Abstract
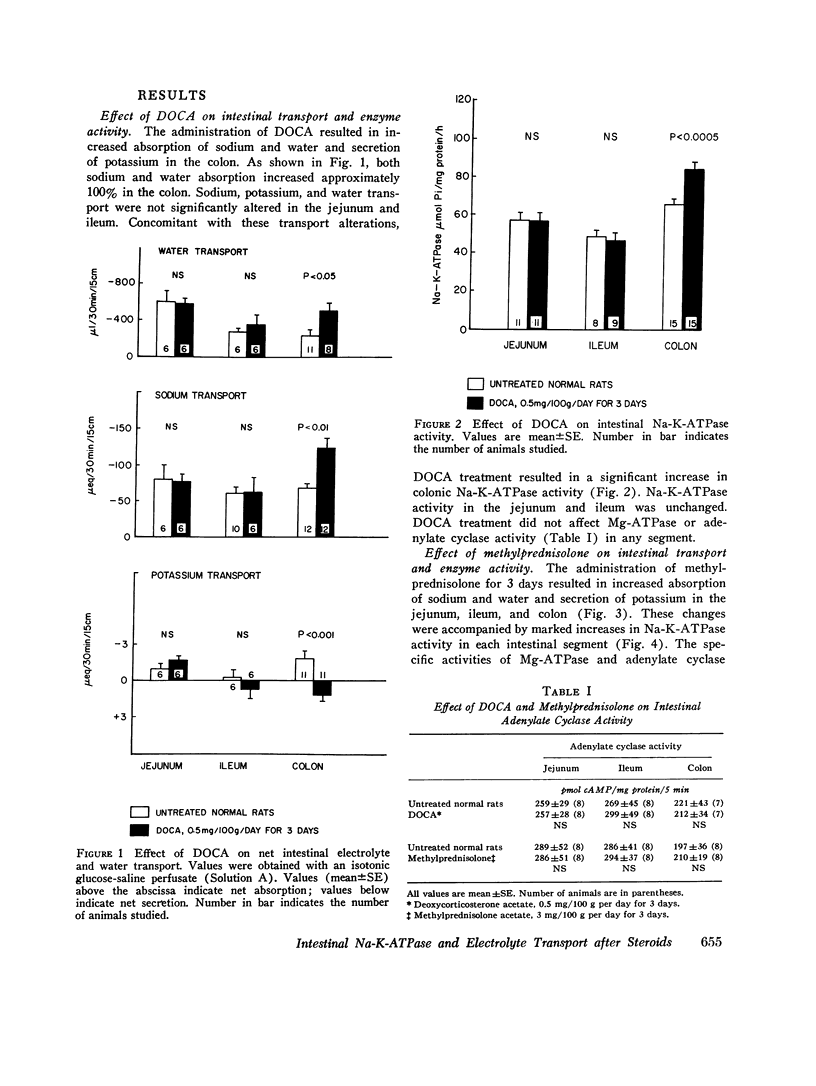
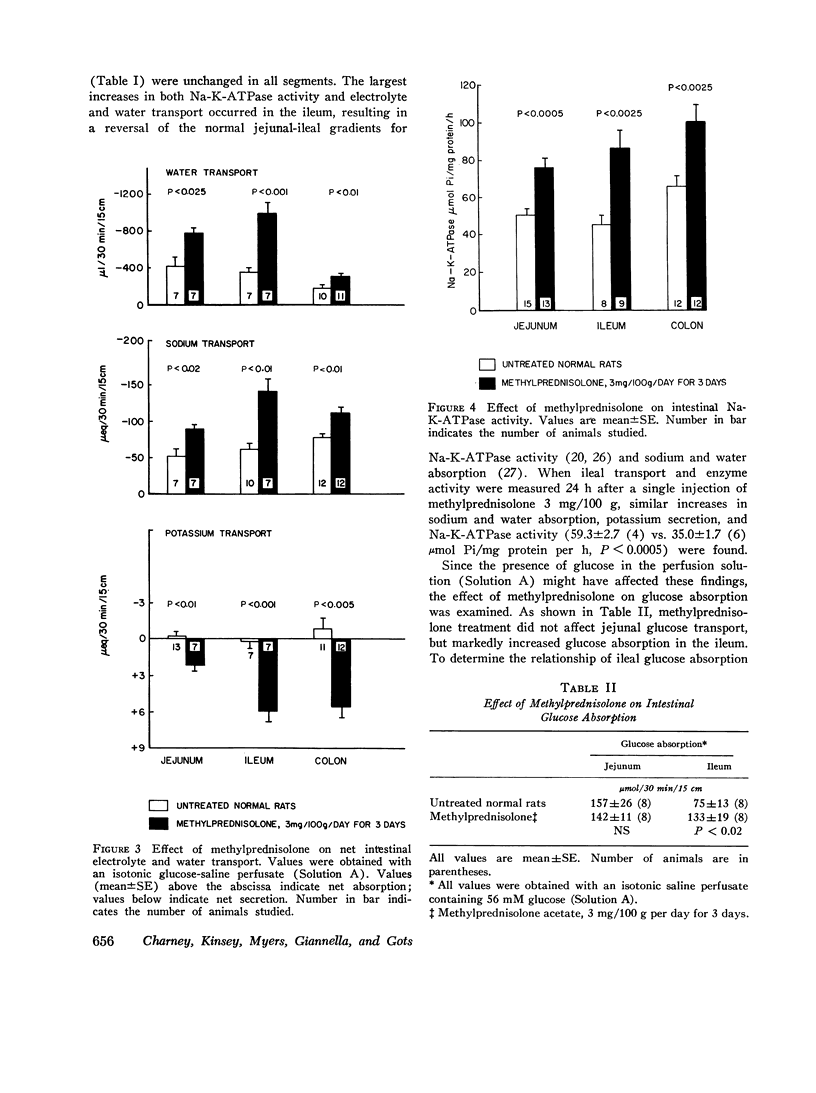
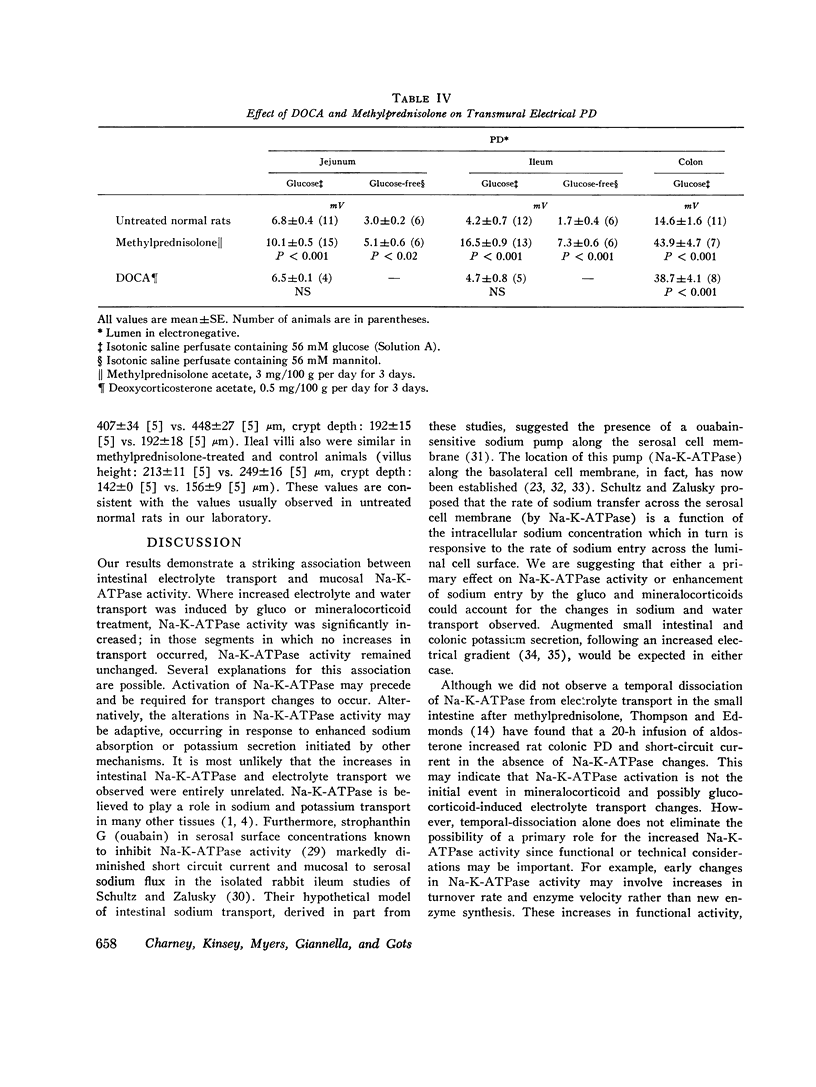
Sodium-potassium-activated adenosine triphosphatase (Na-K-ATPase) is associated with electrolyte transport in many tissues. To help delineate its role in intestinal transport, changes in rat intestinal electrolyte and water transport induced by injecting methylprednisolone acetate 3 mg/100 g or deoxycorticosterone acetate (DOCA) 0.5 mg/100 g per day for 3 days were correlated with changes in Na-K-ATPase activity. Methylprednisolone increased sodium and water absorption, potassium secretion, transmural potential difference, and Na-K-ATPase activity in the jejunum, ileum, and colon. Examination of isolated epithelial cells demonstrated that the jejunal and ileal increase in Na-K-ATPase occurred in both the villus tip and crypermeability, Mg-ATPase, and adenylate cyclase activities were unchanged by methylprednisolone. DOCA increased sodium and water absorption, potassium secretion, transmural potential difference, and Na-K-ATPase activity in the colon alone. Colonic Mg-ATPase and adenylate cyclase activities were unaffected. Jejunal and ileal enzyme activity, electrolyte transport, and permeability were unchanged by DOCA. Methylprednisolone and DOCA were not additive in their effect on colonic Na-K-ATPase activity. Methylprednisolone and DOCA increased electrolyte and water transport and Na-K-ATPase activity concomitantly in specific segments of small intestine and colon. These data are consistent with an important role for Na-K-ATPase in intestinal electrolyte and water transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G., Leblond C. P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am J Anat. 1970 Jan;127(1):15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- BERGER E. Y., KANZAKI G., STEELE J. M. The effect of deoxycorticosterone on the unidirectional transfers of sodium and potassium into and out of the dog intestine. J Physiol. 1960 May;151:352–362. doi: 10.1113/jphysiol.1960.sp006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREITMAN T. R. The feedback inhibition of thymidine kinase. Biochim Biophys Acta. 1963 Jan 8;67:153–155. doi: 10.1016/0006-3002(63)91807-9. [DOI] [PubMed] [Google Scholar]
- Bartter F. C., Delea C. S., Kawasaki T., Gill J. R., Jr The adrenal cortex and the kidney. Kidney Int. 1974 Nov;6(5):272–280. doi: 10.1038/ki.1974.113. [DOI] [PubMed] [Google Scholar]
- Charney A. N., Gots R. E., Giannella R. A. Na+-K+)-stimulated adenosinetriphosphatase in isolated intestinal villus tip and crypt cells. Biochim Biophys Acta. 1974 Nov 15;367(3):265–270. doi: 10.1016/0005-2736(74)90083-2. [DOI] [PubMed] [Google Scholar]
- Charney A. N., Silva P., Besarab A., Epstein F. H. Separate effects of aldosterone, DOCA, and methylprednisolone on renal Na-K-ATPase,. Am J Physiol. 1974 Aug;227(2):345–350. doi: 10.1152/ajplegacy.1974.227.2.345. [DOI] [PubMed] [Google Scholar]
- Charron R. C., Leme C. E., Wilson D. R., Ing T. S., Wrong O. M. The effect of adrenal steroids on stool composition, as revealed by in vivo dialysis of faeces. Clin Sci. 1969 Aug;37(1):151–167. [PubMed] [Google Scholar]
- Cooper H., Levitan R., Fordtran J. S., Ingelfinger F. J. A method for studying absorption of water and solute from the human small intestine. Gastroenterology. 1966 Jan;50(1):1–7. [PubMed] [Google Scholar]
- Diamond J. M. Non-linear osmosis. J Physiol. 1966 Mar;183(1):58–82. doi: 10.1113/jphysiol.1966.sp007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Dowling R. H. Compensatory changes in intestinal absorption. Br Med Bull. 1967 Sep;23(3):275–278. doi: 10.1093/oxfordjournals.bmb.a070571. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J., Richards P. Measurement of rectal electrical potential difference as an instant screening-test for hyperaldosteronism. Lancet. 1970 Sep 26;2(7674):624–627. doi: 10.1016/s0140-6736(70)91397-8. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J. The gradient of electrical potential difference and of sodium and potassium of the gut contents along the caecum and colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):571–588. doi: 10.1113/jphysiol.1967.sp008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratopoulos A. D., Peart W. S., Wilson G. A. The effect of aldosterone on colonic potential difference and renal electrolyte excretion in normal man. Clin Sci Mol Med. 1974 Apr;46(4):489–499. doi: 10.1042/cs0460489. [DOI] [PubMed] [Google Scholar]
- Field M. Intestinal secretion. Gastroenterology. 1974 May;66(5):1063–1084. [PubMed] [Google Scholar]
- Field M., McColl I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am J Physiol. 1973 Oct;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- Fujita M., Matsui H., Nagano K., Nakao M. Asymmetric distribution of ouabain-sensitive ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1971 Apr 13;233(2):404–408. doi: 10.1016/0005-2736(71)90337-3. [DOI] [PubMed] [Google Scholar]
- GILMAN A., KOELLE E., RITCHIE J. M. Transport of potassium ions in therat's intestine. Nature. 1963 Mar 23;197:1210–1211. doi: 10.1038/1971210b0. [DOI] [PubMed] [Google Scholar]
- Hafkenscheid J. C. Occurrence and properties of a (Na + -K + )-activated ATPase in the mucosa of the rat intestine. Pflugers Arch. 1973;338(4):280–294. [PubMed] [Google Scholar]
- Imondi A. R., Balis M. E., Lipkin M. Changes in enzyme levels accompanying differentiation of intestinal epithelial cells. Exp Cell Res. 1969 Dec;58(2):323–330. doi: 10.1016/0014-4827(69)90512-6. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. Physiologic role of sodium-potassium-activated adenosine triphosphatase in the transport of cations across biologic membranes. N Engl J Med. 1968 Feb 1;278(5):253–261. doi: 10.1056/NEJM196802012780506. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. The role of sodium-potassium-activated adenosine triphosphatase in the reabsorption of sodium by the kidney. J Clin Invest. 1967 Dec;46(12):1999–2011. doi: 10.1172/JCI105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Melby J. C. Drug spotlight program: systemic corticosteroid therapy: pharmacology and endocrinologic considerations. Ann Intern Med. 1974 Oct;81(4):505–512. doi: 10.7326/0003-4819-81-4-505. [DOI] [PubMed] [Google Scholar]
- Parkinson D. K., Ebel H., DiBona D. R., Sharp G. W. Localization of the action of cholera toxin on adenyl cyclase in mucosal epithelial cells of rabbit intestine. J Clin Invest. 1972 Sep;51(9):2292–2298. doi: 10.1172/JCI107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. W., Plotkin G. R., Maenza R. M., Solberg L. I., Catlin D. H., Formal S. B. Experimental diarrhea. I. Intestinal water and electrolyte transport in rat salmonella enterocolitis. Gastroenterology. 1971 Jun;60(6):1053–1064. [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Richards P. Clinical investigation of the effects of adrenal corticosteroid excess on the colon. Lancet. 1969 Mar 1;1(7592):437–442. doi: 10.1016/s0140-6736(69)91480-9. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. The difference in sensitivity to cardiac steroids of (Na++K+)-stimulated ATPase and amino acid transport in the intestinal mucosa of the rat and other species. J Physiol. 1970 Jan;206(1):41–60. doi: 10.1113/jphysiol.1970.sp008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Mulholland A. T., Elmslie R. G. Action of aldosterone upon the intestinal transport of potassium, sodium, and water. Gut. 1966 Dec;7(6):686–696. doi: 10.1136/gut.7.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P., Hayslett J. P., Epstein F. H. The role of Na-K-activated adenosine triphosphatase in potassium adaptation. Stimulation of enzymatic activity by potassium loading. J Clin Invest. 1973 Nov;52(11):2665–2671. doi: 10.1172/JCI107460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg H. Proximal and distal tubular function in salt-deprived and in salt-loaded deoxycorticosterone acetate-escaped rats. J Clin Invest. 1973 Feb;52(2):263–272. doi: 10.1172/JCI107182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUELOVE S. C., WITTS L. J. Cortisone and corticotrophin in ulcerative colitis. Br Med J. 1959 Feb 14;1(5119):387–394. doi: 10.1136/bmj.1.5119.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUELOVE S. C., WITTS L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. D., Edmonds C. J. Aldosterone, sodium depletion and hypothyroidism on the ATPase activity of rat colonic epithelium. J Endocrinol. 1974 Sep;62(3):489–496. doi: 10.1677/joe.0.0620489. [DOI] [PubMed] [Google Scholar]
- Torretti J., Hendler E., Weinstein E., Longnecker R. E., Epstein F. H. Functional significance of Na- K-ATPase in the kidney: effects of ouabain inhibition. Am J Physiol. 1972 Jun;222(6):1398–1405. doi: 10.1152/ajplegacy.1972.222.6.1398. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. An electrical method of measuring non-electrolyte permeability. Proc R Soc Lond B Biol Sci. 1969 Mar 18;171(1028):203–225. doi: 10.1098/rspb.1969.0020. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Patterns of non-electrolyte permeability. Proc R Soc Lond B Biol Sci. 1969 Mar 18;171(1028):227–271. doi: 10.1098/rspb.1969.0021. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Knox F. G., Howards S. S., Berliner R. W. Reduced sodium reabsorption by the proximal tubule of Doca-escaped dogs. Am J Physiol. 1969 Apr;216(4):869–875. doi: 10.1152/ajplegacy.1969.216.4.869. [DOI] [PubMed] [Google Scholar]
- Wrong O. Aldosterone and electrolyte movements in the colon. Br Med J. 1968 Feb 10;1(5588):379–380. doi: 10.1136/bmj.1.5588.379-c. [DOI] [PMC free article] [PubMed] [Google Scholar]


