by part of the truncated ArgBP2 molecule.
Figure 12.
Negatively stained images of pure F-actin (Upper Panel) and ArgBPSH3GST bound F-actin (Middle Panel). The Lower Panel demonstrates that purified GST tag portion of ArgBPSH3GST does not bind F-actin.
Figure 13.
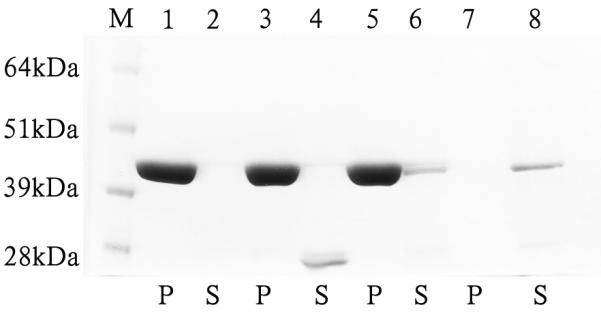
Analysis of interaction of midArgBP2-GST (Arg-15) with F-actin. Amido Black stained 6-12% gel of pellet (p) and supernatant (s) factions resulting from centrifugation of mixtures of actin filaments and midArg15-GST. Lane M: Molecular weight marker, lane 1: pellet from the tube containing actin only; lane 2: supernatant of actin alone; lane 3: pellet of actin from tube containing actin + free GST protein; lane 4: supernatant from the tube containing GST+actin. Note that no GST was detected in lane 3 indicating that GST alone does not bind F-actin. Lane 5: pellet from the reaction tube containing GST-Arg-15; lane 6: supernatant from the tube containing actin + GST-Arg-15; lane 7: pellet of the reaction tube containing GST-Arg-15 protein alone; and lane 8: supernatant from the reaction tube containing only GST-Arg-15. No staining was observed in the pellet from the reaction tube containing GST-Arg-15 suggesting that the GST-Arg-15 alone does not aggregate or precipitated that can be sedimented by ultracentrifugation. The supernatant ~45 kDa bands in lanes 6 and 8 were quantified by using NIH Image-J software and it was found that the intensity of the band in lane 6 from the reaction tube containing both F-actin and GST-Arg-15 is always lower compared to the band in lane 8 (supernatant from the reaction tube containing only GST-Arg-15 fusion protein) although the tubes were treated in an identical way. Pelleted F-actin has lowered the amount of Arg-15 in lane (6) by 33% of Arg-15 in lane 8.
Dynamics of full length and truncated YFP-constructs of ArgBP2 determined with FRAP
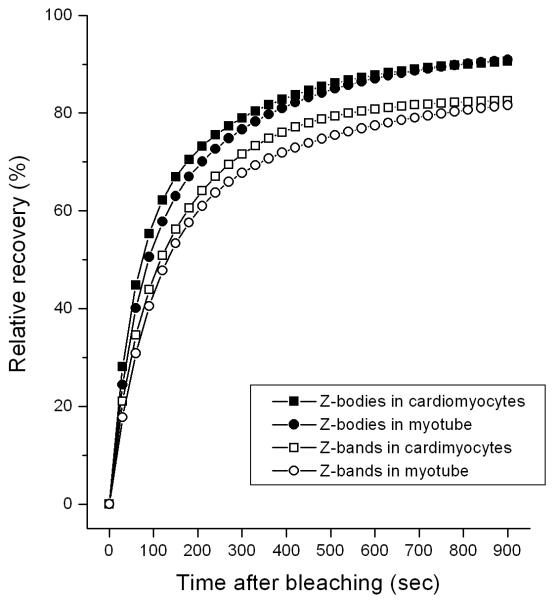
To determine how dynamic YFP-AgrBP2 was in the Z-bodies of premyofibrils and Z-bands of mature myofibrils, transfected quail cardiomyocytes and skeletal myotubes were photobleached. Photobleaching of the full-length construct of YFP-ArgBP2 revealed a rapidly exchange of the protein in both Z-bodies and Z-bands in both cardiomyocytes and in myotubes (Figures 14, 15). The dynamics of YFP-ArgBP2 is the same in the Z-bodies of both types of cells with ~90 % recovery of the prebleach fluorescence intensity 10 minutes after photobleaching (Figure 15). There is a somewhat slower exchange of YFP-ArgBP2 in Z-bands of mature myofibrils than in Z-bodies of premyofibrils, but the exchange indicates that YFP-ArgBP2 is a rapidly exchanging protein in Z-bands, i.e., ~80 % recovery of the prebleach fluorescence intensity 10 minutes after photobleaching in cardiomyocytes and skeletal muscle cells. Table 1 and Figure 15 summarize the nearly identical results in the two different types of muscle cells, cardiac and skeletal. The curves of recovery of the FRAP data are best fitted as two exponential processes (Wang et al., 2005 a; 2007) with two mobile fractions, fast and slow, for each of the Z-bodies and Z-bands in the two different types of muscles (Table 1). The fast mobile fraction decreases as Z-bodies fuse to form Z-bands, a relationship reported for other Z-band proteins (Wang et al., 2005 a). The round aggregates of YFP-ArgBP2 that formed when the protein is over expressed in cardiomyocytes (Figures 5 C - H and 6 D - I) were less dynamic in FRAP experiments (65 % exchange over 20 minutes) compared to the protein localized in Z-bodies (cardiac, 97 %; skeletal 96 %) and Z-bands (cardiac, 86 %; skeletal 82 %) (Table 1).
Figure 14.
Fluorescence Recovery After Photobleaching of YFP-ArgBP2 in a quail cardiomyocyte in tissue culture. The double arrows marked the same myofibril in the cell (a) prebleach, (b) post bleached, (c) one minute post bleached and (d) 10 minutes postbleached. The prebleached intensity is fully recovered by ten minutes indicating that YFP-ArgBP2 is a very dynamic protein in the Z-bands of mature myofibrils. Bar = 10 microns.
Figure 15.
Time course recoveries after photobleaching of YFP-ArgBP2 in Z-bodies and Z-bands in cardiac and skeletal muscle cells. Note that as the Z-bodies fuse to form Z-bands, YFP-ArgBP2 becomes somewhat less dynamic.
Table 1.
FRAP data for Argbp-2 in Z-bodies of premyofibrils and Z-bands of mature myofibrils in cardiomyocytes (C) and skeletal muscle cells (S).
| Z-bodies or Z- bands |
M (fast) (%) |
K (fast) (s−1) |
T1/2 (fast) (s) |
M (slow) (%) |
K (slow) (s−1) |
T1/2 (slow) (s) |
M (%) |
|---|---|---|---|---|---|---|---|
| Z-bodies (C) |
36 ± 6 | 0.031 ± 0.006 |
23 ± 4 | 63 ± 9 | 0.0057 ± 0.0012 |
127 ± 32 |
97 ± 12 |
| Z-bands (C) |
23 ± 12 |
0.043 ± 0.029 |
24 ± 14 | 65 ± 10 | 0.0055 ± 0.0016 |
135 ± 33 |
86 ± 6 |
| Z-bodies (S) |
33 ± 15 |
0.029 ± 0.017 |
31 ± 16 | 63 ± 10 | 0.0063 ± 0.0027 |
130 ± 64 |
96 ± 15 |
| Z-bands (S) |
23 ± 14 |
0.022 ± 0.009 |
35 ± 12 | 58 ± 5 | 0.0042 ± 0.0016 |
190 ± 95 |
82 ± 9 |
The truncated mid-ArgBP2 construct, Arg-15, (Figure 9c) had a very fast exchange rate that recovered in less than two minutes to 100% (Figure 16). This very fast exchange rate is comparable to the exchange rate of free YFP (Figure 16) that we measured at the usual 30-second intervals. It should be noted that there is no localization of YFP to any region of he sarcomere. When we collected our time points in the postbleach period at 0.5-second intervals, we detected that the YFP-Arg-15 exchanged more slowly than free YFP (Figure 17). The longer fragment, Arg-7 that included the SOHO domain together with the mid-ArgBP2 region, recovered to 100% after ten minutes (Figure 16). Analysis of the data revealed that a one step exponential process best explains the recovery of photobleached Arg-15 (Table 2), and the recovery of Arg-7 appears to involve two processes, a fast and a slow one (Table 2).
Figure 16.
A comparison of the recoveries after photobleaching of the full construct of YFP-ArgBP2, free YFP, and two truncated versions of ArgBP2, i.e., Arg-7 and Arg-15. Arg-15 lacks the SOHO present in the Arg-7 construct. Time points are separated by the usual 30-second intervals.
Figure 17.
A comparison of the recoveries after photobleaching at 0.5-second intervals over a 30 second period of free YFP versus Arg-15. Note that Arg-15 exchanges more slowly than free YFP during the first 15 seconds.
Table 2.
FRAP data for Argbp-2 fragments in Z-bands of mature myofibrils in cardiomyocytes
| Argbp-2 fragments |
M (fast) (%) |
K (fast) (s−1) |
T1/2(fast) (s) |
M (slow) (%) |
K (slow) (s−1) |
T1/2(slow) (s) |
M (%) |
|---|---|---|---|---|---|---|---|
| Argbp-2 full |
23 ± 12 |
0.043 ± 0.029 |
24 ±14 | 65 ±10 | 0.0055 ± 0.0016 |
135 ± 33 |
86 ±6 |
| Arg-7 | 37 ± 11 |
0.041 ± 0.008 |
17±3 | 67 ±7 | 0.0051 ± 0.0014 |
143 ± 42 |
103 ± 16 |
| Arg-15 | 106 ± 10 |
0.058 ± 0.011 |
12±3 | - | - | - | 106 ± 10 |
Discussion
Myofibrils not only supply power for the beating heart, but also serve as a scaffold for the spatial distribution of the proteins that modulate and integrate force production and its transmission. The Z-band, a key component of myofibrils (Frank et al., 2006; Lange et al., 2006; Pyle and Solaro, 2004; Sanger and Sanger, 2008, 2010), is itself a scaffold that links the sarcomeric contractile units in series by anchoring the actin and titin filaments of adjacent sarcomeres (Figure 18). In the early stages of Z-band formation during myofibrillogenesis, Z-bodies, the Z-band precursors, associate with the cell membrane before the organization of T-tubules and sarcoplasmic reticulum (Sanger et al., 2004). The proteins that mediate membrane attachment of Z-bodies have not yet been fully identified, although FAK (focal adhesion kinase) and alpha5beta1 integrin appear to play important roles in this process (Quach and Rando, 2006). Costameric proteins like talin and vinculin are late-appearing proteins in Z-band formation in cardiomyocytes (Sanger et al., 2005), and organized distributions of integrin that enable α-actinin linkages to the membrane also arise later during myofibrillogenesis (Bozyczko et al., 1989). We have demonstrated that ArgBP2 binds the Z-body and Z-band proteins actin and alpha-actinin, two of the early appearing proteins in myofibrillogenesis in cardiomyocytes (Rhee et al., 1994; Sanger et al., 2001b; Du et al., 2003, 2008). Our experiments indicate that human cardiac ArgBP2 binds both the chicken striated and smooth muscle isoforms of alpha-actinin.
Figure 18.

Diagram of the Z-band region of a cardiac muscle illustrating the relationships of the thin actin filaments, titin molecules, telethonin, and α-actinin. Through defined binding domains, α-actinin cross links actin filaments and binds the thin filament protein, nebulette, as well as titin filaments. Our hypothesis is that the Z-band protein, ArgBP2, links the myofibril to the cardiomyocyte membrane (T-tubule and the SR = sarcoplasmic reticulum) and to bind molecules of the signal transduction machinery that respond to extracellular stimuli. Drawing is not to scale.
Skeletal muscle cells do not express ArgBP2 in their cells (Wang et al., 1997). It is quite striking that introduction of YFP-ArgBP2 into myotubes not only yields Z-body and Z-band localizations, but also that FRAP experiments yield recovery results that are almost identical to those obtained in cardiomyocytes (Figure 15, Table 1). The Z-bands of cardiac and skeletal myofibrils share many proteins (Clark et al., 2002; Sanger and Sanger, 2008; Sanger et al., 2009). Thus ArgBP2 would bind to many of its normal binding partners in skeletal muscle cells. Gehmlich et al. (2007) have reported the presence of ponsin, a homologue of ArgBP2, in cell lines of skeletal muscle cells. Future work should indicate if ponsin in skeletal muscle cells could contribute to actin dynamics particularly at the Z-bodies of premyofibrils where the barbed end capping protein, CapZ, does not appear to be colocalized (Wang et al., 2005b).
Z-bands also anchor the ends of myofibrils in specialized junctions in the heart, termed intercalated discs, and they link sarcomeres laterally to the cell membrane through costameric proteins (Danowski et al., 1992; Borg et al., 2000). We have demonstrated in this report that ArgBP2 is also present in the costameres of cardiac muscle cells, and that it binds to the costameric protein vinculin present in the Z-bands of cardiomyocytes (Pardo et al., 1983; Danowski et al., 1992). Z-bands are also the attachment sites for the intermediate filaments, and, often, the membranous components of the sarcoplasmic reticulum and transverse tubules (Figure 18) (Sanger et al., 2004). The multifunctional nature of the Z-band is reflected in the variety of proteins that co-localize with this structure: channels, signaling molecules, enzymes, cytoskeletal filament complex that interacts with the cell membrane, and sarcomeric filaments essential for contraction (Clark et al., 2002; Frank et al., 2006; Lange et al., 2006; Sanger and Sanger, 2008). The Z-band of myofibrils is an area of the sarcomere where new proteins and new properties of existing proteins are being discovered (Boateng and Goldspink, 2008; Stout et al., 2008; Sanger and Sanger, 2008).
The fast growing (barbed) ends of actin filaments are concentrated in the Z-bands, suggesting that actin filament nucleation and growth occurs here, although surprisingly this is not established. Pointed end polymerization at the tips of the actin filaments in the A-bands is also possible (Littlefield and Fowler, 1998). The Abl/Arg kinases, which bind ArgBP2, have been implicated in actin filament assembly in various motile events in non-muscle cells (Woodring et al., 2003). Through binding the Abl/Arg kinases that have binding sites for both G- (globular) and F-actin (filamentous), ArgBP2 also could coordinate actin polymerization at the Z-bodies and Z-bands of assembling cardiac myofibrils. The results in this report also indicate that ArgBP2 can itself directly bind F-actin. Future work should demonstrate whether it plays a direct role in the polymerization of actin, or simply plays an important role as a scaffold protein that concentrates the actual molecules involved in actin polymerization.
The most prominent Z-band protein, alpha-actinin (95 kD), forms anti-parallel homodimers that cross-link actin filaments in the myofibrils, and serve as a protein scaffold for a variety of molecules including the enzyme phosphorylase; lipid; actin and integrin; the adhesion proteins: vinculin and zyxin, nebulette, as well as the amino terminal of the giant protein, titin, which extends from mid A-band to Z-band. Recently, several previously unknown Z-band proteins have been identified that bind to α-actinin, including myotilin, cyphers/ZASP/oracle, calsarcins/FATZ/myozenin, myopalladin, and muscle LIM protein (MLP) (Chowrashi et al., 2002; Lange et al., 2006; Frank et al., 2006; Sanger and Sanger, 2008). These α-actinin binding proteins, in turn, target to the Z-band other proteins such as the kinase, PKC; the phosphatase, calcineurin; the transcription factor, NFATc; and telethonin which binds titin and FATZ (see reviews in Clark et al., 2002; Faulkner et al., 2001; Sanger and Sanger, 2008). Roenty et al., (2005) reported that ArgBP2 binds the N-terminus of alpha-actinin, and the N-terminal region of the Z-band protein palladin using yeast two-hybrid analyses. The N-terminus of alpha-actinin, a smaller region of this molecule, is also responsible for its actin-binding properties (Mimura and Asano, 1987). Our data supports the work of Roenty et al. (2005) that ArgBP2 can be added to the list of α-actinin-binding proteins, and based on its role in non-muscle cells, and in our binding experiments described in this report, should play an important role in signaling that takes place at the Z-bodies and Z-bands of cardiomyocytes.
Our data demonstrates that three regions of ArgBP2 target to Z-bodies and Z-bands (Figure 1). Two of those regions of ArgBP2 (mid ArgBP2 and the C-terminus containing the three SH3 domains) bind alpha-actinin and when over expressed in cardiomyocytes they act as dominant negative agents, disassembling Z-bands. The detailed analysis of the different truncated regions of ArgBP2 (Figures 1 and 2) indicates that the addition of neighboring amino acids to the SOHO domain increases the quality of its Z-band targeting. The SOHO domain, (Figure 1) known to bind flotillin (Haglund et al., 2004), targets to the Z-band, and is a candidate for helping to mediate the linking of transverse tubules and sarcolemmic membranes to the Z-bands of cardiac muscle cells. Other regions of ArgBP2 (Figure 1), which do not localize to the Z-bands, may in the future be found to bind other proteins. ArgBP2, a 70 kD scaffolding protein, is at the crossroads of mediating the interactions of structural cross-linking proteins, polymerizing actin filaments, surface membranes and signaling molecules (Figure 18). Although ArgBP2 is in the Z-bodies of premyofibrils and nascent myofibrils in cardiomyocytes, vinculin is only found in a subset of Z-bands of mature myofibrils near the cell surfaces. The binding of ArgBP2 to alpha-actinin would allow targeting to both Z-bodies and all Z-bands.
We have reported that proteins present in the Z-bodies (Wang et al., 2005 a) and thin filaments (Wang et al., 2007, 2008) of the premyofibrils become less dynamic in the Z-bands and thin filaments of mature myofibrils as the Z-bodies of the premyofibrils fuse to form the Z-bands of the mature myofibrils. Our FRAP experiments on YFP-ArgBP2 indicate that it exchanges very rapidly in both Z-bodies and Z-bands. Nevertheless, there is about a 10 % decrease in the dynamics of ArgBP2 in the Z-bands of mature myofibrils. The implication is that ArgBP2 becomes more tightly bound in the Z-bands presumably to a different organization of its binding partners already present in the Z-bodies or more likely to increase binding directly and /or indirectly as other proteins add to the forming Z-bands (Wang et al., 2005 a; Sanger et al., 2005). The analysis of the curves of recovery for ArgBP2 (Table 1) indicates there are two types of processes that are taking place during the recovery phase after photobleaching. The use of truncated ArgBP2 fusion proteins permitted us to obtain an initial test of what domains may be important for the exchange of the full construct of ArgBP2. The photobleaching of Arg-7 and Arg-15 (Figure 16) indicates that the SOHO domain may play a role in the slow exchange rate of ArgBP2, and that the midArg section of ArgBP2, i.e., Arg15, is responsible for the fast exchange phase. However, it is clear that ArgBP2 binds a number of proteins in and associated with the Z-bodies and Z-bands. The transfections of cells with the truncated construct, Arg-b (Figure 2) that lacks the SOHO domain stills localizes to the Z-band. The three SH3 domains may play a role in this Z-band targeting since a truncated construct containing just these SH3 domains (Arg 21 in Figure 1) will also target to the Z-bands (Figure 8). The use of these truncated fusion proteins to the Z-bands also lead to the disassembly of the myofibrils and the absence of premyofibrils at the edges of transfected cardiomyocytes expressing higher levels of truncated ArgBp fragments (e.g., Arg 21 illustrated in Figure 8 D, E). The premyofibrils are the first step in the process of myofibrillogenesis (Rhee et al., 1994; Dabiri et al., 1997; Du et al., 2003, 2008). These results indicate the importance of other regions of the molecule for both myofibrillogenesis and maintenance of the existing myofibrils.
Arg 15, adjacent to the SOHO domain in the mid region (aa 117-229) (Figures 1, 9c), coprecipitated with F-actin and α-actinin (Figures 12, 13). Although midArgBP2 (Arg-15) can bind to actin in this assay, it does not localize along the length of the actin filaments in the I-bands when it is expressed as a GFP fusion protein in cardiomyocytes. These constructs did bind to the Z-bodies of premyofibrils and Z-bands of mature myofibrils (Figures 1, 8 and 9). This could be due to the presence of actin-binding proteins like tropomyosin that bind to the actin filaments in the I-bands. We have previously demonstrated that fluorescently labeled alpha-actinin will bind to newly polymerized naked actin in isolated myofibrils, and this uniform binding of alpha-actinin was blocked in all regions with the exception of the Z-band by the prior addition of tropomyosin (Sanger et al., 1984). All of these results suggest that ArgBP2, like alpha-actinin and Cap Z, may bind the barbed ends of actin filaments in the Z-bands.
Arg-7 (Figure 1) consists of amino acids 54 to 229; this region contains the SOHO domain and half of the region before the start of the three SH3 domains. Cardiomyocytes transfected with Arg-7 reveal Z-band localization that is almost indistinguishable from full-length transfected cardiomyocytes (Figure 9 a versus b). Photobleaching of cells expressing Arg-7 reveal 100 % recovery of Z-band prebleach intensities by ten minutes postbleach (Table 2, Figure 16). This Arg-7 construct reveals a more dynamic character than the full length YFP-ArgBP2 in the Z-bands of cardiomyocytes (Table 2, Figure 16). The other tested construct, Arg-15 is shorter than Arg-7, i.e., amino acids 117 to 229; it lacks the SOHO domain of Arg-7 (Figure 1). While Arg-15 localizes to the Z-bands of mature myofibrils, there is a high background due to unincorporated Arg-15 (Figure 9 a versus c). Photobleaching experiments determined that Arg-15 exchanges as rapidly as Arg-7 by the end of ten minutes (Figure 16; Table 2). There is only one mobile fraction for Arg 15, and it is a fast mobile fraction, comparable in its exchange rates to the fast mobile fractions of Arg-7 and the full-length construct of YFP-ArgBP2 (Figure 16; Table 2). Thus the SOHO domain present in Arg-7 and YFP-ArgBP2, but absent in Arg15 may be involved in the slow exchange reactions.
Summary
Our experiments indicate that ArgBP2 appears early in the first step of myofibrillogenesis, i.e. present in the Z-bodies of premyofibrils. This Z-band protein binds a number of proteins involved in Z-band assembly, attachment of the Z-bands to the cell surfaces, and signaling molecules. Overexpression of the full-length protein or truncated constructs of ArgBP2 leads to a loss of myofibrils, and the absence of premyofibrils, an indication of an inhibition of myofibrillogenesis. Future work using siRNA should indicate ArgBP2's essential role in myofibrillogenesis. We will also need to determine other binding partners of ArgBP2, and its role in signaling processes at the Z-bands of cardiac myofibrils. It will be of interest to determine why this protein is so dynamic in the Z-band, and the role of this molecule, if any, in the fusion of the Z-bodies to form and maintain the Z-bands of mature myofibrils.
Acknowledgment
This work was supported by grants from the American Heart Association, the Muscular Dystrophy Association and NIH.
Grant Sponsor: AHA, MDA, and NIH
References
- Ayoob JC, Sanger JM, Sanger JW. Visualization of the expression of Green Fluorescent Protein (GFP) linked genes. In: Tuan RS, Lo CW, editors. Methods in Molecular Biology : Developmental Biology Protocols. III. Humana Press; 2000a. pp. 153–157. [DOI] [PubMed] [Google Scholar]
- Ayoob JC, Turnacioglu KK, Mittal B, Sanger JM, Sanger JW. Targeting of cardiac titin fragments to Z-bands and dense bodies of living muscle and non-muscle cells. Cell Motil Cytoskeleton. 2000b;45:67–82. doi: 10.1002/(SICI)1097-0169(200001)45:1<67::AID-CM7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ayoob JC, Shaner NC, Sanger JW, Sanger JM. Expression of green or red fluorescent protein (GFP or DsRed) linked proteins in nonmuscle and muscle cells. Mol. Biotechnology. 2001;17:65–71. doi: 10.1385/MB:17:1:65. [DOI] [PubMed] [Google Scholar]
- Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S, Bickel PE, Pessin JE, Saltiel AR. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Ferguson AG, Lucchesi PA, Samarel AM. Pyk2 expression and phosphorylation in neonatal and adult cardiomyocytes. J Mol Cell Cardiol. 2001;33:1017–1030. doi: 10.1006/jmcc.2001.1369. [DOI] [PubMed] [Google Scholar]
- Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovas Res. 2008;77:667–675. doi: 10.1093/cvr/cvm048. [DOI] [PubMed] [Google Scholar]
- Borg TK, Goldsmith EC, Price R, Carver W, Terracio L, Samarel AM. Specialization at the Z line of cardiac myocytes. Cardiovascular Research. 2000;46:277–285. doi: 10.1016/s0008-6363(99)00433-2. [DOI] [PubMed] [Google Scholar]
- Cestra G, Toomre D, Chang S, De Camilli P. The Abl/Arg substrate ArgBP2/nArgBP2 coordinates the function of multiple regulatory mechanisms converging on the actin cytoskeleton. Proc Natl Acad Sci USA. 2005;102:1731–1736. doi: 10.1073/pnas.0409376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowrashi P, Mittal B, Sanger JM, Sanger JW. Amorphin is phosphorylase, phosphorylase is an alpha-actinin-binding protein. Cell Motil Cytoskeleton. 2002;53:125–135. doi: 10.1002/cm.10059. [DOI] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Ann. Rev. Cell Dev. Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc. Natl. Acad. Sci. USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Meth Enzymol. 1999;302:171–186. doi: 10.1016/s0076-6879(99)02017-0. [DOI] [PubMed] [Google Scholar]
- Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118:1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Sanger JM, Linask KK, Sanger JW. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol. 2003;257:382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Du A, Sanger JM, Sanger JW. Cardiac myofibrillogenesis inside intact embryonic hearts. Developmental Biology. 2008;318:236–246. doi: 10.1016/j.ydbio.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life. 2001;51:275–282. doi: 10.1080/152165401317190761. [DOI] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signaling and disease. J. Mol. Med. 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Gehmlich K, Pinotsis N, Hayess K, van der Ven PF, Milting H, El Banayosy A, Korfer R, Wilmanns M, Ehler E, Furst DO. Paxillin and ponsin interact in nascent costameres of muscle cells. J Mol Biol. 2007;369:665–682. doi: 10.1016/j.jmb.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Golson M, Sanger JM, Sanger JW. Inhibitors arrest myofibrillogenesis in skeletal muscle cells at early stages of assembly. Cell Motil Cytoskeleton. 2004;59:1–16. doi: 10.1002/cm.20017. [DOI] [PubMed] [Google Scholar]
- Haglund K, Ivanovic-Dikic I, Shimokawa N, Kruh GD, Dikic I. Recruitment of Pyk2 and Cbl to lipid rafts mediates signals for actin reorganization in growing neurites. J Cell Sci. 2004;117:2557–2568. doi: 10.1242/jcs.01148. [DOI] [PubMed] [Google Scholar]
- Hirotani S, Higuchi Y, Nishida K, Nakayama H, Yamaguchi O, Hikoso S, Takeda T, Kashiwase K, Watanabe T, Asahi M, Taniike M, Tsujimoto I, Matsumura Y, Sasaki T, Hori M, Otsu K. Ca(2+)-sensitive tyrosine kinase Pyk2/CAK beta-dependent signaling is essential for G-protein-coupled receptor agonist-induced hypertrophy. J Mol Cell Cardiol. 2004;36:799–807. doi: 10.1016/j.yjmcc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J Mol Biol. 1963;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- James DJ, Cairns F, Salt IP, Murphy GJ, Dominiczak AF, Connell JM, Gould GW. Skeletal muscle of stroke-prone spontaneously hypertensive rats exhibits reduced insulin-stimulated glucose transport and elevated levels of caveolin and flotillin. Diabetes. 2001;50:2148–2156. doi: 10.2337/diabetes.50.9.2148. [DOI] [PubMed] [Google Scholar]
- Kawabe H, Hata Y, Takeuchi M, Ide N, Mizoguchi A, Takai Y. nArgBP2, a novel neural member of ponsin/ArgBP2/vinexin family that interacts with synapse-associated protein 90/postsynaptic density-95- associated protein (SAPAP) J Biol Chem. 1999;274:30914–30918. doi: 10.1074/jbc.274.43.30914. [DOI] [PubMed] [Google Scholar]
- Kimura A, Baumann CA, Chiang SH, Saltiel AR. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc Natl Acad Sci USA. 2001;98:9098–9103. doi: 10.1073/pnas.151252898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioka N, Ueda K, Amachi T. Vinexin, CAP/ponsin, ArgBP2: a novel adaptor protein family regulating cytoskeletal organization and signal transduction. Cell Struct Funct. 2002;27:1–7. doi: 10.1247/csf.27.1. [DOI] [PubMed] [Google Scholar]
- Lange S, Ehler E, Gautel M. From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol. 2006;16:11–8. doi: 10.1016/j.tcb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Littlefield R, Fowler VM. Defining actin filament length in striated muscle: rulers and caps or dynamic stability? Annu Rev Cell Dev Biol. 1998;14:487–525. doi: 10.1146/annurev.cellbio.14.1.487. [DOI] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, Takai Y. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J Cell Biol. 1999;144:1001–1017. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny AS, Perry CN, Witt CC, Labeit S, Gregorio CC. Muscle-specific RING finger-2 (MURF-2) is important for microtubule, intermediate filament and sarcomeric M-line maintenance in striated muscle development. J. Cell Sci. 2004;117:3175–3188. doi: 10.1242/jcs.01158. [DOI] [PubMed] [Google Scholar]
- Mimura N, Asano A. Further characterization of a conserved actin-binding 27-kDa fragment of actinogelin and alpha-actinins and mapping of their binding sites on the actin molecule by chemical cross-linking. J. Biol Chem. 1987;262:4717–4723. [PubMed] [Google Scholar]
- Pardo JV, Siciliano JD, Craig SW. Vinculin is a component of an extensive network of myoflbril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983;97:1081–1088. doi: 10.1083/jcb.97.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ. Res. 2004;94:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- Quach N, Rando TA. Focal adhesion kinase is essential for costamerogenesis in cultured skeletal muscle cells. Dev Biol. 2006;293:38–52. doi: 10.1016/j.ydbio.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, Sanger JW. The premyofibrils: evidence for its role in myofibrillogenesis. Cell Motil Cyotskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Roenty M, Taivainen A, Moza M, Kruh GD, Ehler E, Carpen O. Involvement of palladin and alpha-actinin in targeting of the Abl/Arg kinase adaptor ArgBP2 to the actin cytoskeleton. Exp Cell Res. 2005;310:88–98. doi: 10.1016/j.yexcr.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM, Jockusch BM. Differences in the stress fibers between fibroblasts and epithelial cells. J Cell Biol. 1983;96:961–969. doi: 10.1083/jcb.96.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Mittal B, Sanger JM. Analysis of myofibrillar structure and assembly using fluorescent labeled contractile proteins. J Cell Biol. 1984;98:825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Mittal B, Pochapin BM, Sanger JW. Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J Cell Biol. 1986;102:2053–2066. doi: 10.1083/jcb.102.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Danowski BA, Sanger JW. Microinjection of fluorescently labeled alpha-actinin into living cells. In: Tuan RS, Lo CW, editors. Methods in Molecular Biology: Developmental Biology Protocols. III. Humana Press; 2000. pp. 449–456. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman N, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Orthop Related Res. 2002;403S:S153–S162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Mittal B, Sanger JM. Formation of myofibrils in spreading chick cardiac myocytes. Cell Motil. 1984;4:405–416. doi: 10.1002/cm.970040602. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM, Franzini-Armstrong C. Assembly of the skeletal muscle cell. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd McGraw-Hill; New York: 2004. pp. 35–65. [Google Scholar]
- Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril. J. Muscle Res. Cell Motility. 2005;26:343–354. doi: 10.1007/s10974-005-9016-7. 2005. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Fishing out proteins that bind to titin. J. Cell. Biol. 2001a;154:21–24. doi: 10.1083/jcb.200106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Myofibrillogenesis in cardiac muscle. In: Dube DK, editor. Myofibrillogenesis. Birkhauser; Boston: 2001b. pp. 3–20. [Google Scholar]
- Sanger JM, Sanger JW. The dynamic Z-bands of striated muscle cells. Science Signal. 2008;1:pe37. doi: 10.1126/scisignal.132pe37. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Wang J, Fan Y, White J, Sanger JM. Assembly and dynamics of myofibrils. Journal of Biomedicine and Biotechnology. 2010;2010:8. doi: 10.1155/2010/858606. Article ID 858606. www.hindawi.com/journals/jbb/2010/858606.html) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Wang J, Holloway JB, Du A, Sanger JM. Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell Motil Cytoskeleton. 2009;66:556–566. doi: 10.1002/cm.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebrands CC, Sanger JM, Sanger JW. Myofibrillogenesis in the presence of taxol. Cell Motil Cytoskeleton. 2004;58:39–52. doi: 10.1002/cm.10177. [DOI] [PubMed] [Google Scholar]
- Simpson DG, Decker ML, Clark WA, Decker RS. Contractile activity and cell-cell contact regulate myofibrillar organization in cultured cardiac myocytes. J Cell Biol. 1993;123:323–336. doi: 10.1083/jcb.123.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran P, Barac A, Szymkiewicz I, Dikic I. Cbl-ArgBP2 complex mediates ubiquitination and degradation of c-Abl. Biochem J. 2003;370:29–34. doi: 10.1042/BJ20021539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AL, Wang J, Sanger JM, Sanger JW. Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging. Cell Motil Cytoskeleton. 2008;65:353–367. doi: 10.1002/cm.20265. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Pyk2/CAKbeta signaling: a novel Ca(2+)-dependent pathway leading to cardiac hypertrophy. J Mol Cell Cardiol. 2004;36:791–793. doi: 10.1016/j.yjmcc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Turnacioglu KK, Mittal B, Dabiri G, Sanger JM, Sanger JW. Zeugmatin Is Part Of The Z-Band Targeting Region Of Titin. Cell Struct Funct. 1997;22:73–82. doi: 10.1247/csf.22.73. B. G. J. M. [DOI] [PubMed] [Google Scholar]
- Urbancikova M, Hitchcock-DeGregori SE. Requirement of amino-terminal modification for striated muscle alpha-tropomyosin function. J Biol Chem. 1994;269:24310–24315. [PubMed] [Google Scholar]
- Voldstedlund M, Vinten J, Tranum-Jensen J. cav-p60 expression in rat muscle tissues. Distribution of caveolar proteins. Cell Tissue Res. 2001;306:265–276. doi: 10.1007/s004410100439. [DOI] [PubMed] [Google Scholar]
- Wang B, Golemis EA, Kruh GD. ArgBP2, a multiple Src homology 3 domain-containing, Arg/Abl- interacting protein, is phosphorylated in v-Abl-transformed cells and localized in stress fibers and cardiocyte Z-disks. J Biol Chem. 1997;272:17542–17550. doi: 10.1074/jbc.272.28.17542. [DOI] [PubMed] [Google Scholar]
- Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005a;61:34–48. doi: 10.1002/cm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sanger JM, Sanger JW. Differential effects of Latrunculin-A on stress fibers and myofibrils in living cultures of skeletal muscle cells: Insights into mechanisms of myofibrillogenesis. Cell Motil Cytoskeleton. 2005b;62:35–47. doi: 10.1002/cm.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sanger JM, Kang S, Thurston H, Abbott LZ, Dube DK, Sanger JW. Ectopic expression and dynamics of TPM1α and TPM1κ in myofibrils of avian myotubes. Cell Motil Cytoskeleton. 2007;64:767–776. doi: 10.1002/cm.20221. [DOI] [PubMed] [Google Scholar]
- Wang J, Thurston H, Essandoh E, Otoo M, Han M, Rajan A, Dube S, Zajdel RW, Sanger JM, Linask KK, Dube DK, Sanger JW. Tropomyosin expression and dynamics in developing avian embryonic muscles. Cell Motil Cytoskeleton. 2008;65:379–392. doi: 10.1002/cm.20267. [DOI] [PubMed] [Google Scholar]
- Woodring PJ, Hunter T, Wang JY. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116:2613–2626. doi: 10.1242/jcs.00622. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu J, Cheng A, Deyoung SM, Chen X, Dold LH, Saltiel AR. CAP interacts with cytoskeletal proteins and regulates adhesion-mediated ERK activation and motility. EMBO J. 2006;25:5284–5293. doi: 10.1038/sj.emboj.7601406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu J, Cheng A, DeYoung SM, Saltiel AR. Identification of CAP as a costameric protein that interacts with filamin C. Mol Biol Cell. 2007;18:4731–4740. doi: 10.1091/mbc.E07-06-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]