Abstract
Background
The ability to modulate emotional responses, or emotion regulation, is a key mechanism in the development of mood disruptions. Detection of a neural marker for emotion regulation thus has the potential to inform early detection and intervention for mood problems. One such neural marker may be the late positive potential (LPP), which is a scalp-recorded event-related potential reflecting facilitated attention to emotional stimuli. In adults, the LPP is reduced following use of cognitive emotion regulation strategies such as reappraisal. No studies to date have examined the LPP in relation to cognitive emotion regulation in children, and whether the LPP is related to parent-report measures of emotion regulation and mood disruptions.
Methods
To examine this question, high-density electroencephalograph (EEG) was recorded from 20 children (M age = 87.8 months, SD = 18.02; 10 girls) while they viewed unpleasant emotional pictures following either a directed negative or neutral interpretation of the picture.
Results
As predicted, the LPP was smaller following neutral versus negative interpretations at posterior recording sites, except for younger girls (aged 5–6). The timing of this effect was later than that reported in studies with adults. For all children, greater modulation of the LPP by neutral interpretations was associated with reduced anxious-depressed symptoms, whereas larger LPPs for both interpretation types were associated with greater mood symptoms and worse parent-reported emotion regulation.
Conclusions
Results suggest that the LPP may represent a clinically relevant neural marker for emotion regulation and mood disruptions.
Keywords: Emotion regulation, EEG, biomarker, ERP, late positive potential
Although there is no single agreed-upon definition of emotion regulation, broadly speaking emotion regulation refers to the ability to modify the intensity and time course of emotional experiences and expressions in order to meet goals and manage arousal (Thompson, 1994). Among the core capacities that characterize emotion regulation, therefore, is the ability to reduce negative emotional responses; this capacity in particular may be a key mechanism in the development of mood disruptions (Cole, Martin, & Dennis, 2004; Gross & John, 2003). For example, difficulty inhibiting an emotional response and excessive attention towards negative emotional information have been associated with mood and anxiety problems (Compton, 2003; Derryberry & Reed, 2002) and may deplete the resources available for more voluntary aspects of emotion regulation (Bishop, Duncan, Brett, & Lawrence, 2004; Hare et al., 2008; Kieras et al., 2000; Simpson et al., 2000).
The study of emotion regulation in adults has largely focused on cognitive strategies that alter how emotional stimuli are attended to and interpreted. Reappraisal is a cognitive strategy that involves reinterpreting a negative situation or stimulus in a less negative way (Gross & John, 2003; Hajcak & Nieuwenhuis, 2006; Kalisch, Wiech, Herrmann, & Dolan, 2006; Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner & Gross, 2005). One example of reappraisal is interpreting a picture of someone with tears streaming down his face as crying for joy rather than sadness. Reappraisal may be a particularly effective emotion regulation strategy because it serves to change the fundamental meaning and personal significance of an emotional stimulus prior to a negative emotional response being elicited Contrasted with less optimal strategies, such as suppression, changing the personal significance of a negative stimulus is efficient and effective in adults, with few physiological or cognitive costs (Gross & Levenson, 1997; Hajcak & Nieuwenhuis, 2006; Richards & Gross, 2000).
Neuroimaging studies have documented the neural bases of emotion regulation via reappraisal (Kalisch et al., 2006; Lévesque et al., 2004; Urry et al., 2006). For example, when adults reinterpret an unpleasant picture in a less negative way, amygdala activity to the unpleasant picture is reduced while activity of lateral prefrontal cortical areas is enhanced (Ochsner et al., 2002). Although neuroimaging studies provide excellent spatial resolution to detect specific cortical areas associated with successful emotion regulation, techniques such as fMRI rely upon the hemodynamic response, which is tracked second by second. Affective processes, however, also occur on the order of milliseconds, and thus neuroimaging data may lack the temporal resolution to capture some of the earliest processes related to emotion regulation (Banaschewski & Brandeis, 2007; Hajcak, Moser, & Simons, 2006). Scalp-recorded event-related potentials (ERPs), because of their excellent temporal resolution (on the order of milliseconds), are particularly well-suited to measuring rapid affective and cognitive processes associated with emotion regulation. For instance, a recent ERP paper examining the time course of these effects suggests significant changes in neural activity approximately 500 ms following the onset of emotion regulation instructions (Hajcak, Dunning, & Foti, 2009). However, there is a dearth of studies using ERPs to examine cognitive emotion regulation strategies, particularly in children.
Identifying biomarkers for emotion regulation in children has the potential to provide an important adjunct to behavioral measures of emotion regulation by revealing mechanisms in emotion regulation, shedding light on the time course of affective processing, and providing a potential marker for regulatory difficulties that can inform early detection and intervention efforts. The goal of the current study was to examine whether directed reappraisals serve to reduce an ERP associated with affective processing in children, and whether this modulation of the neural response is associated with more adaptive emotion regulation.
The late positive potential (LPP) is an ERP that reflects facilitated attention to emotional stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Schupp et al., 2000; Schupp, Junghöfer, Weike, & Hamm, 2004). The magnitude of the LPP is greater when individuals view emotionally arousing compared to neutral pictures. Recently, it has been demonstrated in adults that the LPP to unpleasant pictures is reduced when a more neutral interpretation of the picture is given (Foti & Hajcak, 2008). The reduced LPP following a directed reappraisal therefore may reflect reduced emotional responses due to emotion regulation instructions. Such reductions may result from shifts in meaning as well as the recruitment of prefrontal cortical resources associated with effective cognitive control (Ochsner & Gross, 2005).
Current theories of child temperament and emotion regulation focus on the dual role of reactivity and regulation (Derryberry & Rothbart, 1997; Posner & Rothbart, 2000). That is, emotion regulation emerges out of a child’s tendency to emotionally react to a stimulus combined with the ability to modulate that response. The LPP, because it is enhanced to affective versus neutral stimuli, reflects the arousal properties of an emotional stimulus, but can also be used to track an individual’s responsiveness to that emotional material over time, including the ability to regulate his or her response. Exploring the time course and topography of the LPP may therefore provide insights into whether appraisal influences relatively early initial reactivity to an emotional stimulus or whether reappraisal has an impact on later and presumably more regulated stages of affective processing (Hajcak et al., 2009, 2006; Hajcak & Nieuwenhuis, 2006). For example, in one study with adults (Foti & Hajcak, 2008), the LPP was reduced when unpleasant pictures were described in neutral rather than negative terms beginning in a relatively early time window (from 400 to 1000 ms), maximal at posterior/superior recording sites. In fact, from 1000 ms, unpleasant pictures preceded by neutral descriptions had identical LPPs to neutral pictures. In addition, there was a spatial shift over time such that the LPP was maximal at posterior-superior recording sites in the early window but shifted to being comparable at posterior and anterior recording sites during the middle and late windows. These spatial-temporal patterns are consistent with other studies of the LPP and cognitive emotion regulation in adults (Hajcak & Nieuwenhuis, 2006; Moser, Hajcak, Bukay, & Simons, 2006), but it is unclear whether children show similar patterns. Overall, then, the LPP can be meaningfully divided into multiple time windows which show differential sensitivity to reappraisal and distinct spatial topography from predominantly posterior-superior sites to equally anterior and posterior sites. Moreover, the early modulation of the LPP suggests that rapid and automatic responses to emotional stimuli can be altered by reappraisal and changes in affective meaning.
The current study is based upon a larger project examining the LPP and emotion regulation in children. In another manuscript based on this project (Hajcak & Dennis, 2009) we document similarities as well as differences between adults and children in the timing and topography of the LPP during passive viewing of unpleasant, pleasant, and neutral emotional pictures (Foti & Hajcak, 2008; Hajcak & Foti, 2008; Hajcak & Nieuwenhuis, 2006; Schupp et al., 2004). Like adults, children showed an enhanced LPP to unpleasant and pleasant compared to neutral pictures by 500 ms; in adults, however, these differences tend to be significant as early as 200–300 ms after a picture is presented. In addition, although both adults and children show a shift over time from predominantly posterior cortical activity to equally distributed anterior-superior activity, changes in children’s early LPP due to the emotional content of pictures included both occipital and parietal recording sites and thus may be more occipitally maximal than in adults. This later and more broadly distributed recruitment of cortical activity during emotion processing may be due in part to ongoing changes in cortical development (Casey, Giedd, & Thomas, 2000; Segalowitz & Davies, 2004).
To date, no studies have examined whether the LPP is sensitive to directed cognitive emotion regulation in children. The LPP may be particularly well suited for examining cognitive emotion regulation in children because of its excellent temporal resolution and relative ease of administration compared to functional neuroimaging. Therefore, the goal of the current study was to build upon our previous demonstration that the LPP in children is sensitive to emotional content during passive picture viewing (Hajcak & Dennis, 2009) by examining changes in the LPP during directed cognitive emotion regulation. We will test whether changes in the LPP due to reappraisal are associated with independent measures of child positive and negative emotion regulation and with internalizing problems including symptoms of anxiety and depression, somatic complaints, and withdrawal.
Because girls have been shown to be at greater risk for mood disruptions later in life (Brooks-Gunn & Warren, 1989), it was also of interest to examine whether boys and girls differ in the ability to recruit cortical resources in the context of emotion regulation. For example, girls may show higher reactivity to negative emotional stimuli both in terms of subjective arousal and in terms of enhanced limbic versus prefrontal brain activity (Hall, Witelson, Szechtman, & Nahmias, 2004). This neural pattern implies that girls compared to boys recruit fewer cognitive control resources during affective processing, and thus regulatory capacity may be reduced. Thus, we tested whether girls compared to boys show greater reactivity and reduced regulation while viewing unpleasant pictures that are described in either negative or neutral terms: greater reactivity would be indicated by larger amplitude LPPs when presented with unpleasant pictures described in negative terms, whereas reduced regulation would be indicated by larger LPPs when presented with unpleasant pictures described in neutral terms. Because ERPs related to effortful cognitive processes, like reappraisal, vary with age due to increased cognitive control in older children (Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006), we also examined whether gender differences in the LPP were moderated by child age.
There were three hypotheses: (a) Neutral compared to negative descriptions of unpleasant pictures will reduce the amplitude of the LPP during picture viewing; (b) Gender differences will emerge such that girls compared to boys will show larger amplitude LPPs – enhanced reactivity may be marked by greater LPPs following negative descriptions and reduced regulation may be marked by greater LPPs following neutral descriptions; these gender effects may be enhanced among younger children due to relatively immature cognitive development; and (c) Reduced modulation of the LPP by neutral interpretations and larger amplitude LPPs overall will be associated with maternal report of greater emotion regulation problems and mood symptoms in children.
Method
Participants
Participants were twenty-five 5- to 10-year-old children (12 male, 13 female). There were ten 5-to 6-year-olds, six 7- to 8-year-olds, and four 9- to 10-year-olds. We chose to include this age range because early school age is a period marked by the cognitive and neural development needed to perform well under the attentional processing demands imposed by the experimental task (Casey et al., 2000). Data from 5 subjects (3 females) were excluded due to movement artifacts leading to poor quality EEG recording. The final sample of 20 was comprised of 10 males (mean age = 85.60 months, SD = 14.95) and 10 females (mean age = 90.00 - months, SD = 21.24). All participants’ families were paid $50 for their participation.
Stimulus materials
A total of 30 developmentally appropriate pictures were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005). These pictures depicted unpleasant scenes (e.g., disaster scenes, frightening animals).1
The task was administered on a Pentium D class computer, using Presentation software (Neurobehavioral Systems, Inc., Albany, CA) to control the presentation and timing of all stimuli. Each picture was displayed in color and occupied the entirety of a 19-in (48.26 cm) monitor. At a viewing distance of approximately 24 in (60.96 cm), each picture occupied approximately 40° of visual angle horizontally and vertically.
Procedure and measures
After consent procedures (parents signed consent documents and children gave verbal consent), electroencephalograph (EEG) sensors were attached and the participant was given detailed task instructions. Following passive viewing of the pictures used in the reappraisal paradigm, participants took part in the 15-minute directed interpretation procedure. Children were instructed, ‘For our next game we’re going to see these same pictures. Listen to the stories and think of the pictures so that they match the stories. Remember to stay still and look at the screen. Try to match the story to the picture.’ Each picture was presented for 2000 ms, following which an audio recording of either a negative or neutral interpretation of the story1 was played for approximately 5–7 seconds while a fixation mark (+) was presented on the blank screen. The same picture was then presented again for 2000 ms. EEG to the second picture presentation was used to generate LPP following negative and neutral interpretations. In this way, participants were provided with interpretations that could influence stimulus meaning rather than be asked to generate an interpretation. Each of the 30 pictures was presented twice along with the same interpretation. However, to prevent a potential confound based on the effect of the initial interpretation type, one block of 30 pictures started with either an initial negative or neutral interpretation, and the other block with the other type of interpretation. Following the initial picture, the order of picture presentation was completely random. Participants received equal numbers of both negative and neutral interpretations (15 each, presented twice). Whether a given picture was associated with a negative or neutral interpretation alternated between subjects.
Psychophysiological recording and data reduction
EEG was continuously recorded using the Active Two BioSemi system (BioSemi, Amsterdam, Netherlands). Recordings were taken from 64 scalp electrodes based on the 10/20 system, as well as two electrodes placed on the left and right mastoids. The electrooculogram (EOG) generated from blinks and eye movements was recorded from four facial electrodes: two approximately 1 cm above and below the subject’s left eye, one approximately 1 cm to the left of the left eye, and one approximately 1 cm to the right of the right eye. As per BioSemi’s design, the ground electrode during acquisition was formed by the Common Mode Sense active electrode and the Driven Right Leg passive electrode.
EEG and EOG signals were sampled at 512 Hz. All data were re-referenced off-line to an average mastoid reference and filtered with a high pass frequency of .2 Hz and a low pass frequency of 30 Hz. The EEG was segmented for each trial, beginning 500 ms before each picture onset and continuing for 2500 ms. The raw EEG epochs were passed through a computerized artifact scan batch to correct artifacts using Brain Electrical Source Analysis version 5.1 (BESA; MEGIS Software GmbH, Munich, Germany). After artifact correction, trials that still included EEG or EOG activity with a voltage step of more than 50 μV between sampling points, or trials with voltages remaining above ± 100 μV, were excluded from further analysis.
ERPs were constructed by separately averaging the two interpretation types (negative, neutral). For each ERP average, the mean amplitude in the 500 ms window prior to picture onset served as the baseline. For analyses of the LPP, the spatial dimensions of the data set were reduced by creating eight clusters of electrodes with five electrodes in each. There were three two-level regional clusters: left versus right hemisphere, anterior versus posterior, and inferior versus superior (Dien & Santuzzi, 2005). The left/right anterior-superior clusters included electrodes AF3/4, F1/2, F3/4, FC1/2, and FC3/4; the left/right anterior-inferior clusters were defined by electrodes AF7/8, F5/6, F7/8, FC5/6, and FT7/8; the left/right posterior-superior clusters included CP1/2, CP3/4, P1/2, P3/4, and PO3/4; the left/right posterior-inferior clusters included CP5/6, P5/6, P7/8, PO7/8, and TP7/8. Figure 1 shows the electrode positions. Based on visual inspection, the LPP was defined as the mean amplitude in three time windows following stimulus onset for the picture after the directed interpretation: 300–600 ms (early window), 600–1000 ms (middle window) and 1000–2000 ms (late window). Trial acceptance rates ranged from 50 to 86% of trials (15–26 trials) for each interpretation type, M trials = 19.85 (66.17%), SD = 2.93 (9.75%). Trial acceptance rates did not differ between negative and neutral interpretations (67% and 71% respectively), and did not differ due to child age (younger children, ages 5–6: 65% and older children, ages 7–10: 67%) or gender (girls 64% and boys 68%).
Figure 1.
Diagram of electrode positions
Maternal report of child emotion regulation
Mothers completed the Emotion Regulation Checklist (Shields & Cicchetti, 1998). This 24-item questionnaire concerns children’s reactions to emotional challenges and regulation of a wide range of emotions. Two subscales were calculated based on measure developers’ guidelines, which have been shown to converge with other measures of emotion regulation (Shields & Cicchetti, 1998): emotion dysregulation (α = .70), which was comprised of items including ‘is prone to angry outbursts/tantrums easily’ and ‘exhibits wide mood swings’; and positive regulation (α = .60), comprised of items including ‘can say when s/he is feeling sad, angry or mad, fearful or afraid.’ Items were rated on a 1–4 scale.
Maternal report of child mood disruptions
The Child Behavior Checklist (Achenbach & Maruish, 1999) was used to measure child mood disruptions. It is a standardized assessment of child internalizing and externalizing problems. Parents responded to 118 items describing child behavior, and rated each item as being not true to very true for their child on a 0 to 2 scale. Given the hypothesized association between emotion regulation and mood disruptions, we examined t-scores from the internalizing and externalizing problems subscales as well as three internalizing subscales: Anxious-Depressed (e.g., feelings of worthlessness, fear or nervousness, and crying), Withdrawn-Depressed Behavior (e.g., lacking energy, enjoying few activities, and being socially withdrawn), and Somatic Complaints (e.g., nightmares, dizziness, and vomiting).
Results
The late positive potential (LPP)
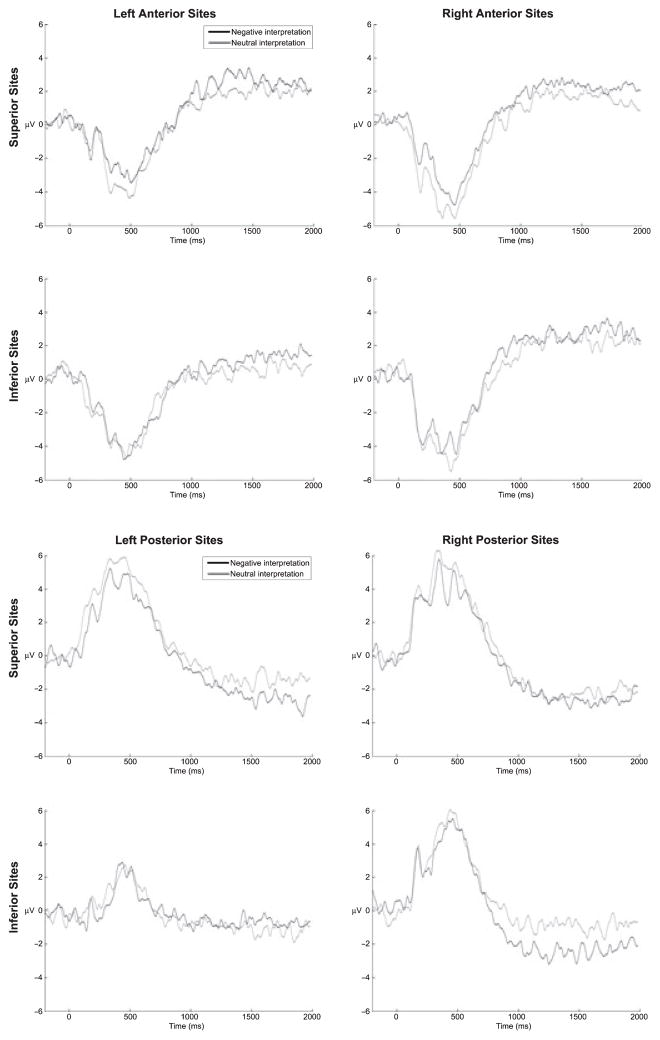
Figure 2 presents the stimulus-locked LPPs at anterior and posterior electrode clusters (the mean amplitude of the 5 electrodes included in each cluster).
Figure 2.
Stimulus-locked ERPs elicited by unpleasant pictures after negative or neutral interpretations at anterior-superior and anterior-inferior electrode clusters (top half of figure), and at posterior-superior and posterior-inferior electrode clusters (bottom half of the figure). Stimulus-onset occurred at 0 ms
Children were divided into younger and older groups based on a median split. There were 10 younger children (aged 5–6) and 10 older children (aged 7–10). There were 5 boys and 5 girls in each age group. A 2 (Child Gender) × 2 (Child Age) 2 (Left Hemisphere–Right Hemisphere) × 2 (Anterior-Posterior) × 2 (Inferior-Superior) × 2 (Negative Interpretation – Neutral Interpretation) repeated measures ANOVA was conducted separately for the early LPP, middle LPP, and late LPP. Greenhouse–Geisser corrections were applied to p values associated with multiple-df, repeated measures comparisons.
Early window (300 to 600 ms)
The overall amplitude of the LPP was larger at posterior and superior recording sites (Anterior-Posterior × Inferior-Superior F(1,16) = 5.12, p < .05, partial η2 = .24). The difference between superior and inferior recording sites, however, was more pronounced in left versus right posterior sensors (Anterior-Posterior × Inferior-Superior × Hemisphere F(1,16) = 5.01, p < .05, partial η2 = .24; all post-hoc tests p < .05). Interpretation Type interacted with Child Gender, Hemisphere, and Anterior-Posterior spatial dimensions (F(1,16) = 4.41, p = .05, partial η2 = .22), but post-hoc comparisons did not reach significance, indicating that LPP in the early window did not reliably vary with Interpretation Type. Other main effects and interactions did not reach significance (all p’s > .05).
Middle window (600 to 1000 ms)
The amplitude of the LPP in the middle window was larger at posterior and superior recording sites (Anterior-Posterior × Inferior-Superior F(1,16) = 7.93, p = .01, partial η2 = .33) and in the right hemisphere (Hemisphere F(1,16) = 5.44, p < .05, partial η2 = .25). There was a significant interaction between Interpretation Type and Child Gender (F(1,16) = 5.32, p < .05, partial η2 = .25), indicating that only boys showed larger LPP amplitudes following negative versus neutral interpretations (t(9) = 2.19, p = .05; boys’ negative M = 7.55, SD = 4.03 versus neutral M = 3.85, SD = 5.23 and girls’ negative M = 1.93, SD = 7.03 versus neutral M = 5.65, SD = 5.17). Counter to prediction, boys versus girls showed larger LPP amplitudes following negative interpretations (t(18) = 2.17, p < .05) and showed a greater difference between negative and neutral interpretations (t(18) = 2.39, p < .05).2
However, the significant Interpretation Type by Child Gender by Child Age interaction (F(1,16) = 5.05, p < .05, partial η2 = .24) showed that these gender differences were attributable to younger girls. Younger girls showed smaller LPP amplitudes following negative interpretations and larger LPP amplitudes following neutral interpretations compared to older girls and younger and older boys (all p’s < .05). When younger girls were excluded from analyses, both boys and girls showed larger LPP amplitudes following negative versus neutral interpretations (t(14) = 2.08, p = .057; negative M = 5.48, SD = 5.14 versus neutral M = 2.75, SD = 4.87) and the differences between girls and boys for LPPs following negative interpretations and for the negative – neutral interpretation difference score did not reach significance (all t’s < 1). There were no significant age or gender differences in LPP amplitudes following neutral interpretations.
In addition, in the middle window Interpretation Type interacted with all spatial dimensions and Child Gender (F(1,16) = 8.81, p < .01, partial η2 = .36), but not with Child Age. Post-hoc tests showed that for boys alone, the LPP was smaller following neutral versus negative interpretations at left posterior-superior recording sites (t(9) = −2.17, p = .05) and at left and right posterior-inferior recording sites (t(9) = −4.18, p < .01 and t(9) = −5.33, p < .01, respectively). Among girls, Interpretation Type (negative versus neutral) did not modulate the LPP in the middle window. As predicted, however, when girls were compared to boys, girls showed significantly diminished LPP difference scores in posterior recording sites (negative – neutral interpretations; t(19) = 5.71, p < .05), but counter to prediction, also showed smaller amplitude LPPs after negative interpretations in the right posterior hemisphere (t(19) = −4.23, p = .05).
In summary, the magnitude of the LPP was greater following negative versus neutral interpretations for posterior recording clusters in the middle window, but only among boys. Moreover, boys versus girls showed greater magnitude LPPs following unpleasant interpretations, and girls showed reduced LPP differences scores; Child Age effects, however, suggest that these gender differences were attributable to younger girls.
Late window (1000 to 2000 ms)
In the late window, the LPP was largest at anterior recording sites (Anterior-Posterior F(1,16) = 29.45, p < .001, partial η2 = .65), particularly in right superior versus left superior sensors (Anterior-Posterior × Inferior-Superior × Hemisphere F(1,16) = 6.21, p < .05, partial η2 = .28; all post-hoc tests p < .05). Other main effects and interactions did not reach significance. Thus, by the late window, the LPP was maximal at right superior-anterior sensors but was not sensitive to interpretation type.
Maternal report of child affective individual differences
Table 1 reports mean scores for child internalizing and externalizing problems, specific mood symptoms (withdrawn, anxious-depressed, and somatic complaints), and emotion regulation (positive regulation and dysregulation). Because boys consistently showed the expected modulation of the LPP via reappraisal (in the middle window), we examined whether boys and girls differed in terms of maternal report of child mood problems and emotion regulation. No significant gender differences emerged.
Table 1.
Descriptive statistics for mood disruptions and emotion regulation variables
| M | SD | Range | |
|---|---|---|---|
| Internalizing | 46.80 | 10.71 | 29–65 |
| Externalizing | 50.75 | 12.75 | 28–69 |
| Anxious-depressed | 52.60 | 3.46 | 50–62 |
| Withdrawn | 51.95 | 5.03 | 50–70 |
| Somatic complaints | 54.45 | 6.56 | 50–70 |
| Positive regulation | 3.29 | .47 | 2–4 |
| Dysregulation | 2.12 | .54 | 1.13–3.13 |
Associations between LPP and emotional well-being
To examine associations between the LPP and mood disruptions or emotion regulation, LPP amplitudes after negative or neutral interpretations and negative – neutral interpretation difference scores in the early, middle, and late windows were correlated with maternal report of child affective individual differences.
Although the effect of interpretation type on the LPP was not consistent across all children or all time windows, the difference score may still reflect meaningful individual differences in children’s reactivity to and regulation of their response to affectively charged pictures and thus may be related to emotional well-being. In addition, examining correlations with LPP amplitudes for each interpretation type separately will allow us to capture affective processing under distinct emotional conditions and aid interpretation of correlations between the difference score and emotional well-being.
Based on analyses of the spatial distribution of the LPP, average LPP amplitudes were calculated for the early and middle windows at posterior-superior recording sites and for the late window at anterior-superior recording sites. Averages were computed separately for negative and neutral interpretations. This yielded a total of six scores (3 levels of time window × 2 levels of interpretation type) and three difference scores (negative – neutral interpretation for each time window). Outliers (scores greater or less than two SD from the mean) were excluded from analyses. Zero-order correlations are reported in Table 2.
Table 2.
Correlations between mean LPP amplitudes and maternal report of child mood disruptions and emotion regulation LPP Child mood disruptions and emotion regulation
| LPP | Child mood disruptions and emotion regulation |
||||||
|---|---|---|---|---|---|---|---|
| Internalizing problems | Externalizing problems | Anxious/depressed | Somatic complaints | Withdrawn/depressed | Positive regulation | Dysregulation | |
| Early window | |||||||
| Neutral interpretation | .28 | .25 | .49* | .05 | −.08 | .18 | .08 |
| Negative interpretation | .06 | .13 | .20 | −.09 | −.05 | .19 | .02 |
| Negative – Neutral | −.37 | −.23 | −.52* | −.20 | .06 | −.05 | −.09 |
| Middle window | |||||||
| Neutral interpretation | .14 | .11 | .30 | .04 | −.05 | .12 | .23 |
| Negative interpretation | −.13 | .28 | −.16 | −.31 | .27 | −.44* | .54* |
| Negative – Neutral | −.21 | .15 | −.35 | −.29 | .25 | −.44* | .28 |
| Late window | |||||||
| Neutral interpretation | .39 | .22 | .24 | .11 | .48* | −.07 | .15 |
| Negative interpretation | .46* | .21 | .12 | .55* | .26 | −.15 | .14 |
| Negative – Neutral | .04 | −.02 | −.10 | .35 | −.19 | −.06 | −.02 |
p < .05.
During the early window, larger LPP amplitudes following neutral interpretations were associated with greater anxious-depressed symptoms. Larger difference scores (LPP negative – LPP neutral), on the other hand, were associated with reduced anxious-depressed symptoms.
During the middle time window, larger LPP amplitudes following negative interpretations were associated with reduced positive regulation and greater dysregulation. Larger difference scores were also associated with reduced positive regulation. This effect appeared to be due to changes in the LPP following negative interpretations, since the LPP following neutral interpretations was not significantly correlated with positive regulation.
During the late time window, larger LPP amplitudes following negative interpretations were associated with more somatic complaints, whereas higher LPP amplitudes following neutral interpretations were associated with greater withdrawal.
Discussion
This was the first study to demonstrate that a cognitive emotion regulation strategy modulated the LPP in children. Specifically, the LPP to unpleasant pictures was decreased when unpleasant pictures were described in a more neutral versus negative manner. This effect was significant in the middle window for all children except younger girls (aged 5–6). In addition, the amplitude of the LPP following directed interpretations was associated with child emotional well-being: larger amplitudes were correlated with increased symptoms of anxiety, depression, somatic complaints, and withdrawal, and greater signs of emotion dysregulation. Taken together, results suggest that the LPP during cognitive emotion regulation may be a clinically relevant biomarker for emotional reactivity and regulation capacities related to mood disruptions. In addition, although this study’s small sample size limited our ability to explore the effects of gender and age and requires that we interpret findings with caution, the LPP may be sensitive to gender and age differences in the development and pathophysiology of reactivity, regulation, and mood problems. The goal of this study was to provide a preliminary examination of the LPP as a new biomarker for emotion regulation in children. Future research should examine links between the LPP and emotional well-being and emotional disruptions with larger samples, clinical samples, and in studies with a longitudinal design.
The timing of changes in the LPP due to directed interpretations showed both similarities and differences with adults (Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006; Moser et al., 2006). In the current study, only the LPP during the middle window (600 to 1000 ms) was decreased after neutral versus negative interpretations. Adults appear to show modulation of the LPP by reappraisal both as early as 400 ms and as late as 3000 ms (Foti & Hajcak, 2008). However, in the current study, the LPP was measured only up to 2000 ms, and thus the presence of later sensitivity of the LPP to cognitive emotion regulation cannot be evaluated. Compared to previous studies with adults, then, children (except for younger girls) showed a similar decrease in the LPP following neutral interpretations, although the timing of this effect was later than in adults and limited to the 600–1000 ms window. This suggests that children may have greater difficulty initiating shifts in meaning via appraisal at the earlier and relatively automatic stages of affective processing. That is, they may be less practiced and less efficient in using cognitive reappraisal. In addition, children may not be able to sustain these shifts in meaning through to more elaborated and effortful stages of processing. This question, however, requires further study, including a comparison sample of adults and extended measurement into later stages of the LPP in children.
In terms of general spatial-temporal patterns, children, like adults, showed maximal LPP amplitudes at posterior-superior recording sites in early time windows (Foti & Hajcak, 2008; Hajcak, Dunning, & Foti, 2007; Hajcak & Nieuwenhuis, 2006), but by the late window (1000–2000 ms), the LPP was broadly distributed across anterior recording sites. Because cognitive emotion regulation did not modulate the LPP when it was maximal in anterior recording sites, it may be that children show reduced recruitment of prefrontally mediated cognitive control resources. Although the current study did not detect age-related differences in the anterior distribution of the LPP, other studies have shown age-related changes in anterior ERPs, suggesting that older children are better able to recruit cognitive resources in the service of emotion regulation (Ladouceur, Dahl, & Carter, 2007; Lewis et al., 2006; Lewis, Todd, & Honsberger, 2007).
The sample size of the current study was small and thus gender effects should be interpreted with caution. It was hypothesized that, if girls are more reactive to and less efficient at regulating their responses to unpleasant emotional stimuli (Hall et al., 2004), then this might be reflected in greater LPP amplitudes in response to both negative and neutral interpretations. However, results only supported this hypothesis in part, and just for younger girls. Compared to younger girls, both boys and older girls showed larger LPP amplitudes after negative interpretations in the middle time window, suggesting greater attentional processing and reactivity. On the other hand, as predicted, younger girls showed relatively larger LPP amplitudes to pictures following neutral interpretations and diminished LPP difference scores (negative – neutral interpretations) suggesting reduced regulation.
Thus, overall, younger girls showed smaller LPPs to unpleasant interpretations, larger LPPs to neutral interpretations, and reduced LPP difference scores compared to both boys and older girls. This raises several possibilities. One is that younger girls might have experienced the pictures and their negative interpretations as more distressing and thus spontaneously shifted their attention away from the task, resulting in smaller LPPs.
In addition, younger girls may have had difficulty reappraising via the neutral interpretations, and thus LPPs remained larger compared to the other children. This may indicate relative cognitive immaturity among this group of 5–6-year-old girls, or just decreased task engagement, both of which would decrease their ability to benefit from reappraisal. Future research should examine cognitive emotion regulation applied to pleasant, unpleasant, and neutral emotional picture to further explore this possibility. Although the interaction between child gender and age emerged during the middle time window, it was not consistent (e.g., child age did not moderate the interaction between child gender and spatial dimensions of the LPP). Thus, to fully evaluate age and gender effects, it will be critical for future research to include larger samples of girls across a range of ages. In the current study, although results are suggestive, we did not have adequate power to fully examine neural and behavioral differences between girls and boys.
Correlations with maternal report of child mood disruptions and emotion regulation provide further evidence that the LPP is sensitive to clinically relevant affective processes. Modulation of the LPP by neutral interpretations was consistent only for boys in the middle time window; thus correlations between difference scores and emotional well-being for all children and across time windows should be interpreted with caution. However, those boys and girls who showed relatively smaller LPPs to neutral versus negative interpretations in the early time window showed decreased maternal report of anxious-depressed symptoms. LPP amplitudes to both neutral and negative interpretations were also significantly correlated with child emotional well-being: The LPP during the early window was associated with anxious-depressed symptoms, the LPP during the middle window with emotion regulation and dysregulation, and the LPP during the late window with somatic complaints and withdrawal. Interestingly, in the middle window, greater difference scores were correlated with reduced positive regulation, although in this case larger difference scores were likely due to greater LPPs to negative interpretations rather than effective down-regulation of the LPP via neutral interpretations. In addition, no correlations with externalizing problems emerged, suggesting specific associations with affective individual differences.
The specificity of these associations between each time window and distinct outcomes is intriguing. It may be that enhanced affective processing in earlier and more automatic stages (the early window) is a core mechanism in the development of mood-related symptoms such as anxiety; in contrast, more elaborated and conscious affective processing in later time windows may be more relevant to symptoms involving behavioral regulation (somatic complaints and withdrawal). Moreover, it seems that the LPP during the middle window, when effects of interpretation type on LPP emerged, were specifically associated with the types of emotion regulation behaviors that parents observe in their children. Future research should examine the unique function of affective processing from 600 to 1000 ms in relation to the ability to adapt to emotional challenges.
To our knowledge, this is among the first studies to have documented associations between the LPP and emotion regulation, although previous research has shown enhanced LPPs in clinical populations, such as those with specific phobia (Miltner et al., 2005). Because of the small sample size, these findings are preliminary and require further replication. However, these early results highlight the potential of the LPP as a biomarker for emotion regulation that relates to risk for specific mood and behavioral problems. Future research should systematically examine the LPP in relation to a range of clinical symptoms and multiple emotion regulation strategies. This is an important goal because such research might aid in the identification of mechanisms of emotion regulation that create risk for specific disorders (Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Lewis et al., 2006; Stieben et al., 2007).
The current study used a method similar to that used in studies of reappraisal (Ochsner et al., 2002; Ochsner & Gross, 2005). One important difference was that the interpretation in the current study was not truly antecedent focused (Gross, 1998) because pictures were presented prior to stories so that children could better attend to and understand the interpretations. This method, however, does capture the ability to volitionally interrupt an ongoing emotional response, which may be particularly compromised among children who are impulsive or prone to mood disorders. Future research should compare a range of antecedent- and response-focused strategies, including reappraisal, directed attention, and suppression in order to examine whether this methodological factor influenced results.
Because we did not gather self-report data on children’s mood, we are missing an important source of information about children’s subjective affective vulnerabilities. Another limitation was that, in order to keep the task brief so as to not overtax young children’s attentional capacities, there was not a neutral picture viewing condition; thus it is unclear whether the LPP showed valence and arousal-dependent modulation. Also, although the stimuli used in this study have been rated by adults, children did not subjectively rate the stimuli. Piloting indicated children at this age have difficulty rating arousal and valence using the self-assessment mannequin or SAM rating system. Thus it is not clear that unpleasant pictures were perceived as such by the children – although previous findings suggest that they do (cf., Hajcak & Dennis, 2009).
In summary, this is the first study to examine the sensitivity of the LPP to directed cognitive emotion regulation in young children. The excellent temporal sensitivity of the LPP allowed us to detect potential similarities and differences between children and adults in the recruitment of early and later cognitive mechanisms in emotion regulation. Future research should examine whether the LPP is sensitive to individual differences in the development of emotion and emotion regulation over time, and whether it reflects mechanisms in the development of mood and anxiety disorders. Results of this early study hold the promise of identifying the LPP as a clinically useful measure to detect early risk or resilience for problems with emotion regulation.
Key points.
It is known that the late positive potential (LPP) is enhanced when viewing emotionally arousing stimuli and reduced when adults use cognitive emotion regulation strategies, such as reappraisal.
The current study is the first to document in children that manipulating the meaning of unpleasant stimuli via reappraisal reduces the LPP to those unpleasant stimuli.
The current study is also the first to show that the degree to which the LPP is sensitive to reappraisal is related to individual differences in emotion dysregulation, mood problems, and internalizing problems.
Clinical implications include the use of the LPP as a biomarker for affective risk, which might inform early detection and intervention.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health (NIH) Grants 5T34 GM007823, 5K01 MH075764-02, and 5S06GM060654-04, the latter two awarded to T.D. This publication was also made possible by Grant RR03037 from the National Center for Research Resources (NCRR), a component of the NIH.
Abbreviations
- LPP
late positive potential
- ERP
event-related potential
Footnotes
Conflict of interest statement: No conflicts declared.
Online Appendix A presents negative and neutral interpretations for each picture. The following IAPS pictures were used: 1050, 1120, 1201, 1300, 1321, 1930, 2120, 2130, 2688, 2780, 2810, 2900, 3022, 3230, 3280, 5970, 6190, 6300, 7380, 9050, 9250, 9404, 9421, 9470, 9480, 9490, 9582, 9594, 9600, and 9611.
Online Appendix B shows the scalp distribution of the negative minus neutral interpretation difference waveforms for boys (top) and girls (bottom) in the time window of the early LPP, middle LPP, and late LPP (from left to right).
The following supplementary material is available for this article:
Appendix A. Negative and neutral interpretations for each picture (Word document)
Appendix B. Scalp distribution of the negative minus neutral interpretation difference waveforms (Word document)
This material is available as part of the online article from:
http://www.blackwell-synergy.com/doi/abs/10.1111/j.1469-7610.2009.02168.x
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Achenbach TM, Maruish ME. The Child Behavior Checklist and related instruments. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 429–466. [Google Scholar]
- Banaschewski T, Brandeis D. Annotation: What electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. Journal of Child Psychology and Psychiatry. 2007;48:415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP. Biological and social contributions to negative affect in young adolescent girls. Child Development. 1989;60:40–55. doi: 10.1111/j.1467-8624.1989.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Compton RJ. The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews. 2003;2:115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225– 236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Dien J, Santuzzi A. Application of repeated measures ANOVA to high-density ERP datasets: A review and tutorial. In: Handy TC, editor. Event-related potentials: A methods handbook. Cambridge, MA: The MIT Press; 2005. pp. 57–82. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Neural response to emotional pictures is unaffected by concurrent task difficulty: An event-related potential study. Behavioral Neuroscience. 2007;121:1156–1162. doi: 10.1037/0735-7044.121.6.1156. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Automatic and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology. 2009;120:505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective and Behavioral Neuroscience. 2006;6:291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hall GBC, Witelson SF, Szechtman H, Nahmias C. Sex differences in functional activation patterns revealed by increased emotion processing demands. Neuroreport. 2004;15:219–223. doi: 10.1097/00001756-200402090-00001. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go–nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18:1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieras DE, Meyer DE, Ballas JA, Lauber EJ, Braver TS, Cohen JD, et al. VII: Computational modeling of control. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance. XVIII. Cambridge, MA: The MIT Press; 2000. pp. 679–751. [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2005. [Google Scholar]
- Lévesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Todd RM, Honsberger MJM. Event-related potential measures of emotion regulation in early childhood. Neuroreport: For Rapid Communication of Neuroscience Research. 2007;18:61–65. doi: 10.1097/WNR.0b013e328010a216. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Trippe RH, Krieschel S, Gutberlet I, Hecht H, Weiss T. Event-related brain potentials and affective responses to threat in spider/snake-phobic and non-phobic subjects. International Journal of Psychophysiology. 2005;57:43–52. doi: 10.1016/j.ijpsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: The cognitive costs of keeping one’s cool. Journal of Personality and Social Psychology. 2000;79:410–424. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: An electrophysiological strategy. Brain and Cognition. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: The contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27:381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Ongür D, Akbudak E, Conturo TE, Ollinger JM, Snyder AZ, et al. The emotional modulation of cognitive processing: An fMRI study. Journal of Cognitive Neuroscience. 2000;12:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59:25. [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.