Abstract
The intestinal epithelium contains a rapidly proliferating and perpetually differentiating epithelium. The principal functional unit of the small intestine is the crypt-villus axis. Stem cells located in the crypts of Lieberkühn give rise to proliferating progenitor or transit amplifying cells that differentiate into the four major epithelial cell types. The study of adult gastrointestinal tract stem cells has progressed rapidly with the recent discovery of a number of putative stem cell markers. Substantial evidence suggests that there are two populations of stem cells: long-term quiescent (reserved) and actively cycling (primed) stem cells. These are in adjoining locations and are presumably maintained by the secretion of specific proteins generated in a unique microenvironment or stem cell niche surrounding each population. The relationship between these two populations, and the cellular sources and composition of the surrounding environment remains to be defined, and is an active area of research. In this review we will outline progress in identifying stem cells and defining epithelial-mesenchymal interactions in the crypt. We will summarize early advances using stem cells for therapy of gastrointestinal disorders.
Introduction: Intestinal epithelial renewal and the crypt stem cell
The intestinal epithelium contains a rapidly proliferating and perpetually differentiating epithelium. The principal functional unit of the small intestine is the crypt-villus axis (Figure 1). Stem cells located in the crypts of Lieberkühn give rise to proliferating progenitor or transit amplifying cells that differentiate into the four major epithelial cell types. These include columnar absorptive cells or enterocytes, mucous secreting goblet cells, enteroendocrine cells and Paneth cells. Enterocytic, goblet and enteroendocrine cell differentiation takes place during migration upward from the crypt to the tips of the adjacent villi or surface epithelium, whereas Paneth cells complete their differentiation at the crypt base1, 2 3. The colon, devoid of villi, is lined by a flat surface epithelium with similar crypt invaginations, although Paneth cells are not present4. The crypt-villus unit is surrounded by supporting lamina propria/mesenchymal cells 5.
Figure 1.
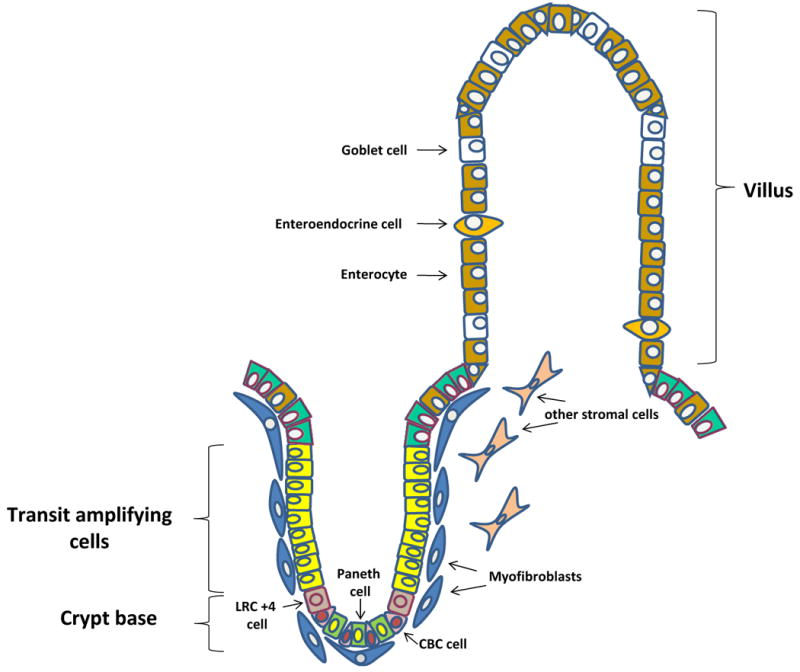
Schematic illustration of the crypt–villus axis, the functional unit of the intestinal epithelium. Stem cells, located in the crypt base, give rise to transit amplifying cells that differentiate into the four major epithelial cell types: enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. The colon lacks villi and is lined by a flat surface epithelium with similar crypt invaginations. The crypt-villus unit is surrounded by supporting lamina propria/mesenchymal cells, including subepithelial myofibroblasts. Two putative populations of stem cells exist; 1. a quiescent/reserved population that consists of label retaining cells (LRC +4) located above the basally situated Paneth cell and 2. an actively cycling/primed population that consists of crypt base columnar (CBC) cells.
The study of adult gastrointestinal tract stem cells has progressed rapidly with the recent discovery of a number of putative stem cell markers. Substantial evidence suggests that there are two populations of stem cells: long-term quiescent (reserved) and actively cycling (primed) stem cells. In this model, baseline regeneration is accomplished by a “primed subpopulation” of active stem cells, whereas quiescent stem cells function as a reserve subpopulation that responds to injury 6, 7. These are in adjoining locations and are presumably maintained by the secretion of specific proteins generated in a unique microenvironment or stem cell niche surrounding each population 6, 7. The relationship between these two populations, and the cellular sources and composition of the surrounding environment/niche remains to be defined, and is an active area of research.
The precise location of the gut stem cell is still a matter of some debate. Early studies suggested its location at the base of the intestinal crypt, supplying proliferative progenitors required for epithelial regeneration.8, 9 More recent studies have confirmed these data, indicating the presence of actively cycling cells in the crypt base that have the qualities of stem cells and appear to be responsible for intestinal homeostasis 6, 7, 10. On the other hand, preferential DNA label retention of agents such as bromodeoxyuridine or [3H] thymidine localized the intestinal stem cell (ISC) to the 4+ position above the crypt base and above the Paneth cells 11-13. These are the quiescent stem cells thought to be responsible for repair following injury6, 7. The number of stem cells per intestinal crypt is commonly estimated to be between 4 and 6 cells per crypt.
In this review we will outline progress in identifying stem cells and defining epithelial-mesenchymal interactions in the intestinal stem cell niche. We will summarize early advances using stem cells for therapy of gastrointestinal disorders
Adult intestinal stem cells
Intestinal stem cells are defined as long-lived cells that exhibit self-renewal and multi-potency, or the ability to differentiate into multiple cell types. Correct identification of intestinal stem cells requires demonstration of these properties in vitro and in vivo.
Identification: Morphology, clonal marking and lineage tracing
Prior to the isolation of definitive stem cell markers, indirect strategies were employed to identify stem cells, including DNA label retention13. This mode of stem cell identification was based on the hypotheses 1. That stem cells were quiescent and 2. stem cells hold on to the original DNA strands and assign newly synthesized strands to transit amplifying daughter cells, a hypothesis originally proposed by Cairns in 1975 9. Although the precise mechanism for label retention remains unclear, studies have demonstrated asymmetric DNA template segregation in intestinal stem cells, using specific labels for new and old chromatids13. The utility of label retention as a means for marking intestinal stem cells marker is limited by the possibility that non-stem postmitotic cells may also be label-retaining4. In fact, clonal analysis by Bjerknes and Cheng 8, 12 suggested that undifferentiated, proliferative, non-label retaining cells called crypt base columnar cells (CBC) residing below the +4 position in between the Paneth cells had stem cell properties.
Initial attempts to identify intestinal stem cells and demonstrate crypt monoclonality also depended heavily on in vivo assays that exploited the proliferative capacity of the intestinal crypts. For example, regeneration assays in response to radiation or chemotherapeutic agents such as 5-fluorouracil, using X chromosome inactivation as a surrogate marker 4 were utilized. Interpretation of these studies was difficult secondary to the inability to clearly distinguish the contribution of short lived progenitor cells from stem cells.
Lineage tracing, in which stem cell activity is retrospectively evaluated by investigation of cellular descendents, has demonstrated two stem cell populations in the crypt base.
Intestinal stem cell markers
Two putative stem cell markers that have been rigorously evaluated in vivo are leucine-rich repeat containing G-protein-coupled receptor (Lgr5)14 and Bmi115. Others, including Dcamkl1 (double cortin and calcium/calmodulin-dependent protein kinase-like-1), Musashi-1, a microtubule associated kinase, β1-integrin, phosphorylated PTEN, 14-3-3£, phosphorylated Akt, and sFRP-5 (secreted frizzled-related protein 5), a Wnt antagonist, have been proposed as intestinal stem cell markers based on their position in the crypt base, just above Paneth cells (reviewed in 16, 17). Other markers of the CBC population, include olfactomedin 4 (Olfm4) and Achaete scutelike 2 (Ascl2)18, 19.
The Lgr5 gene, also known as Gpr49, was initially identified in colon cancer cells as a Wnt target gene, and is also over expressed in ovarian and liver cancers. It is an orphan G protein-coupled receptor with a large leucine-rich extracellular domain and seven transmembrane domain9. Although the molecular function of Lgr5 is unknown, Lgr5 deficient mice have tongue and lower jaw malformations that lead to aerophagia and early neonatal death9. Analyses of mice with a lac-Z or an enhanced green fluorescent protein (EGFP) knock-in allele17, as well as in situ hybridization studies, showed that Lgr5 expression is limited to the CBC cells. Immunostaining of murine small intestine to detect GFP, for instance, demonstrates that Lgr5 is present only in CBC cells located between Paneth cells (Figure 2a). Of note, bromodeoxyuridine labeling studies indicate that these are rapidly cycling cells. Finally, distinct from +4 cells, CBC cells are much less sensitive to low dose (1 Gray) irradiation14.
Figure 2.
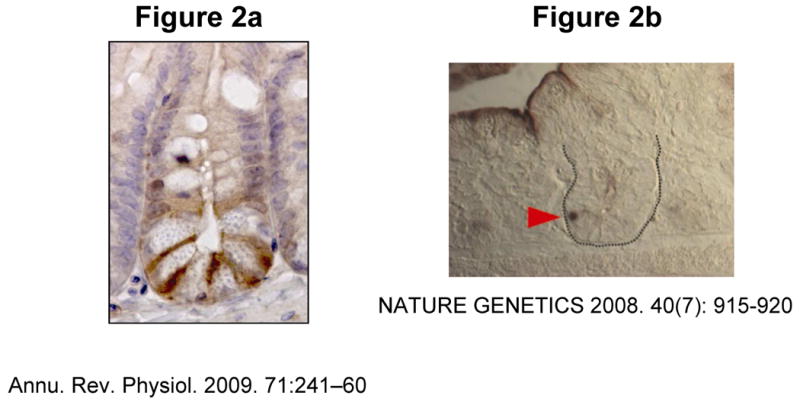
Figure 2A. The intestinal stem cell marker Lgr5, is expressed in CBC (crypt base columnar) cells located in between Paneth cells. [ANNUAL REVIEW OF PHYSIOLOGY by Laurens G. van der Flier and Hans Clevers. Copyright 2009 by ANNUAL REVIEWS, INC.. Reproduced with permission of ANNUAL REVIEWS, INC. in the format Journal via Copyright Clearance Center].
Figure 2B. The intestinal stem cell marker Bmi1 (red arrowhead) is expressed in the crypt base above the Paneth cells [Reprinted by permission from Macmillan Publishers Ltd: Nature Genetics 40(7): 915-920, 2008].
Lineage tracing/gene fate mapping using an inducible Cre knock-in allele in combination with the Rosa26-lacZ reporter strain, has demonstrated in vivo that Lgr5+ CBC cell fulfills the definition of the stem cell in the small intestine and colon. These cells undergo self-renewal and are multipotent, giving rise to all intestinal epithelial lineages; epithelial clones were also noted to be long-lived14. Sorted Lgr5+ cells can form crypt-villus organoids in culture20. Gene signature evaluation of purified Lgr5 positive stem cells has led to the identification of Achaete scutelike 2 (Ascl2)19, a basic helix-loop-helix transcription factor. Ascl2 is detected in adenomas. Its expression in the intestinal epithelium is under the control of the Wnt pathway and is limited to Lgr5+ stem cells. Gain- and loss-of-function studies have demonstrated that Ascl2 is essential for maintenance and regulation of Lgr5 intestinal stem cells19.
Wnt pathway activation leads to the expression of target genes active in crypt cell proliferation as well as in colorectal cancer. Interestingly, examination of large adenomas that developed in Lgr5-GFP mice crossed with mice with a Cre inducible floxed Apc allele demonstrated that unlike other Wnt target genes, expression of Lgr5-GFP is restricted to a few cells; suggesting the role of this marker in identification of a putative cancer stem cell 9, 21.
Markers for the +4 stem cell population have also been investigated. Lineage tracing/gene fate mapping and cell ablation using an inducible Cre knock-in allele to the Bmi1 locus, has demonstrated that Bmi1, a member of the Polycomb group gene family, is a marker of the ISC population located at the 4+ position in the small intestine crypt. Small intestinal in situ hybridization with a Bmi1 probe, demonstrates that Bmi1 mRNA expression occurs only in crypt bottoms located above Paneth cells (Figure 2b, red arrow). Bmi1+ cells, specific to the proximal small intestine, are long-lived and multipotent, giving rise to all epithelial differentiated cell lineages 15 and thus fulfilling the criteria that define stem cells. However, this marker occasionally identifies cells intermingled with Paneth cells at the crypt base; analysis of Lgr5 stem cells has shown that Bmi1RNA is enriched in this population of stem cells, thus expression of these two markers may not be exclusive to one stem cell population19.
Low levels of Lgr5 mRNA and protein in CBC cells render it difficult to use as a marker18. Gene signature evaluation of Lgr5 + stem cells has led to the identification of Olfactomedin-4 (Olfm4), a member of the bone morphogenetic protein (BMP) antagonist family expressed in murine small intestine but not in murine colon18. In humans, however, Olfm4 is highly expressed in both colon and small intestine crypt base columnar cells as well as in a subset of cells within adenocarcinomas18. Unlike Ascl2, Olm4 is not detected in adenomas, suggesting it is not expressed under control of the Wnt pathway19. Prominin1 is another marker that co-localizes with Lgr5+ cells but is also found in proliferating progenitor cells22.
In vitro culture of intestinal stem cells
Recent studies have reported novel methodology that permits the long term maintenance of stem/early progenitor cells and the generation of crypt-villus structures with the architecture of intestinal crypts20, 23. Lgr5+ stem cells can be maintained in long-term culture and differentiate into crypt-villus like units 20 if grown in laminin-rich Matrigel in the presence of the Wnt agonist R-spondin 1, the Bmp antagonist Noggin, a Notch agonist peptide, epidermal growth factor and the Rho kinase inhibitor Y-27632 which inhibits embryonic stem cell anoikis20, 24. A single Lgr5+ stem cell thus can give rise to crypt-villus-like structures in the presence of the appropriate extracellular signals, which are derived from the underlying mesenchyme in vivo. This highlights the contribution of the adjacent mesenchyme to maintenance of the stem cells. Interestingly, although the isolated cells and structures were exposed to the same extracellular factors, expression of nuclear β-catenin remained highest at the crypt base, and crypt–villus domains were established, suggesting that the differential response to extracellular signals along the crypt-villus axis 20 is intrinsic to the epithelium.
However, others have shown that mesenchymal derived cells are required for long term culture of intestinal stem cells 23. Utilizing a microenvironment consisting of an air-liquid interface 3D collagen gel to improve oxygenation, as well as myofibroblasts and stem cell niche signaling molecules, long-term culture of intestine and colon has been established23. Intact Wnt and Notch signaling, recognized as active in the ISC niche, were confirmed to be required for the in vitro cultures. Growth of myofibroblasts correlated with the long term viability and proliferation of the cultures. The presence of ISCs was confirmed by examining Lgr5 and Bmi1 expression. Successful stem cell propagation using this culture system should advance the study of intestinal stem cell niche and ISC-niche interactions.
Stem cell niche: the tissue restricted microenvironment
Intestinal stem cell niche characterization has thus far lagged behind other organs such as the hematopoietic and neural systems. The stem cell niche is a complex, dynamic milieu that adapts in response to environmental cues25. This stromal microenvironment surrounding the stem cell, consists of extracellular matrix, neural cells, lymphocytes, macrophages, endothelial cells, fibroblasts, smooth muscle cells, and myofibroblasts 23 25, 26. Niche generated signals most likely work in concert with intrinsic stem cell properties to regulate stem cell behavior26. A key property of the stem cell niche is its capability of regulating stem cell proliferation and differentiation even in the absence of stem cells 27, 28.
Constituents of the stem cell niche
The stem cell niche likely contains several different types of cells; each with a specific contribution to the regulation of stem cell behavior. One of the most extensively studied components is the intestinal subepithelial myofibroblast, which supports ISCs and affects adjacent epithelial cells 2, 26. Located in the lamina propria, in a strategic position adjacent to the crypts, intestinal subepithelial myofibroblasts regulate stem cell behavior through the elaboration of growth factors and cytokines 2, 29, 30. Intercellular signaling pathways, such as Bmp, Wnt, and Notch, are conserved in stem cell biology across tissues and species. The microenvironment surrounding one of the putative intestinal stem cells, the Lgr5+ stem cell, is characterized by prominent Wnt activity and inhibition of Bmp signaling with the presence of Bmp inhibitors noggin and gremlin. On the other hand, the microenvironment surrounding the +4 intestinal stem cell is characterized by the Wnt inhibitor sFRP5 and BMP46.
Endothelial cells that are part of the surrounding crypt microvasculature, enteric neurons, intraepithelial lymphocytes (IELs) and microbes 2 have also been cited as niche constituents. The intestine also houses indigenous microbes, several components of which regulate crypt cell proliferation as well as activity of the enteric nervous system 2.
Finally, in the Drosophila midgut, intestinal stem cell progenitor cells appear to generate a transient niche cell via Notch signaling 31; these niche cells in turn regulate establishment of the gut stem cell via Decapentaplegic (Bmp2/4 homolog) signaling 31. These findings suggest a role for epithelial cells in generating the stem cell niche and provide an additional model for regulation of intestinal stem cell behavior. Investigation of this novel mechanism in the mammalian intestine is ongoing.
An intriguing but poorly understood component of the niche is the mesenchymal stem cell (MSC). This cell type is characterized by a fibroblast-like phenotype and the ability to adhere to plastic tissue culture plates. MSCs have demonstrated immunomodulatory potential through secretion of prostaglandin E2 and improvement of vasculogenesis. This population has been isolated from a variety of sources including bone marrow, adipose tissue, and intestinal tissue and depending on the growth conditions, can differentiate into multiple mesenchymal lineages32. Several ongoing studies are investigating the role of MSCs as therapy for inflammatory bowel disease, graft versus host disease, radiation induced enteritis and colitis (see below).
Epithelial-mesenchymal interactions: Bmp and Hedgehog signaling
Epithelial-mesenchymal interactions, critical to proper gut development and intestinal organogenesis, are conserved throughout evolution. Diffusible Wnt, Hedgehog (Hh), transforming growth factor-β and bone morphogenetic proteins are implicated in molecular cross-talk between epithelium and underlying mesenchyme4, 33. Mesenchymal Bmps act on small intestinal epithelium in a paracrine fashion to inhibit crypt cell proliferation and crypt fission 34, 35. Recent studies suggest a role for Bmp antagonists, such as gremlin and chordin, as crucial components of the intestinal epithelial stem cell niche 29. Mutations in mesenchymal genes such as the transcription factor forkhead homolog 6 (Fkh6) and transcription factor Nkx2-3, result in an increase in crypt cell proliferation17. Bmp signaling is altered in each of these models17. Deletion of epimorphin, a mesenchymal/myofibroblast protein, and member of the syntaxin family of membrane-bound intracellular vesicle docking proteins, results in protection from dextran-sodium sulfate induced colitis and inflammation induced carcinogenesis, in association with disruption in Bmp signaling 36, 37. Epithelial derived ligands in turn signal to the underlying mesenchyme. For example, Hh ligands produced in the epithelium, signal to the underlying mesenchyme which expresses signaling targets Gli-1, Ptch-1, and Hhip 38-40.
Transgenic mice expressing the pan Hh inhibitor Hhip show that Hh signaling is paracrine, from epithelium to Ptch-1-expressing mesenchymal cells including intestinal subepithelial myofibroblasts (ISEMFs), resulting in increased epithelial proliferation 35 and inflammation in the setting of long-term inhibition 38. In turn, active Hh signaling has also been identified as antagonistic to Wnt signaling, promoting colonic epithelial differentiation 41-43.
The relevance of epithelial-mesenchymal interactions in the study of the intestinal stem cell becomes most obvious during attempts at in vitro evaluation of ISCs. Isolated intestinal epithelium survives anoikis/apoptosis only if interaction with constituents of extracellular matrix, including collagen, is restored4. In addition, as described above, the ability to culture LGR5+ cells and generate the crypt/villus axis in vitro is dependent on the inclusion of stem cell niche derived soluble factors.
Signaling in the crypt: Wnt pathway
Wnt signaling is central to maintenance of the intestinal stem cell, as evidenced by depletion of stem cells in the setting of Wnt signaling abrogation. Deletion of TCF4, an end target of Wnt signaling25, or β-catenin mutation, results in diminution of the proliferative compartment and loss of entire crypts3. Similarly, transgenic expression of the secreted Wnt inhibitor Dickkopf-1 (Dkk-1) leads to reduced small intestinal epithelial proliferation and loss of crypts 44. A similar but even more profoundly abnormal phenotype is produced by adenoviral expression of DKK-1 in adult mice 45. Transgenic expression of the Wnt agonist R-spondin, on the other hand, results in augmentation of crypt proliferation17. Bmp signals generated in the mesenchyme in turn inhibit stem cell self-renewal through modulation of Wnt activity30.
Evaluation of Wnt target gene expression in the small intestinal epithelium has demonstrated active Wnt signaling in the crypt base 17. Similarly gene expression profiling along the human colonic crypt villus axis has demonstrated differential expression of Wnt signaling pathway along the crypt-villus axis of the human colonic crypt29. The crypt top was characterized by APC, WNT5B, and TCF4; while the crypt bottom, the putative stem cell niche, was characterized by expression of AXIN2, TCF3, and several secreted WNT inhibitors including DKK3, SFRP1, SFRP2, FZD2, FZD3, FZD7, and FZDB29. Similarly, members of the BMP pathway contribute to the colonic epithelial stem cell niche by modulation of the Wnt pathway; the crypt base was characterized by the BMP antagonists GREM1, GREM2, AND CHRDL1 which were expressed by surrounding pericryptal subintestinal myofibroblasts 29. This combination of signaling characterizes the stem cell niche and regulates intestinal stem cell behavior 29.
Stem cell therapy for gastrointestinal disorders
Recent breakthroughs in the identification of intestinal stem cell markers will undoubtedly fuel novel research that will focus on treatment of gastrointestinal disorders. Diseases in which there is impaired regeneration or function of the small intestine or colon, such as in short bowel syndrome, inflammatory bowel disease, ischemic bowel, or following irradiation, are most likely to be the first targets for novel therapies. However, at the present time, stem-cell based therapies for gastrointestinal disorders have predominantly focused on the use of bone marrow derived stem cells and mesenchymal stem cells, to treat diverse disorders such as inflammatory bowel disease and graft vs. host disease in humans.
Bone marrow derived stem cells have been used to treat refractory Crohn's disease in humans, with induction of remission following autologous hematopoietic stem cell transplantation 46, 47 48 49. Long-term remissions have been reported 50. Although the precise mechanisms for healing remain undefined, animal models suggest that this occurs through enhanced mucosal repair, as bone marrow cells contribute activated myofibroblasts and epithelial cells, and promote neovascularization 51-53.
Mesenchymal stem cells differentiate into several tissues including bone, cartilage and fat and are thought to be important for tissue repair 32. A single patient with a perforated colon and peritonitis due to tissue toxicity from allogeneic hematopoietic stem cell transplantation was treated with two treatment courses of mesenchymal stem cells with resolution of peritonitis 54, although the patient died several weeks later from disseminated fungal infection. One patient with severe graft-versus-host disease and refractory diarrhea received haploidentical mesenchymal stem cells, resulting in at least a one year remission 55. Mice in which inflammatory bowel disease was induced by trinitrobenzene sulfonic acid, showed reduced disease severity when treated with human adipose-derived mesenchymal stem cells. Diminished severity of colitis was associated with down-regulation of Th-1 immune responses56. Similarly, infusion of human bone–marrow derived mesenchymal stem cells into irradiated mice accelerated recovery from radiation induced intestinal damage57. These studies suggest potential therapeutic benefit for mesenchymal stem cells as enhancers of epithelial repair in a variety of gastrointestinal disorders.
Although not covered in this review, another exciting area of research focuses on enteric nervous system stem cells and regeneration. For example, enteric nervous system stem cells were derived from human gut mucosal biopsies from children. Transplantation into aganglionic chick and human gut in organ culture showed formation of enteric neurons, glia and organized ganglia 58
Conclusions: what does the future hold?
Remarkable progress has occurred in the past several years in identifying and characterizing adult intestinal stem cells and their interactions with mesenchymal cells of the stem cell niche. Although therapeutic trials for intestinal disorders in animal models have lagged behind those in other organs, the next few years will likely see rapid and substantial progress in the translation of these critical basic biological discoveries. Regenerative therapies will be developed using hematopoietic, mesenchymal and adult intestinal stem cells cultured in the appropriate niche environments. Eventual expansion into human trials is highly likely, particularly for debilitating disorders such as short bowel syndrome, radiation induced injury, or refractory, unremitting Crohn's disease, for which there are few or no effective therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin DC. Small intestine: anatomy and structural anomalies. In: Yamada T, editor. Textbook of Gastroenterology. Chichester, West Sussex, U.K.: Wiley-Blackwell; 2009. pp. 1085–1107. [Google Scholar]
- 2.Mills JC, Gordon JI. The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci U S A. 2001;98:12334–6. doi: 10.1073/pnas.231487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol. 2006;419:337–83. doi: 10.1016/S0076-6879(06)19014-X. [DOI] [PubMed] [Google Scholar]
- 5.Abud HE, Watson N, Heath JK. Growth of intestinal epithelium in organ culture is dependent on EGF signalling. Exp Cell Res. 2005;303:252–62. doi: 10.1016/j.yexcr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 8.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. IV. Effects of resecting 30% of the small intestine. Am J Anat. 1981;160:93–103. doi: 10.1002/aja.1001600108. [DOI] [PubMed] [Google Scholar]
- 9.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–21. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–9. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batlle E. A new identity for the elusive intestinal stem cell. Nat Genet. 2008;40:818–9. doi: 10.1038/ng0708-818. [DOI] [PubMed] [Google Scholar]
- 13.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–8. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 14.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 15.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–20. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2009;6:724–37. doi: 10.1038/nrgastro.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 18.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–7. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 19.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–12. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 22.Verstappen J, Katsaros C, Torensma R, Von den Hoff JW. A functional model for adult stem cells in epithelial tissues. Wound Repair Regen. 2009;17:296–305. doi: 10.1111/j.1524-475X.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 23.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–6. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 25.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 27.Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16:693–9. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–30. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 29.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104:15418–23. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 31.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–3. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–9. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 33.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–15. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- 34.Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–6. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 35.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang L, Iordanov H, Swietlicki EA, Zheng Q, Jiang S, Tang Y, Levin MS, Rubin DC. Epimorphin(-/-) mice have increased intestinal growth, decreased susceptibility to dextran sodium sulfate colitis, and impaired spermatogenesis. J Clin Invest. 2006;116:1535–46. doi: 10.1172/JCI25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, Deschryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest. doi: 10.1172/JCI40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zacharias WJ, Li X, Madison BB, Kretovich K, Kao JY, Merchant JL, Gumucio DL. Hedgehog is an Anti-Inflammatory Epithelial Signal for the Intestinal Lamina Propria. Gastroenterology. doi: 10.1053/j.gastro.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y, Swietlicki EA, Jiang S, Buhman KK, Davidson NO, Burkly LC, Levin MS, Rubin DC. Increased apoptosis and accelerated epithelial migration following inhibition of hedgehog signaling in adaptive small bowel postresection. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1280–8. doi: 10.1152/ajpgi.00426.2005. [DOI] [PubMed] [Google Scholar]
- 40.Buhman KK, Wang LC, Tang Y, Swietlicki EA, Kennedy S, Xie Y, Liu ZY, Burkly LC, Levin MS, Rubin DC, Davidson NO. Inhibition of Hedgehog signaling protects adult mice from diet-induced weight gain. J Nutr. 2004;134:2979–84. doi: 10.1093/jn/134.11.2979. [DOI] [PubMed] [Google Scholar]
- 41.van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, Nielsen C, Gaffield W, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–82. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 42.van den Brink GR, Hardwick JC. Hedgehog Wnteraction in colorectal cancer. Gut. 2006;55:912–4. doi: 10.1136/gut.2005.085902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, Offerhaus GJ, van den Bergh Weerman MA, Boeckstaens GE, Hommes DW, Hardwick JC, Hahn H, van den Brink GR. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the Hedgehog pathway. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 44.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–13. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–71. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ditschkowski M, Einsele H, Schwerdtfeger R, Bunjes D, Trenschel R, Beelen DW, Elmaagacli AH. Improvement of inflammatory bowel disease after allogeneic stem-cell transplantation. Transplantation. 2003;75:1745–7. doi: 10.1097/01.TP.0000062540.29757.E9. [DOI] [PubMed] [Google Scholar]
- 47.Kreisel W, Potthoff K, Bertz H, Schmitt-Graeff A, Ruf G, Rasenack J, Finke J. Complete remission of Crohn's disease after high-dose cyclophosphamide and autologous stem cell transplantation. Bone Marrow Transplant. 2003;32:337–40. doi: 10.1038/sj.bmt.1704134. [DOI] [PubMed] [Google Scholar]
- 48.Craig RM, Traynor A, Oyama Y, Burt RK. Hematopoietic stem cell transplantation for severe Crohn's disease. Bone Marrow Transplant. 2003;32 1:S57–9. doi: 10.1038/sj.bmt.1703945. [DOI] [PubMed] [Google Scholar]
- 49.Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N, Kletzel M, Whitington PF, Burt RK. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552–63. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 50.Brittan M, Alison MR, Schier S, Wright NA. Bone marrow stem cell-mediated regeneration in IBD: where do we go from here? Gastroenterology. 2007;132:1171–3. doi: 10.1053/j.gastro.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 51.Brittan M, Chance V, Elia G, Poulsom R, Alison MR, MacDonald TT, Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984–95. doi: 10.1053/j.gastro.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Komori M, Tsuji S, Tsujii M, Murata H, Iijima H, Yasumaru M, Nishida T, Irie T, Kawano S, Hori M. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–18. doi: 10.1111/j.1067-1927.2005.130114.x. [DOI] [PubMed] [Google Scholar]
- 53.Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–54. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 54.Ringden O, Uzunel M, Sundberg B, Lonnies L, Nava S, Gustafsson J, Henningsohn L, Le Blanc K. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–6. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 55.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–89. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 57.Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, Chapel A, Sache A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 58.Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–25. e1–3. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]


