Abstract
Adipocytes are insulin sensitive cells that play a major role in energy homeostasis. Obesity is the primary disease of fat cells and a major risk factor for the development of Type II diabetes, cardiovascular disease, and metabolic syndrome. Obesity and its related disorders result in dysregulation of the mechanisms that control adipocyte gene expression and function. To identify potential novel therapeutic modulators of adipocytes, we screened 425 botanical extracts for their ability to modulate adipogenesis and insulin sensitivity. We observed that less than 2% of the extracts had substantial effects on adipocyte differentiation of 3T3-L1 cells. Two of the botanical extracts that inhibited adipogenesis were extracts from St. John’s Wort (SJW). Our studies revealed that leaf and flower, but not root, extracts isolated from SJW inhibited adipogenesis as judged by examining PPARγ and adiponectin levels. We also examined the effects of these SJW extracts on insulin sensitivity in mature 3T3-L1 adipocytes. Both leaf and flower extracts isolated from SJW substantially inhibited insulin sensitive glucose uptake. The specificity of the observed effects was demonstrated by showing that treatment with SJW flower extract resulted in a time and dose dependent inhibition of insulin stimulated glucose uptake. SJW is commonly used in the treatment of depression. However, our studies have revealed that SJW may have a negative impact on adipocyte related diseases by limiting differentiation of preadipocytes and significantly inducing insulin resistance in mature fat cells.
Introduction
The use of herbal supplements is on the rise around the world, but insufficient data exist on the efficacy of most botanical products. Many countries have increased their efforts to subject botanicals to rigorous scientific research. Our laboratory participated in a blinded screening study to evaluate the effects of over four hundred botanical extracts on adipocyte differentiation. We observed that less than 2% of the extracts had substantial effects on adipocyte differentiation. Two of the botanical extracts that inhibited adipogenesis were extracts from St. John’s Wort (SJW). SJW is commonly used in the treatment of depression and a variety of other conditions; however its effects on adipocytes have not been studied.
Adipocytes are highly specialized insulin sensitive cells that play a major role in energy homeostasis in vertebrate organisms. Obesity is the primary disease of fat cells and a major risk factor for the development of non-insulin dependent diabetes mellitus (NIDDM), cardiovascular disease, metabolic syndrome and hypertension. Since SJW is readily available and consumed by millions of people and both obesity and diabetes are world-wide epidemics; understanding the effects of SJW on adipocyte development and function is worthwhile. Adipocytes are the primary sites of lipid storage, are insulin sensitive, and secrete endocrine hormones and compounds which alter these properties could be protective or causative of obesity and type II diabetes. Our studies demonstrated that leaf and flower extracts, but not root extracts, from SJW were capable of inhibiting adipocyte differentiation of 3T3-L1 cells. Moreover, these SJW flower and leaf extracts were shown to inhibit insulin sensitive glucose uptake in mature fat cells. In summary, our results suggest that SJW has profound effects on adipocyte development and function.
Materials and Methods
Materials
Dulbecco’s Modified Eagle’s Media (DMEM) was purchased from Invitrogen. Bovine and fetal bovine (FBS) sera were purchased from Hyclone. Both the PPARγ monoclonal antibody and the STAT 5A polyclonal antibody were purchased from Santa Cruz. The adiponectin antibody was a rabbit polyclonal obtained from Affinity Bioreagents. HRP-conjugated secondary antibodies were purchased from Jackson Immunoresearch. Enhanced chemiluminescence (ECL) kit was purchased from Pierce. Nitrocellulose was purchased from BioRad. St. John’s Wort extracts were prepared by the Rutgers University Botanical Research Center. Briefly, plant samples were air dried and extracted with 80% ethanol (1:5 w/v) three times, infused each time for 24 h, and evaporated in a rotary evaporator to thick aqueous suspension. The extracts were further dried under vacuum and stored in amber glass vials at −20 °C. Prior to use, extracts were re-suspended in DMSO.
Cell Culture
Murine 3T3-L1 preadipocytes were plated and grown to 2 days post confluence in DMEM with 10% bovine serum. Medium was changed every 48 hours. Cells were induced to differentiate by changing the medium to DMEM containing 10% fetal bovine serum, 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 1.7 μM insulin. At this time, cells were treated with a 1000X stock of the botanical extracts suspended in DMSO. Cells were also treated with DMSO alone. After 48 hours this medium was replaced with DMEM supplemented with 10% FBS and treated again with botanical extracts or vehicle (DMSO).
Preparation of Whole Cell Extracts
Monolayers of 3T3-L1 preadipocytes or adipocytes were rinsed with phosphate-buffered saline (PBS) and then harvested in a non-denaturing buffer containing 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EGTA, 1 mM EDTA, 1% Triton-X 100, 0.5% Igepal CA-630 (Nonidet P-40), 1 μM PMSF, 1 μM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, and 10 μM leupeptin, and 2 mM sodium vanadate. Samples were extracted for 30 minutes on ice and centrifuged at 10,000 × g at 4°C for 15 minutes. Supernatants containing whole cell extracts were analyzed for protein content using a BCA kit (Pierce) according to the manufacturer’s instructions.
Gel Electrophoresis and Western Blot Analysis
Proteins were separated in 7.5% polyacrylamide (acrylamide from National Diagnostics) gels containing sodium dodecyl sulfate (SDS) according to Laemmli [1] and transferred to nitrocellulose membrane in 25 mM Tris, 192 mM glycine, and 20% methanol. Following transfer, the membrane was blocked in 4% fat-free milk for 1 hour at room temperature. Results were visualized with HRP-conjugated secondary antibodies and enhanced chemiluminescence.
Determination of 3H-labeled 2-Deoxyglucose Uptake
The assay of 2- [3H] deoxyglucose was performed as previously described [2]. Prior to the assay, mature 3T3-L1 adipocytes were serum deprived for 2 h and treated with various doses of botanical extracts for different periods of time. Next, the cells were incubated in the presence or absence of insulin (10 nM) for 10 min. Glucose uptake was initiated by addition of 2- [3H] deoxyglucose at a concentration of 0.1 mM 2-deoxyglucose in 1 μCi 2- [3H] deoxyglucose in Krebs-Ringer-Hepes buffer and incubated for 3 min at room temperature. Glucose uptake is reported as [3H] radioactivity, corrected for nonspecific diffusion (5 μM cytochalasin B) and normalized to total protein content as determined by BCA analysis. Nonspecific uptake and absorption was always less than 10% of the total uptake. Uptake measurements were performed in triplicate under conditions where hexose uptake was linear.
Results
In a blinded study, we screened 425 botanical extracts to examine their ability to modulate adipocyte differentiation of 3T3-L1 cells. We observed that less than 2% of the extracts had substantial effects on adipocyte differentiation. After the screening was complete and identification of the extracts was unblinded, we noted that two of the botanical extracts that inhibited adipogenesis were extracts from St. John’s Wort (SJW). As shown in Figure 1, three extracts from SJW were examined for their ability to inhibit the induction of adipocyte specific proteins such as PPARγ and adiponectin. Extracts from the root (R) of SJW had no effect on adipocyte development, but extracts from the leaves (L) and flowers (F) blocked the induction of PPARγ and adiponectin expression. In addition, the normal induction in STAT5A expression which accompanies differentiation [3] was also inhibited. Botanical extracts obtained from SJW were resuspended in DMSO and diluted 1000 fold into the cell culture media. Vehicle treatment of DMSO had no effect on adipocyte development. In addition, there was no observable cell death (data not shown) and MAPK expression was examined to indicate equivalent levels of protein in each sample.
Figure 1. Leaf and Flower extracts from SJW inhibit adipocyte differentiation of 3T3-L1 cells.
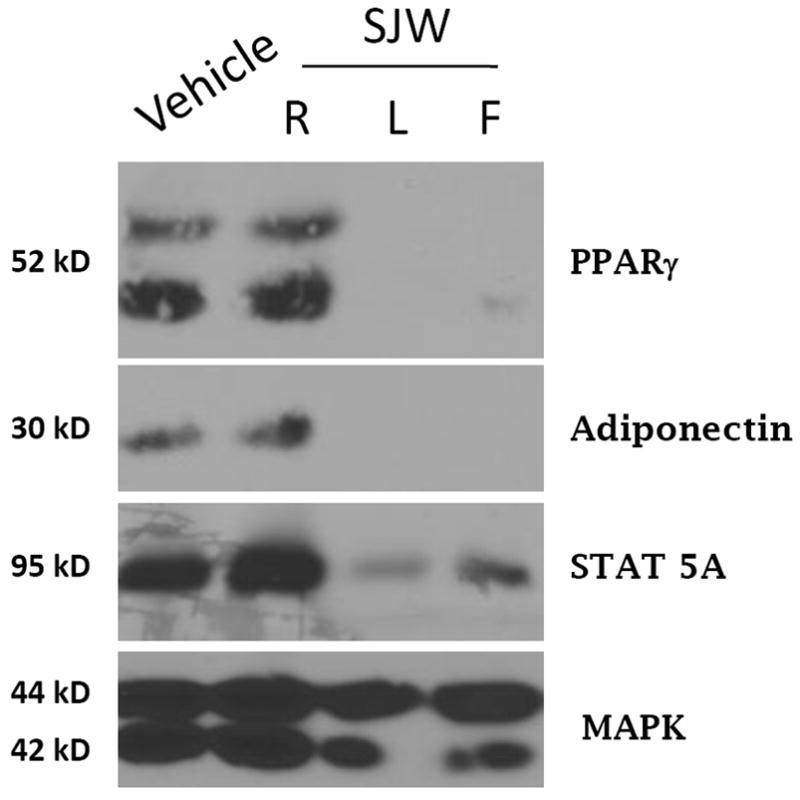
Whole cell extracts were prepared from 3T3-L1 cells one week after they were induced to differentiate with the normal induction cocktail in the presence or absence of 50 μg/ml of SJW extracts obtained from SJW. Cells were also treated with a 1000 X stock of DMSO (vehicle). One hundred ug of each extract was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western blot analysis. The detection system was horse-radish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Pierce). This is a representative experiment independently performed three times.
To assess the specificity of this inhibitory effect, we examined the ability of various doses of the flower extract to modulate adipocyte differentiation. As shown in Figure 2, treatment with a flower extract of SJW resulted in a dose dependent inhibition of adipogenesis as indicated by an inhibition in the induction of PPARγ and adiponectin expression. As indicated in Figure 1, a dose of 50 μg/ml completely blocked the induction of PPARγ and adiponectin. In addition, a dose of 25 μg/ml significantly attenuated the induction of PPARγ and blocked the induction of adiponectin. Treatment with 12 μg/ml did not inhibit the induction of PPARγ, but substantially reduced the induction of adiponectin expression. Treatment with the lowest dose of flower extract (6 μg/ml) or treatment with DMSO (V) had no effect on the induction of PPARγ and adiponectin as compared to untreated (CTL) cells. MAPK expression does not change during the differentiation of 3T3-L1 cells and is shown to indicate equivalent levels of protein in each sample.
Figure 2. A flower extract from SJW results in a dose dependent inhibition of adipogenesis in 3T3-L1 cells.
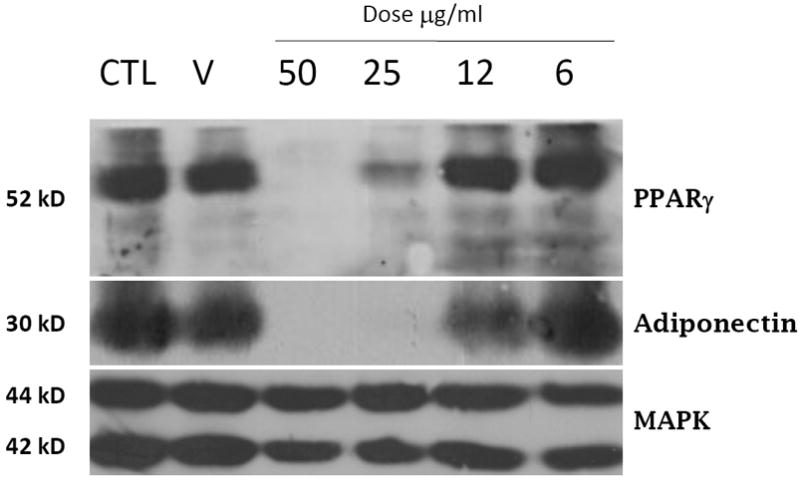
Whole cell extracts were prepared from 3T3-L1 cells one week after they were induced to differentiate with the normal induction cocktail in the presence of various doses of a SJW flower extract. Cells were also treated with a 1000X stock of DMSO (V). One hundred ug of each extract was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western blot analysis. Samples were processed and results were visualized as described in Figure Legend 1. This is a representative experiment independently performed three times.
Because two of the SJW extracts affected adipocyte development, we examined the ability of these SJW extracts to modulate adipocyte function. Fully differentiated 3T3-L1 adipocytes were treated with SJW extracts for 90 minutes and then glucose uptake was examined in the presence and absence of an acute stimulation with insulin. As shown in Figure 3, vehicle (control) treated cells were highly responsive to insulin treatment which resulted in a 4.5 fold increase in glucose uptake. None of the SJW extracts had any effect on glucose uptake in the absence of insulin. However, treatment with a leaf or flower extract resulted in a significant inhibition of insulin stimulated glucose uptake. To assess the specificity of these effects, mature 3T3-L1 adipocytes were pretreated overnight with the various doses of SJW flower extract indicated in Figure 4A. These studies revealed a dose dependent inhibition in insulin sensitive glucose uptake. Mature adipocytes were also pretreated for various times with 25 μg/ml flower extract of SJW. After a 1 or 2 hour treatment, there was a substantial inhibition of insulin sensitive glucose uptake. An 18 hour pretreatment consistently resulted in an even greater inhibition of insulin stimulated glucose uptake.
Figure 3. Leaf and Flower, but not root, extracts from SJW induce insulin resistance in fully differentiated 3T3-L1 adipocytes.
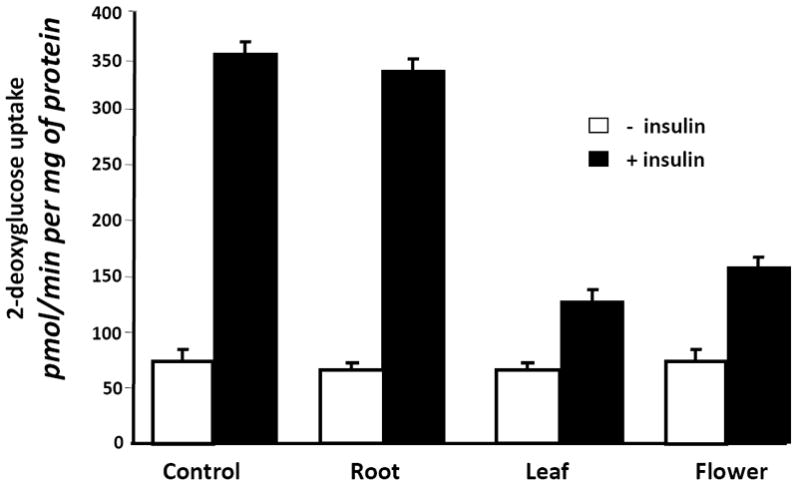
Fully differentiated 3T3-L1 adipocytes were serum deprived and treated overnight (20 hours) with 25 μg/ml of the indicated SJW extract. After the pretreatment, cells were stimulated with insulin (10 nM) for 10 min. Glucose uptake was initiated by addition of 2-[3H] deoxyglucose. The glucose uptake values shown represent the mean +/- S.E. of triplicate determinations from four independent experiments.
Figure 4. A flower extract of SJW induces insulin resistance in cultured murine adipocytes in a dose and time dependent manner.
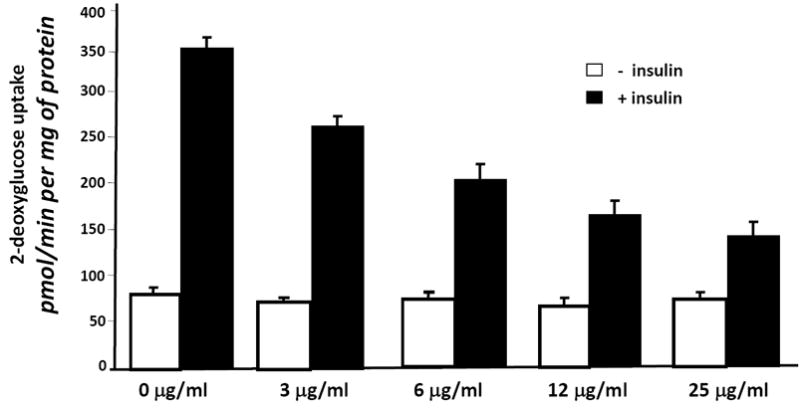
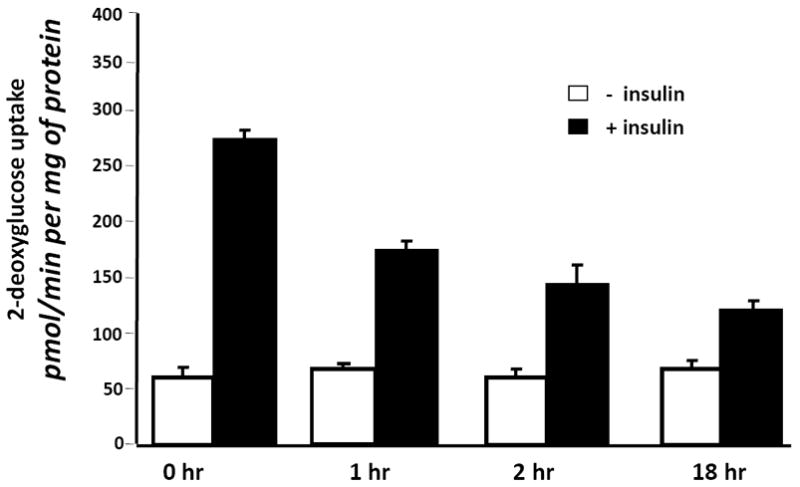
(A) Fully differentiated 3T3-L1 adipocytes were serum deprived and pretreated for 90 minutes with various doses of a SJW flower extract. (B) Fully differentiated 3T3-L1 adipocytes were serum deprived and pretreated for the indicated times with 25 μg/ml of a SJW flower extract. After the pretreatment, cells were stimulated with insulin (10 nM) for 10 min. Glucose uptake was initiated by addition of 2-[3H] deoxyglucose. The glucose uptake values shown represent the mean +/- S.E. of triplicate determinations from four independent experiments.
Discussion
SJW is a common commercially available botanical that is widely used as an over the counter treatment for depression. In a blinded screening study, we observed that extracts obtained from the flower and leaf of SJW, but not the roots, were capable of inhibiting the adipogenesis of murine preadipocytes. Of note, a current hypothesis is that Type 2 diabetes can be viewed as a failure to appropriately expand fat mass in the context of a positive energy balance [4]. In light of this notion, the ability of SJW to inhibit adipogenesis may not be metabolically favorable. Several studies suggest that inhibiting adipocyte development can be a causative factor in the development of insulin resistance [5; 6]. In addition to its ability to inhibit adipocyte development, SJW extracts can also have negative effects on mature fat cells. We observed that SJW flower extracts resulted in a time and dose dependent inhibition of insulin stimulated glucose uptake in mature mouse fat cells. Although less insulin sensitive, we also observed that these extracts could inhibit insulin stimulated glucose uptake in cultured human fat cells (data not shown). Based on our in vitro studies, our results suggest that SJW may have some negative effects on adipocyte development and function. There is a substantial body of literature indicating that SJW can interfere with the action of numerous drugs (reviewed in [7]). It is well established that SJW lowers the circulating concentrations and pharmacological effects of a number of drugs and oral contraceptives [8]. Hyperforin, a psychoactive constituent of SJW, has been to shown to potently activate the pregnane X receptor [9; 10] which is known to activate various set of genes that are involved in the metabolism and excretion of drugs [11].
Although our studies on SJW are limited to in vitro observations, there is some data to support a role of SJW in insulin resistance. A recent paper demonstrated that a protein found in SJW can modulate MCP-1 promoter expression and result in increased MCP-1 expression [12]. It is well known that macrophage derived inflammatory mediators are substantially increased in of obesity and Type 2 diabetes in mice and humans [reviewed in 13]. Moreover, MCP-1 has been shown to regulate the infiltration of macrophages into adipose tissue and play a part in insulin resistance [14]. Since SJW has been shown to increase MCP-1 expression [12], it is reasonable to hypothesize that this popular herb may also be capable of inducing insulin resistance in vivo, perhaps via induction of MCP-1. In summary, a screening process to identify botanical extracts that regulated adipocyte development resulted in the observation that extracts from leaf or flower of SJW inhibit adipogenesis and insulin sensitive glucose uptake in fat cells. Obesity and diabetes are world-wide epidemics and SJW is readily available over the counter and consumed by millions of people wide. Overall, our studies suggest that SJW may have a negative impact on adipocyte related diseases by limiting differentiation of preadipocytes and significantly inducing insulin resistance in mature fat cells.
Acknowledgments
We are grateful to Pat Arbour-Reily and Ursula A. White for technical assistance and preparation of the manuscript.
This work was supported by pilot project funding from the Pennington Biomedical Botanical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 2.Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991;266:21839–21845. [PubMed] [Google Scholar]
- 3.Stephens JM, Morrison RF, Pilch PF. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem. 1996;271:10441–10444. doi: 10.1074/jbc.271.18.10441. [DOI] [PubMed] [Google Scholar]
- 4.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65:S7–12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, van de WE, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang NY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci U S A. 2008;105:6139–6144. doi: 10.1073/pnas.0801981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kober M, Pohl K, Efferth T. Molecular mechanisms underlying St. John’s wort drug interactions. Curr Drug Metab. 2008;9:1027–1037. doi: 10.2174/138920008786927767. [DOI] [PubMed] [Google Scholar]
- 8.Di YM, Li CG, Xue CC, Zhou SF. Clinical drugs that interact with St. John’s wort and implication in drug development. Curr Pharm Des. 2008;14:1723–1742. doi: 10.2174/138161208784746798. [DOI] [PubMed] [Google Scholar]
- 9.Wentworth JM, Agostini M, Love J, Schwabe JW, Chatterjee VK. St John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 10.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- 12.Mukerjee R, Deshmane SL, Darbinian N, Czernik M, Khalili K, Amini S, Sawaya BE. St. John’s Wort protein, p27SJ, regulates the MCP-1 promoter. Mol Immunol. 2008;45:4028–4035. doi: 10.1016/j.molimm.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 14.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]


