Abstract
Practice of a motor task leads to an increase in amplitude of motor-evoked potentials (MEP) in the exercised muscle. This is termed practice-dependent plasticity, and is abolished by the NMDA antagonist dextromethorphan and the GABAA agonist lorazepam. Here, we sought to determine whether specific subtypes of GABAA circuits are responsible for this effect on MEPs by comparing the action of the non-selective agonist, lorazepam with that of the selective GABAA-alpha1 receptor agonist, zolpidem. In 7 healthy subjects, transcranial magnetic stimulation (TMS) was used to quantify changes in amplitude of motor-evoked potentials (MEP) after practice of a ballistic motor task. In addition we measured how the same drugs affected the excitability of a number of MEP amplitudes and cortical inhibitory circuits (short-interval intracortical inhibition (SICI), short-interval afferent inhibition (SAI) and long-interval intracortical inhibition (LICI)). This allowed us to explore correlations between drug effects on measures of cortical excitability and practice dependent plasticity of MEPs.
As previously reported, lorazepam increased SICI and decreased SAI, while zolpidem only decreased SAI. The new findings were that practice-dependent plasticity of MEPs was impaired by lorazepam but not zolpidem, and that this was negatively correlated with lorazepam-induced changes in SICI but not SAI. This suggests that the intracortical circuits involved in SICI (and not neurons expressing GABAA-alpha1 receptor subunits that are implicated in SAI) may be involved in controlling the amount of practice-dependent MEP plasticity.
Keywords: plasticity, motor cortex, transcranial magnetic stimulation, GABA, SICI
Introduction
Pharmacological interventions coupled with transcranial magnetic stimulation (TMS) methods have made it possible to study a number of inhibitory circuits in the human cerebral cortex and to test how they are involved in particular types of motor behaviour. The present paper focuses on the role of subtypes of the GABAA receptor in synaptic plasticity induced when subjects learn a new motor task.
Butefisch et al. (2000) initially showed that if subjects practice an isolated thumb movement in a particular direction then the amplitude of MEPs evoked in agonist muscles is larger after practice than before. Since this was blocked by NMDA receptor antagonists, it was presumed to involve LTP-like changes in the efficacy of glutamatergic synapses in motor cortex. The authors also found that the effect was blocked by pretreatment with lorazepam, a non-selective GABAA agonist. The latter was compatible with reports in the animal literature that emphasised the role of GABA in regulating motor cortical plasticity (Hess et al. 1996) as well as with other investigations of synaptic plasticity in humans (Ziemann et al. 1998a; Ziemann et al. 1998b; Ziemann et al. 2001; Pleger et al. 2003). Taken together these results suggest that LTP-like plasticity is enhanced when GABA inhibition is reduced. However, there is no information on whether specific subtypes of receptor are preferentially involved in the effect.
The present experiments examined this question by comparing the effects on practice dependent synaptic plasticity of the non-selective GABAA agonist lorazepam with the selective GABAA-alpha1 receptor agonist, zolpidem. We predicted that as both drugs are GABA agonists then both of them might potentially interfere with plasticity. However, if the GABAA-alpha1 receptor was not involved then plasticity would be reduced only by lorazepam whereas it would be unaffected by zolpidem.
In a parallel set of experiments we also asked which neural circuits might be most involved in controlling levels of synaptic plasticity. A number of inhibitory intracortical circuits have been identified using transcranial magnetic stimulation (TMS): short-interval intracortical inhibition (SICI), short-interval afferent inhibition (SAI) and long-interval intracortical inhibition (LICI) (Kujirai et al. 1993; Wassermann et al. 1996; Tokimura et al. 2000; Sailer et al. 2002). SICI is believed to involve GABAA receptor neurotransmission (Ziemann 2004; Florian et al. 2008) and LICI is believed to involve GABAB receptor neurotransmission (McDonnell et al. 2006; Florian et al. 2008). Studies with the drug zolpidem indicate that the GABAA-alpha1 receptor is associated with the pathway mediating SAI whereas the SICI pathway is not (Di Lazzaro et al. 2006; Di Lazzaro et al. 2007). We argued that if particular pathways are responsible for controlling plasticity then changes in the amount of plasticity produced by lorazepam or zolpidem would correlate with their effects on SICI, LICI or SAI.
Methods
Study Design
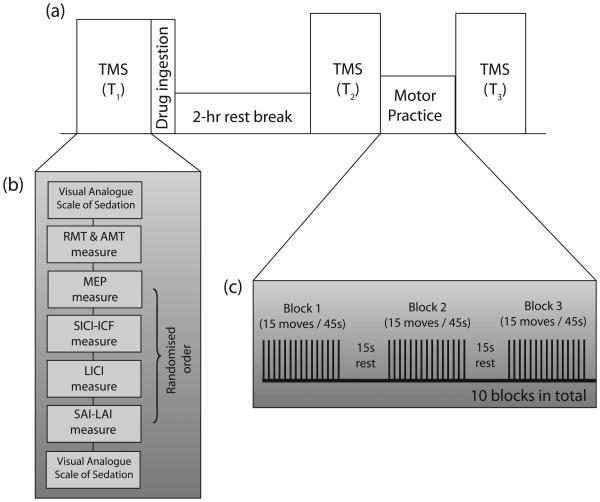
The study was structured as a double-blind randomised controlled cross-over trial with two drug arms. 2.5mg lorazepam or 10mg zolpidem was prepared by the pharmacy of the National Hospital for Neurology and Neurosurgery into unmarked containers. A placebo arm would be easily unblinded and would also not control for the effects of sedation so no placebo arm was used. Subjects had TMS measurements at three time points: T1, T2 and T3. The allocated drug was given at the end of T1. TMS measurements at T2 occur 2 hours after drug ingestion coinciding with the peak plasma concentration of lorazepam and zolpidem: lorazepam, 1.5–2.5 hours (Kyriakopoulos et al. 1978); zolpidem, 0.75–2.6 hours (Salva and Costa 1995). After T2, subjects performed the motor practice task (see below) and after completion of the task, proceeded to have TMS measurements again at T3. The study design is summarised in Fig 1.
Fig 1.
Study design (a) Timeline of a single experimental session with three timepoints: T1, T2 and T3, corresponding to baseline, during drug peak levels and after task practice; (b) multiple neurophysiological measurements were made during each timepoint; and (c) task practice consisted of 15 paced thumb abduction movements repeated over 10 blocks with frequent rest breaks.
For the subjects' second session, they received the other drug. The order was randomised and balanced (4 subjects received lorazepam in the first session). The inter-session interval was 27.7 days (range 10-50 days).
Subjects
7 healthy subjects were recruited and informed written consent was obtained (Age = 30.3 ± 3.8 years (SD), 1 female). All subjects were right-handed (not formally measured) and were not on any medication. The experiments conformed to the Declaration of Helsinki and were carried out with approval of the local Ethics Committee.
Transcranial Magnetic stimulation
Surface EMG was recorded from the left abductor pollicis brevis muscles (APB), the left first dorsal interossei (FDI) and the left flexor carpi radialis (FCR) with Ag/AgCl electrodes using a tendon-belly montage. EMG signals were amplified with Digitimer D360 amplifiers (Digitimer, Welwyn Garden City, UK) with 1000x gain and band-pass filtered (30-1000Hz for MEP) and sampled at 5kHz using a CED1401 laboratory interface and Signal software (Cambridge Electronic Design, Cambridge, UK).
Magnetic stimuli were delivered with two Magstim-200 magnetic stimulator (The Magstim Co., Whitland, UK) connected by a Y-cable. A figure-of-8 coil (diameter 80 mm) was adjusted over the optimal scalp position to evoke an MEP in the right APB with the coil handle pointed postero-laterally at a 45° angle to the sagittal plane. The resting motor threshold (RMT) was defined as the lowest intensity capable of inducing at least 5 out of 10 MEPs of >50μV peak-to-peak amplitude. The active motor threshold (AMT) was defined as the lowest intensity capable of inducing at least 5 out of 10 MEPs of >200μV peak-to-peak amplitude during an active tonic contraction of thumb APB.
The settings for the various TMS measures are as follows:
The corticospinal excitability was measured at rest at 150%RMT over 10 trials.
Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were measured with the test MEP amplitude set at ~1-mV and the conditioning stimulus set at 80%AMT or 100%AMT. The interstimulus interval was 3ms (for SICI), 8ms and 15ms (for ICF). 10 trials were recorded for each condition and the test condition.
Long-interval intracortical inhibition (LICI) was measured with the test MEP amplitude set at ~1-mV and the conditioning stimulus set at 110%RMT or 120%RMT. The interstimulus interval was 100ms (for LICI). 10 trials were recorded for each condition and the test condition.
Short-latency afferent inhibition (SAI) and long-latency afferent inhibition (LAI) was measured with the test MEP amplitude set at ~1-mV. Electrical stimulation (200μs pulse width) was delivered to the median nerve using a Digitimer DS7A Constant Current Stimulator (Digitimer, Welwyn Garden City, Herts, UK) at twice or thrice sensory threshold. The interstimulus interval was 22ms (for SAI) and 100ms (for LAI). 10 trials were recorded for each condition and the test condition.
The order of the various TMS measurements were randomised (Fig 1b). In addition to the TMS measurements, all subjects filled a visual analogue scale (VAS) of arousal before and after each time point. The visual analogue scale was a 24cm horizontal line with the left extreme marked ‘Wide awake’ and the right extreme marked ‘Fast asleep’.
Motor practice
The motor task consists of a paced ballistic thumb abduction task similar to that used in previous studies (Muellbacher et al. 2002). This motor task has been demonstrated to be dependent on the primary motor cortex, is associated with changes in MEP amplitude and movement representation (Classen et al., 1998; Muellbacher et al. 2001) and can be disrupted in the first hour after motor practice (Muellbacher et al. 2002). In this study, the subject's left forearm, hand and fingers were secured in a wooden frame leaving the thumb free to abduct in the horizontal plane. A piezoresistive monoaxial accelerometer (Model SA-105 vibrometer, Fribourg, Switzerland) was attached on the lateral aspect of the left thumb proximal phalanx with the maximal vector being thumb abduction. The accelerometer signal was sampled at 5000Hz and not filtered.
The task consisted of paced ballistic thumb abduction to a loud auditory tone played at 0.333Hz. 15 thumb abductions were performed per block for 10 blocks, with a 15-second rest break between blocks (Fig 1c). If the subjects missed any movement, they were required to perform a ‘replacement movement’. In total, subjects practiced 150 movements for 10 minutes. Subjects were motivated with verbal encouragement by a blinded investigator. Visual feedback of the acceleration from the previous trial was provided on a computer screen to the subject and the subject was told to attend to the feedback on the computer screen not to their hand as subjective judgement of thumb acceleration is often inaccurate.
Data analysis
Peak-to-peak amplitude of MEP was used as the primary measure of corticospinal recruitment. The other intracortical measures are represented as follows:
SICI and ICF were represented as peak-to-peak amplitude of conditioned stimulus normalised to test stimulus.
LICI was represented as peak-to-peak amplitude of conditioned stimulus normalised to test stimulus.
SAI and LAI was represented as peak-to-peak amplitude of conditioned stimulus normalised to test stimulus. The values for 2x and 3x sensory threshold stimulation were averaged and represented as a single value as there was no evidence of interaction.
Blinding was conducted by an independent researcher and all data analysis was conducted by a blinded researcher.
Statistical analysis
Statistical analysis was performed using SPSS 12.0 software (SPSS, SPSS Inc). To compare the effects of drug arms in the various intracortical measures, repeated-measures analysis of variation (ANOVA) was used; the various factors used in the ANOVAs are described in the text. The threshold for statistical significance (α) was set at p<0.05.
Results
Subjects correctly identified the drug taken on 6 out of 14 sessions (42.9%) which is not above chance (two-tailed Fisher's exact test, p>0.05). The RMT, AMT, sensory threshold and conditioning stimulus intensities at baseline, after drug ingestion and after practice are shown in Table 1, 2 and 3 respectively. There were no statistically significant differences in RMT, AMT, sensory threshold or test stimulus amplitude between the drug arms.
Table 1.
Subject characteristics at T1 (baseline) where all % values represent % maximum stimulator output unless stated otherwise. Values represent means ± standard deviation.
| Drug arm | RMT | AMT | Sensory threshold |
SICI/ICF | LICI | SAI | |||
|---|---|---|---|---|---|---|---|---|---|
| TS Intensity |
TS Amplitude |
TS Intensity |
TS Amplitude |
TS Intensity |
TS Amplitude |
||||
| Lorazepam | 39.9 ± 6.5 % |
31.1 ± 4.5 % |
0.21 ± 0.05 mA |
49.9 ± 8.5 % |
0.64 ± 0.35 mV |
49.9 ± 8.5 % |
0.61 ± 0.40 mV |
50.4 ± 8.7 % |
0.74 ± 0.53 mV |
| Zolpidem | 40.0 ± 5.2 % |
29.1 ± 5.7 % |
0.19 ± 0.06 mA |
50.1 ± 7.3 % |
0.85 ± 0.29 mV |
50.1 ± 6.5 % |
0.79 ± 0.27 mV |
50.1 ± 7.0 % |
0.98 ± 0.15 mV |
Table 2.
Subject characteristics at T2 (after drug ingestion) where all % values represent % maximum stimulator output unless stated otherwise. Values represent means ± standard deviation.
| Drug arm | RMT | AMT | Sensory threshold |
SICI/ICF | LICI | SAI | |||
|---|---|---|---|---|---|---|---|---|---|
| TS Intensity |
TS Amplitude |
TS Intensity |
TS Amplitude |
TS Intensity |
TS Amplitude |
||||
| Lorazepam | 40.6 ± 6.3 % |
30.4 ± 4.4 % |
0.20 ± 0.03 mA |
52.1 ± 8.6 % |
0.66 ± 0.38 mV |
53.3 ± 8.5 % |
0.76 ± 0.68 mV |
52.6 ± 8.7 % |
0.52 ± 0.29 mV |
| Zolpidem | 39.9 ± 5.6 % |
30.1 ± 5.5 % |
0.17 ± 0.04 mA |
50.5 ± 7.9 % |
0.78 ± 0.29 mV |
51.0 ± 8.4 % |
0.73 ± 0.27 mV |
50.6 ± 8.3 % |
0.71 ± 0.31 mV |
Table 3.
Subject characteristics at T3 (after training) where all % values represent % maximum stimulator output unless stated otherwise. Values represent means ± standard deviation.
| Drug arm | RMT | AMT | Sensory threshold |
SICI/ICF | LICI | SAI | |||
|---|---|---|---|---|---|---|---|---|---|
| TS Intensity |
TS Amplitude |
TS Intensity |
TS Amplitude |
TS Intensity |
TS Amplitude |
||||
| Lorazepam | 40.4 ± 7.7 % |
30.6 ± 5.3 % |
0.22 ± 0.06 mA |
50.6 ± 8.8 % |
0.85 ± 0.71 mV |
50.7 ± 8.6 % |
0.82 ± 0.70 mV |
51.4 ± 8.2 % |
0.66 ± 0.50 mV |
| Zolpidem | 40.6 ± 5.0 % |
30.0 ± 5.6 % |
0.18 ± 0.04 mA |
50.3 ± 7.4 % |
0.78 ± 0.36 mV |
49.4 ± 8.4 % |
0.72 ± 0.30 mV |
49.9 ± 7.9 % |
0.66 ± 0.33 mV |
Drug induced changes in cortical circuits
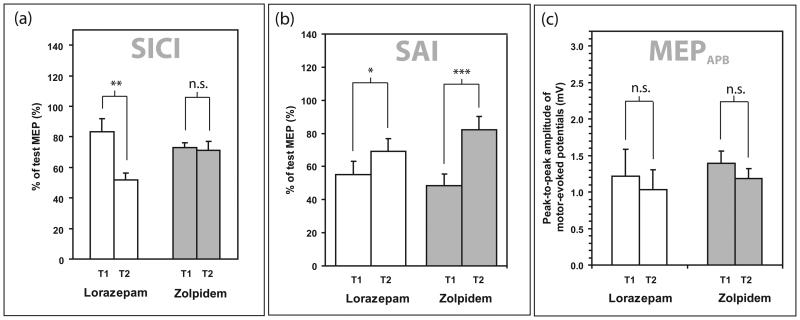
As previously reported (Di Lazzaro et al. 2006), lorazepam increased SICI, while zolpidem did not affect SICI (Fig 2). The difference in effect on SICI of lorazepam and zolpidem was statistically significant on three-factorial ANOVA (within-subject factors INTENSITY, TIME, DRUG) with an interaction of TIME x DRUG (F(1,12)=7.227, p=0.020). Post-hoc testing with Bonferroni correction showed a significant difference between T1 and T2 for the lorazepam group (p=0.002) but not in the zolpidem group (p=0.802). Post-hoc testing of the SICI at T2 showed a significant difference between the lorazepam and zolpidem arms (student's paired t-test, p=0.041).
Fig 2.
The effect of drug on the (a) short-interval intracortical inhibition (SICI); (b) short-interval afferent inhibition (SAI) (c) corticospinal excitability in the abductor pollicis brevis (APB) muscle. In all cases, the unfilled bars represent the lorazepam sessions and the filled bars represent the zolpidem sessions. Comparisons were by post-hoc student's paired t-tests with * represents p<0.05; ** represents p<0.01; *** represents p<0.001 and n.s. represents p>0.05.
The reduction of SAI by both drugs was also statistically significant as previously reported (Di Lazzaro et al. 2007). Three-factorial ANOVA (within-subject factors INTENSITY, TIME, DRUG) showed a significant interaction of TIME × DRUG (F(1,12)=7.23, p=0.02). Post-hoc testing with Bonferroni correction showed a significant difference between T1 and T2 for the lorazepam group (p=0.018) and for the zolpidem group (p<0.001), but no significant differences between the two drugs at T1 (p=0.539). Post-hoc testing of the SAI at T2 did not show any significant difference between the lorazepam and zolpidem arms (student's paired t-test, p=0.118).
The effect of the drugs on MEP amplitudes at 150%RMT was not significant (p>0.05 in both drug arms). Likewise there was no significant effect on LICI at 110% RMT and 120% RMT (supplementary figure 1). There was no significant drug-induced effect on other intracortical circuits (ICF and LAI) and data are enclosed as supplementary material.
All subjects experienced lethargy after ingestion of the drug. Their responses on the visual analogue scale for sedation (before zolpidem 3.7cm ± 3.3SD, after zolpidem 10.4cm ± 4.9SD; before lorazepam 3.4cm ± 3.3SD, after lorazepam 10.6cm ± 5.6SD, student's paired t-test p<0.01) were not significantly different after drug ingestion between drug arms (student's paired t-test p=0.91).
Performance during motor practice
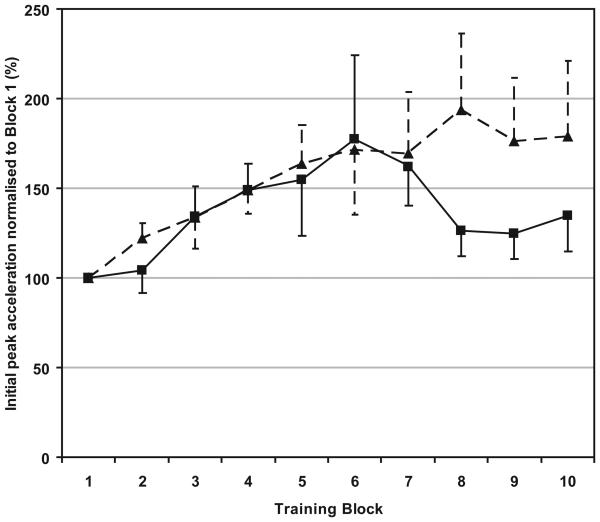
All subjects completed 10 blocks of 15 movements training. Peak acceleration during the ten minutes is shown in Fig 3. On a two-factorial ANOVA of DRUG × BLOCK, there was an effect of BLOCK (F(1,9)=2.94, p=0.004) indicating a progressively stronger initial peak acceleration of thumb abduction despite sedation. There appeared to be a fatiguing effect in the zolpidem group after block 7-10 but there were no significant differences between drug sessions (F(1,12)=0.583, p=0.460) and there was also no significant interaction of DRUG x BLOCK (F(1,9)=0.868, p=0.447).
Fig 3.
Motor performance as represented by initial peak acceleration of thumb abduction over ten 1-minute blocks of practice. In all cases, the solid line and squares represent the zolpidem sessions and the dashed line and triangles represent the lorazepam sessions.
Practice-induced changes in MEP
The MEP changes after practice are shown in Fig 4a and Fig 4b. A three-factorial repeated-measures ANOVA was performed with the within-subject factors: TIME, MUSCLE and DRUG showing an interaction of TIME × MUSCLE × DRUG (F(1,12)=5.21, p=0.042). Post-hoc testing with Bonferroni correction showed that in the trained APB muscle, MEP amplitudes enlarged in the zolpidem group (p=0.005) but not in the lorazepam group (p=0.750). Interestingly, in the untrained ADM muscle of the zolpidem group, there was no significant increase in MEP amplitude (p=0.634). There was no statistically significant effects of practice on SICI, LICI, ICF, SAI and LAI (p>0.05 in all ANOVAs) and data is available as supplementary figure 2.
Fig 4.
The effect of training on the (a) corticospinal excitability in the trained abductor pollicis brevis (APB) muscle and (b) in an untrained hand muscle, the abductor digiti minimi (ADM) muscle. In all cases, the unfilled bars represent the lorazepam sessions and the filled bars represent the zolpidem sessions. Comparisons were by post-hoc student's paired t-tests with ** represents p<0.01 and n.s. represents p>0.05.
In summary after zolpidem, MEP amplitude in the APB muscle increased after task practice, while after lorazepam, there was no increase after task practice.
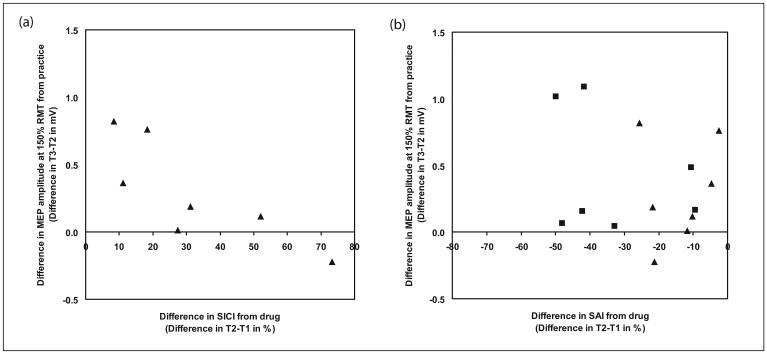
Correlation of drug-induced change in SICI and SAI with effects on practice-induced plasticity of MEPs
The change of SICI from T1 to T2 for the lorazepam sessions (i.e. lorazepam-induced SICI change) was significantly, and negatively, correlated with the change of MEP amplitude from T2 to T3 (Spearman's rank correlation, rho =−0.86, p=0.01). Thus subjects who showed the largest increases in SICI had the smallest changes in MEP amplitude after motor practice. The change of SAI from T1 to T2 (i.e. drug-induced SAI change) was not correlated with the difference of MEP amplitude from T2 to T3 (Spearman's rank correlation, rho = −0.04, p=0.94 for lorazepam sessions and Spearman's rank correlation, rho = 0.07, p=0.88 for zolpidem sessions). This correlation can be seen on Figure 5 where positive values of SICI on the right represent stronger inhibition while negative values of SAI on the left represent weaker inhibition.
Fig 5.
(a) Correlation analysis between lorazepam-induced SICI change (squares) and practice-induced MEP change; (b) correlation analysis between zolpidem-induced SAI change (squares) and lorazepam-induced SAI change (triangles) with practice-induced MEP change.
Surprisingly, there was also a tendency for drug-induced SAI change to correlate with drug-induced change in the visual analogue scale for the level of sedation (Spearman's rank correlation, rho=−0.529, p=0.052). There is no correlation between performance in the task practice and the amount of MEP increase (Spearman's rank correlation, p=0.34 for the zolpidem arm of the experiment).
Discussion
In summary, this study confirms previous reports that non-specific enhancement of GABAA transmission with lorazepam blocks practice-induced MEP plasticity (Butefisch et al. 2000; Ziemann et al. 2001) and that the selective GABAA-alpha1 receptor agonist, zolpidem reduces SAI but has no effect on SICI (Di Lazzaro et al. 2007). Our novel finding is that zolpidem does not affect practice-induced MEP plasticity. Together the data imply that the SAI circuit is not an important controller of practice-induced MEP plasticity. In contrast, the inhibition of practice-induced MEP plasticity by lorazepam correlates with the increase of SICI induced by lorazepam. This suggests that the circuits involving SICI may be important controllers of practice-induced MEP plasticity.
Drug induced changes in cortical circuits
Our results showing a lack of effect of zolpidem on SICI confirm a previous study (Di Lazzaro et al. 2006). In both studies, the dose of zolpidem was similar, and was calculated to be specific to the GABAA-alpha1 receptor (Mohler et al. 2002; Mohler et al. 2005). The conclusion is that the effect of lorazepam on SICI is not due to activation of GABAA-alpha1 receptors. As previously noted (Di Lazzaro et al. 2007), the specificity of the effect on SICI contrasts with that of the SAI circuit which was affected by both lorazepam and zolpidem.
The lack of effect on MEP amplitude by lorazepam in this study compared to previous studies (Boroojerdi et al. 2001; Kimiskidis et al. 2006) can be explained by the low intensity used to assess corticospinal excitability (~60% MSO) compared to previous studies (>65% MSO in Kimiskidis et al., 2006). Additionally, Kimiskidis et al., 2006 also found an effect on cortical silent period (a measure considered analogous to LICI) at higher intensities. Again, higher intensities were not assessed in this current study, so no conclusions can be made about these inhibitory circuits or GABAB receptors.
Practice-dependent MEP plasticity
This study confirms previous reports that this practice-dependent plasticity is blocked by the GABA agonist lorazepam (Butefisch et al. 2000). Such effects have direct parallels in the animal literature. Direct recordings in the primary motor cortex of rats after motor training suggest that motor training is associated with LTP of the excitatory synapses onto pyramidal neurons in layer II/III of the primary motor cortex (Rioult-Pedotti et al. 1998; Rioult-Pedotti et al. 2000). In addition, induction of LTP in the same synapses by direct electrical stimulation requires GABA activity to be reduced by prior administration of an antagonist, bicuculline (Hess et al. 1996). Thus, it may be that the enhancement of GABAergic activity in humans by lorazepam is the principal cause of the reduction in practice-dependent plasticity (Butefisch et al. 2000).
This conclusion is strengthened by the new data reported here showing that the subjects in whom lorazepam increased SICI were less likely to show practice-dependent plasticity of MEPs than those in whom the drug-induced effects on SICI were weak. The fact that zolpidem had little effect on SICI and little effect on plasticity may even indicate that the inhibitory connections activated during the SICI paradigm are a primary controller of practice-dependent MEP changes. In contrast the inhibitory effects produced by the SAI circuit may be much less relevant, since the changes in SAI were not correlated with changes in plasticity. It would also be consistent with the fact that administration of scopolamine, a muscarinic antagonist that reduces SAI (Di Lazzaro et al. 2000) also reduces (rather than increases) practice-dependent plasticity (Sawaki et al. 2002). Since scopolamine does not affect SICI, its influence on learning is probably via a different mechanism to the GABA-related effects discussed here.
One important drawback of this study is the inability to use a true placebo due to the behavioural effects of these two sedative drugs. Without the placebo arm, it is not possible to quantify precisely how much of the inhibition of practice-dependent plasticity is related to drowsiness and how much is related to GABA-ergic agonism as plasticity is known to be affected by attention (Stefan et al., 2002). Additionally, without a placebo arm, it is not possible to conclusively state that there is no role to play for GABAA-alpha1 receptors or SAI in practice-dependent plasticity as there might still be a smaller degree of inhibition of practice-dependent plasticity. Nonetheless, it is possible to conclude that there is a difference in degree if inhibition of practice-dependent plasticity between SICI and SAI.
It is interesting to note that although there was no significant MEP enhancement in the lorazepam arm of the experiment after practice, there was clear improving motor performance during the practice. Thus, additional factors or mechanisms beyond practice-dependent plasticity of MEPs are likely to be playing a role in the performance improvement in the lorazepam arm.
Although this study has shown an association for SICI in practice-dependent plasticity like previous studies (Ziemann et al., 1998; Ziemann et al., 2002), it is also possible that the correlation between the practice-dependent plasticity and the drug-induced effect on SICI may not necessarily be causally linked as lorazepam may be enhancing GABA-ergic signalling in other independent circuits which have yet to be identified and it is these circuits that are predominantly inhibiting practice-dependent plasticity. This remains a possibility although a less parsimonious one.
Finally, why is it that increasing GABAergic signalling in the SICI pathway affects practice-dependent plasticity to a greater degree than SAI? Any inhibition should reduce excitability in the cortex, and make it more difficult to produce LTP (Hess et al. 1996; Glazewski et al. 1998; Steele and Mauk 1999; Casasola et al. 2004). However inhibitory synapses have different effects depending on the spatial localisation of the synapses on the pyramidal cell with inhibitory synapses to the perisomatic region being more potent but less selective than inhibitory synapses to the distal dendrites (Miles et al. 1996, Segev and Burke 1998; Markram et al. 2004). The predominant expression of zolpidem-sensitive GABA receptors to the perisomatic region of pyramidal cells (Klausberger et al. 2002; Xiang et al. 2002) and zolpidem-insensitive GABA receptors to the distal dendrites (Ali and Thomson 2008) suggest that SICI and SAI may synapse at different locations on the pyramidal cell and thus modulate practice-dependent plasticity to differing degrees.
As muscarinic receptor antagonists also affect SAI (Di Lazzaro et al. 2000), SAI inhibitory interneurons may be similar to fast-spiking basket cells described in animal studies which also express GABAA-alpha1 receptor and muscarinic acetylcholine receptors and synapse onto the perisomatic region of the pyramidal cell (Nusser et al. 1996; Kawaguchi and Kubota 1997; Fritschy et al. 1998; Kubota and Kawaguchi 2000; Klausberger et al. 2002; Kawaguchi et al. 2006; Ali and Thomson 2008; Ascoli et al. 2008) and thus the perisomatic inhibition by SAI may be less relevant to practice-dependent plasticity which predominantly occurs in layer II/III synapses (Rioult-Pedotti et al. 1998; Rioult-Pedotti et al. 2000). Thus, the similarities between SAI and inhibition by fast-spiking basket cells are suggestive, but this still remains unproven.
Conclusion
In summary, we demonstrate that practice-dependent plasticity of MEPs is limited to differing degrees by different GABA-ergic intracortical circuits. The use of GABA-subunit selective drugs allows for dissection of the physiological functions of various inhibitory intracortical circuits.
Acknowledgements
We would like to thank Ms Rima Gupta, Clinical Trials Pharmacist at the National Hospital for Neurology and Neurosurgery, London, for preparation and dispensing the drug. This study received support from the Medical Research Council and the Wellington Fund.
References
- 1.Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18(6):1260–71. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- 2.Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003;23(29):9664–74. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boroojerdi B, Ziemann U, Chen R, Butefisch CM, Cohen LG. Mechanisms underlying human motor system plasticity. Muscle Nerve. 2001;24(5):602–13. doi: 10.1002/mus.1045. [DOI] [PubMed] [Google Scholar]
- 5.Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97(7):3661–5. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casasola C, Montiel T, Calixto E, Brailowsky S. Hyperexcitability induced by GABA withdrawal facilitates hippocampal long-term potentiation. Neuroscience. 2004;126(1):163–71. doi: 10.1016/j.neuroscience.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26(14):3713–20. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79(2):1117–23. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 9.Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111(5):794–9. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118(10):2207–14. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575(Pt 3):721–6. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florian J, Muller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586(2):495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390(2):194–210. [PubMed] [Google Scholar]
- 14.Glazewski S, Herman C, McKenna M, Chapman PF, Fox K. Long-term potentiation in vivo in layers II/III of rat barrel cortex. Neuropharmacology. 1998;37(4-5):581–92. doi: 10.1016/s0028-3908(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 15.Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol. 2007;97(3):2215–29. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75(5):1765–78. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi Y, Karube F, Kubota Y. Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cereb Cortex. 2006;16(5):696–711. doi: 10.1093/cercor/bhj015. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7(6):476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 19.Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, Kazis A, Mills KR. Lorazepam-induced effects on silent period and corticomotor excitability. Exp Brain Res. 2006;173(4):603–11. doi: 10.1007/s00221-006-0402-1. [DOI] [PubMed] [Google Scholar]
- 20.Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22(7):2513–21. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20(1):375–86. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyriakopoulos AA, Greenblatt DJ, Shader RI. Clinical pharmacokinetics of lorazepam: a review. J Clin Psychiatry. 1978;39(10 Pt 2):16–23. [PubMed] [Google Scholar]
- 24.Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126(Pt 4):866–72. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- 25.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290(5489):131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 26.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173(1):86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 28.Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16(4):815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 29.Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300(1):2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 30.Mohler H, Fritschy JM, Vogt K, Crestani F, Rudolph U. Pathophysiology and pharmacology of GABA(A) receptors. Handb Exp Pharmacol. 2005;(169):225–47. doi: 10.1007/3-540-28082-0_9. [DOI] [PubMed] [Google Scholar]
- 31.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136(4):431–8. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- 32.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415(6872):640–4. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 33.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93(21):11939–44. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pleger B, Schwenkreis P, Dinse HR, Ragert P, Hoffken O, Malin JP, Tegenthoff M. Pharmacological suppression of plastic changes in human primary somatosensory cortex after motor learning. Exp Brain Res. 2003;148(4):525–32. doi: 10.1007/s00221-002-1324-1. [DOI] [PubMed] [Google Scholar]
- 35.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 36.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1(3):230–4. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 37.Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544(Pt 2):617–29. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salva P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29(3):142–53. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- 39.Sawaki L, Boroojerdi B, Kaelin-Lang A, Burstein AH, Butefisch CM, Kopylev L, Davis B, Cohen LG. Cholinergic influences on use-dependent plasticity. J Neurophysiol. 2002;87(1):166–71. doi: 10.1152/jn.00279.2001. [DOI] [PubMed] [Google Scholar]
- 40.Segev I, Burke R, editors. Methods in Neuronal Modeling. MIT Press; Cambridge, Massachusetts: 1998. [Google Scholar]
- 41.Steele PM, Mauk MD. Inhibitory control of LTP and LTD: stability of synapse strength. J Neurophysiol. 1999;81(4):1559–66. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- 42.Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92(1):66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- 43.Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt 2):503–13. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109(1):158–63. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- 45.Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281(5379):985–8. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 46.Xiang Z, Huguenard JR, Prince DA. Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J Neurophysiol. 2002;88(2):740–50. doi: 10.1152/jn.2002.88.2.740. [DOI] [PubMed] [Google Scholar]
- 47.Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–29. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998a;18(3):1115–23. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 1998b;18(17):7000–7. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124(Pt 6):1171–81. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]