Abstract
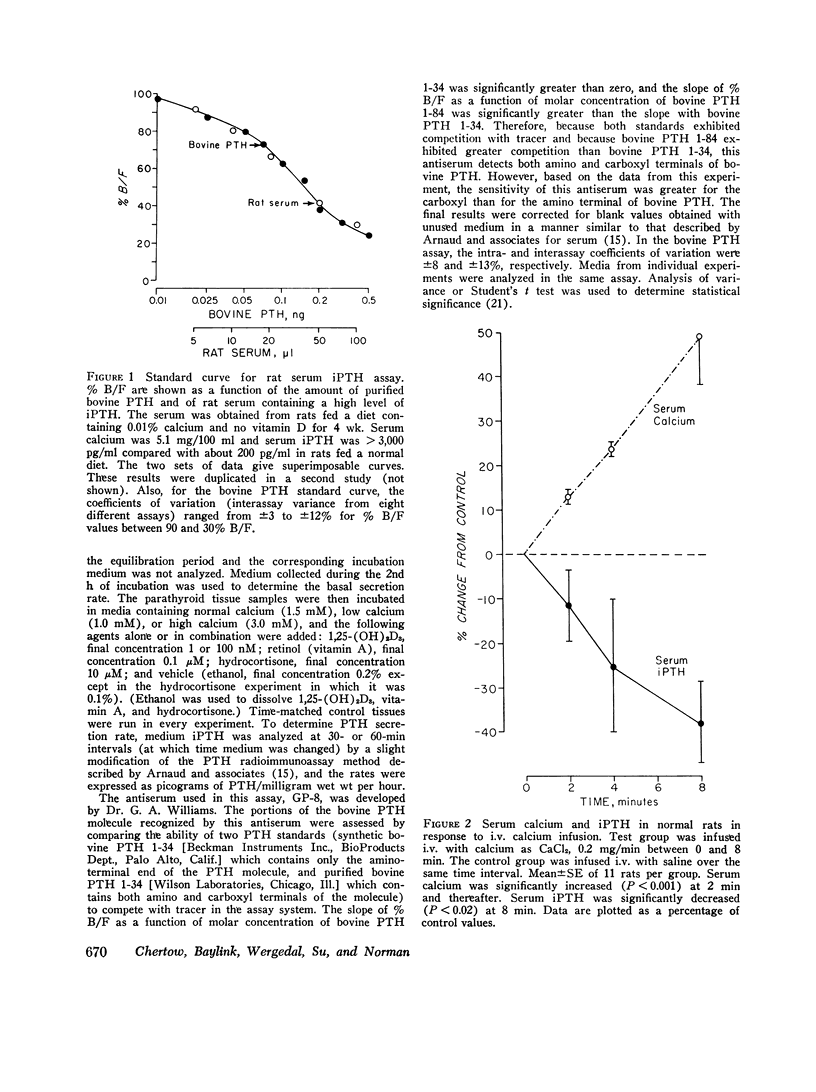
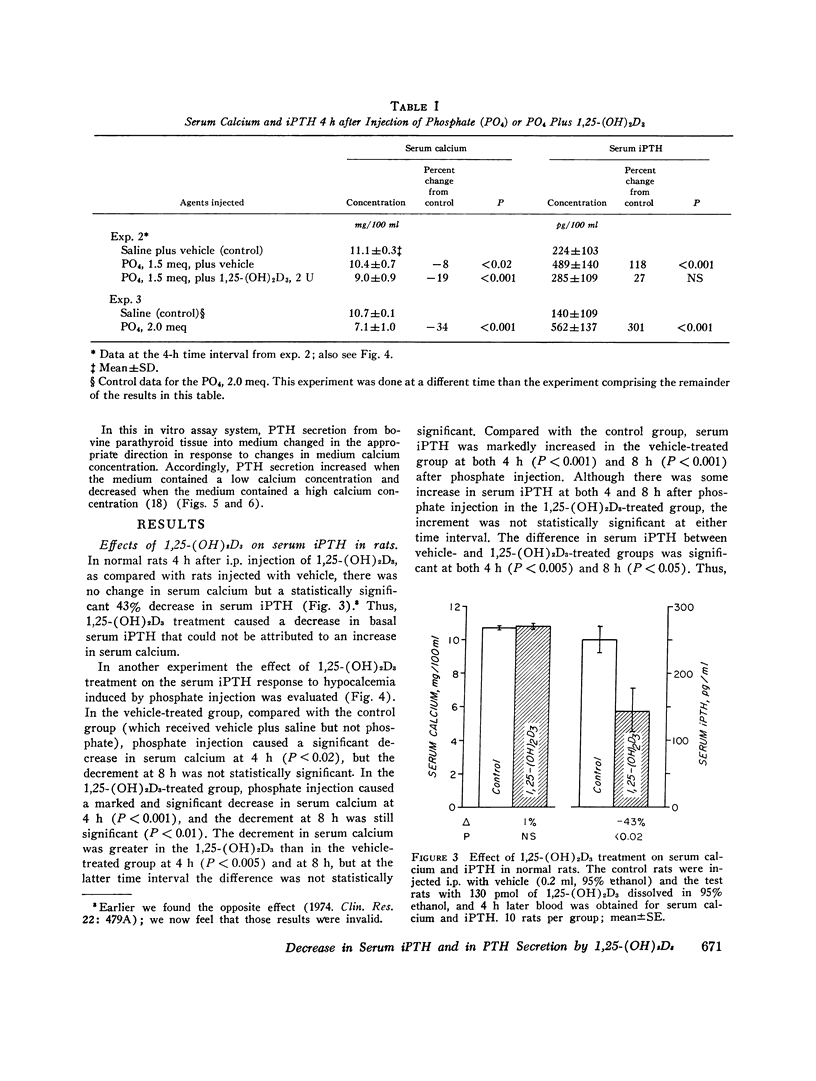
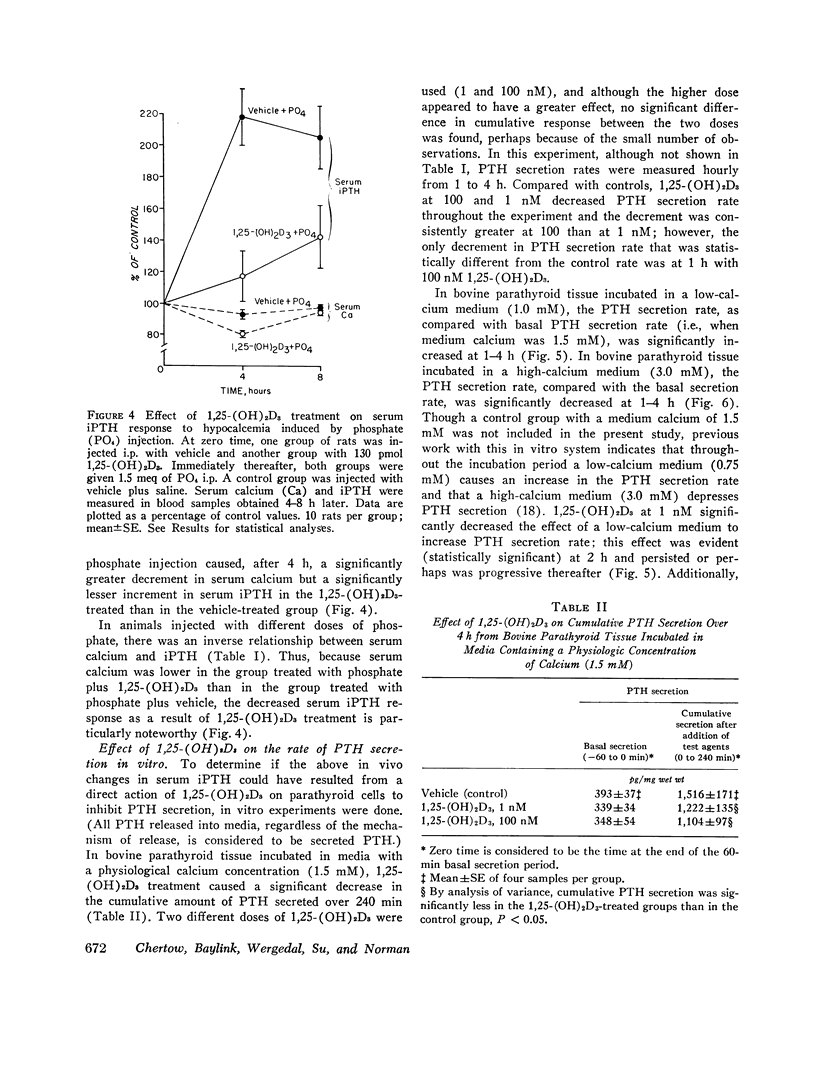
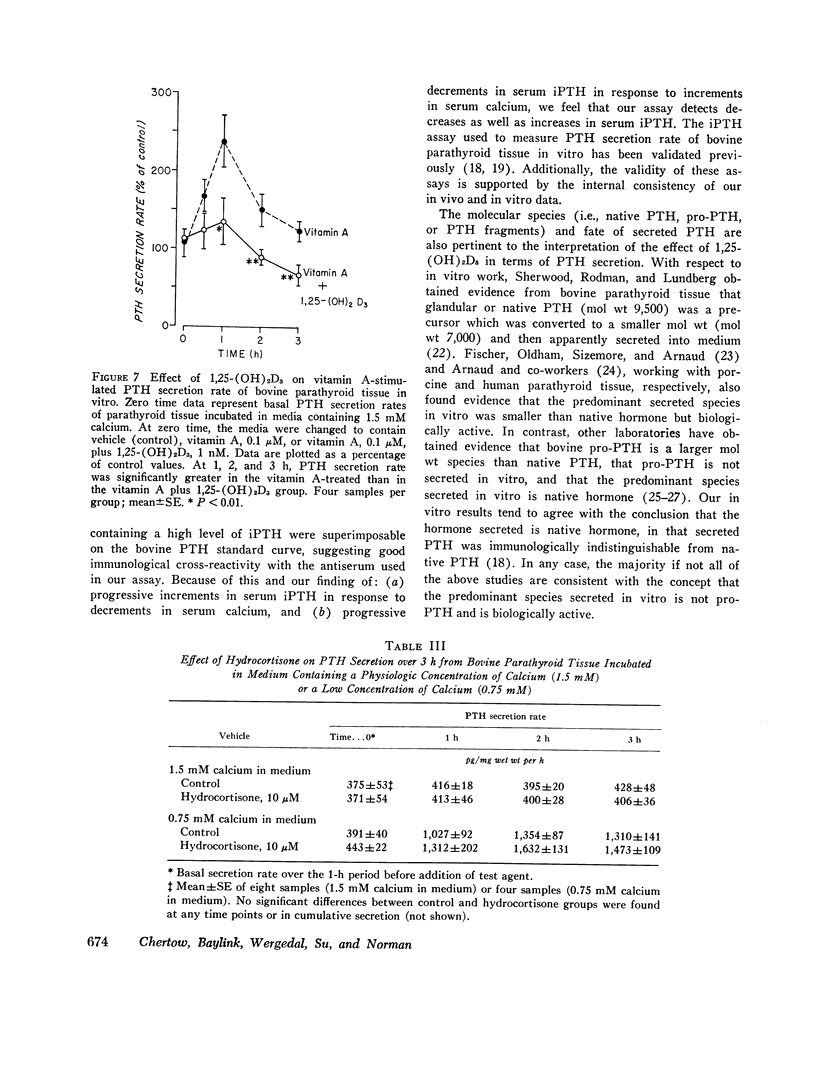
The present study determined the effects of 1,25-dihydroxycholecalciferol on serum immunoactive parathyroid hormone and on parathyroid hormone secretion in vitro. Rats injected i.p. with 1,25-dihydroxycholecalciferol, 130 pmol (2 U)/140 g body wt, which is probably a physiologic dose, had a significant 43% decrease in serum immunoreactive parathyroid hormone at 4 h. In addition, this dose of 1,25-dihydroxycholecalciferol inhibited the serum immunoreactive parathyroid hormone response to hypocalcemia induced by phosphate injection. Because the increment in serum immunoreactive parathyroid hormone was less but the decrement in serum calcium more in phosphate plus 1,25-dihydroxycholecalciferol-treated than in phosphate plus vehicle-treated rats, the impaired serum immunoreactive parathyroid hormone response to 1,25-dihydroxycholecalciferol could not be attributed to the change in serum calcium. In studies of parathyroid hormone secretion from bovine parathyroid tissue in vitro, the concentration of 1,25-dihydroxycholecalciferol used for most experiments was 1nM, which is in the range found in rat serum. 1,25-Dihydroxycholecalciferol at 1 or 100 nM significantly inhibited parathyroid hormone secretion when medium calcium concentration was normal (1.5 mM), high (3.0 mM), and low (1.0 mM). Maximum inhibition ranged from 19 to 74%; inhibition was generally seen after 2 h of incubation; and inhibition was sustained or progressive thereafter. Vitamin A, 0.1 muM, caused a marked stimulation of parathyroid hormone secretion. 1,25-Dihydroxycholecalciferol at 1 nM markedly reduced (44%) the effect of vitamin A to stimulate parathyroid hormone secretion. This effect of 1,25-dihydroxycholecalciferol was maximal at 1 h and persisted thereafter. Another steroid, hydrocortisone, 10 muM, did not inhibit parathyroid hormone secretion, suggesting that the 1,25-dihydroxycholecalciferol effect was not a nonspecific inhibitory effect on parathyroid cells. Because other workers have shown that parathyroid hormone directly stimulates 1,25-dihydroxycholecalciferol secretion, our results are consistent with the concept that there is a feedback loop where parathyroid hormone directly stimulates secretion of 1,25-dihydroxycholecalciferol, which in turn directly inhibits secretion of parathyroid hormone.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Sherwood L. M. Regulation of parathyroid hormone secretion by adrenyl cyclase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):396–401. doi: 10.1016/s0006-291x(72)80064-0. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D. Hyperparathyroidism and renal failure. Kidney Int. 1973 Aug;4(2):89–95. doi: 10.1038/ki.1973.87. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D., Sizemore G. W., Oldham S. B., Fischer J. A., Tsao H. S., Littledike E. T. Human parathyroid hormone: glandular and secreted molecular species. Am J Med. 1971 May;50(5):630–638. doi: 10.1016/0002-9343(71)90118-5. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D., Tsao H. S., Littledike T. Radioimmunoassay of human parathyroid hormone in serum. J Clin Invest. 1971 Jan;50(1):21–34. doi: 10.1172/JCI106476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylink D., Stauffer M., Wergedal J., Rich C. Formation, mineralization, and resorption of bone in vitamin D-deficient rats. J Clin Invest. 1970 Jun;49(6):1122–1134. doi: 10.1172/JCI106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Iodoinsulin used to determine specific activity of iodine-131. Science. 1966 Apr 8;152(3719):205–207. doi: 10.1126/science.152.3719.205. [DOI] [PubMed] [Google Scholar]
- Billitteri A., Raoul Y. Antagonisme des vitamines A et D au niveau des lysosomes: recherches chez la souris in vivo. C R Seances Soc Biol Fil. 1968 Oct 19;162(3):657–661. [PubMed] [Google Scholar]
- Billitteri A., Raoul Y. Antagonisme entre les vitamines A et D au niveau des membranes. Bibl Nutr Dieta. 1969;13:162–170. [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Chertow B. S., Buschmann R. J., Henderson W. J. Subcellular mechanisms of parathyroid hormone secretion: ultrastructural changes in response to calcium, vitamin A, vinblastine, and cytochalasin B. Lab Invest. 1975 Feb;32(2):190–200. [PubMed] [Google Scholar]
- Chertow B. S., Williams G. A., Kiani R., Stewart K. L., Hargis G. K., Flayter R. L. The interactions between vitamin A, vinblastine, and cytochalasin B in parathyroid hormone secretion. Proc Soc Exp Biol Med. 1974 Oct;147(1):16–19. doi: 10.3181/00379727-147-38272. [DOI] [PubMed] [Google Scholar]
- Chu L. L., MacGregor R. R., Anast C. S., Hamilton J. W., Cohn D. V. Studies on the biosynthesis of rat parathyroid hormone and proparathyroid hormone: adaptation of the parathyroid gland to dietary restriction of calcium. Endocrinology. 1973 Oct;93(4):915–924. doi: 10.1210/endo-93-4-915. [DOI] [PubMed] [Google Scholar]
- Corradino R. A. Embryonic chick intestine in organ culture. A unique system for the study of the intestinal calcium absorptive mechanism. J Cell Biol. 1973 Jul;58(1):64–78. doi: 10.1083/jcb.58.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELUCA H. F., ENGSTROM G. W., RASMUSSEN H. The action of vitamin D and parathyroid hormone in vitro on calcium uptake and release by kidney mitochondria. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1604–1609. doi: 10.1073/pnas.48.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. A., Oldham S. B., Sizemore G. W., Arnaud C. D. Calcium-regulated parathyroid hormone peptidase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2341–2345. doi: 10.1073/pnas.69.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., DeLuca H. F. Metabolism of 1,25-dihydroxycholecalciferol in the rat. J Clin Invest. 1972 Nov;51(11):2900–2906. doi: 10.1172/JCI107114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Powell D., Murray T. M., Mayer G. P., Potts J. T., Jr Parathyroid hormone: secretion and metabolism in vivo. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2986–2991. doi: 10.1073/pnas.68.12.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Tregear G. W., Stevens E. D., Dee P. C., Potts J. T., Jr Radioimmunoassay for proparathyroid hormone. Endocr Res Commun. 1974;1(1):1–17. doi: 10.1080/07435807409053812. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Spierto F. W., MacGregor R. R., Cohn D. V. Studies on the biosynthesis in vitro of parathyroid hormone. II. The effect of calcium and magnesium on synthesis of parathyroid hormone isolated from bovine parathyroid tissue and incubation medium. J Biol Chem. 1971 May 25;246(10):3224–3233. [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Studies on the mechanism of action of calciferol VII. Localization of 1,25-dihydroxy-vitamin D3 in chick parathyroid glands. Biochem Biophys Res Commun. 1975 Feb 17;62(4):781–788. doi: 10.1016/0006-291x(75)90391-5. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):803–804. doi: 10.1073/pnas.68.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Tulloch B. R., Hope-Gill H., Clarke P. V., Vydelingum N., Fraser T. R. Mode of insulin action. Lancet. 1975 Jan 18;1(7899):144–147. doi: 10.1016/s0140-6736(75)91435-x. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T. FAT-SOLUBLE VITAMINS AND BIOLOGICAL MEMBRANES. Nature. 1964 Oct 10;204:156–160. doi: 10.1038/204156a0. [DOI] [PubMed] [Google Scholar]
- Lumb G. A., Stanbury S. W. Parathyroid function in human vitamin D deficiency and vitamin D deficiency in primary hyperparathyroidism. Am J Med. 1974 Jun;56(6):833–839. doi: 10.1016/0002-9343(74)90812-2. [DOI] [PubMed] [Google Scholar]
- Marcus R., Aurbach G. D. Adenyl cyclase from renal cortex. Biochim Biophys Acta. 1971 Aug 20;242(2):410–421. doi: 10.1016/0005-2744(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Martin T. J., Greenberg P. B., Melick R. A. Nature of human parathyroid hormone secreted by monolayer cell cultures. J Clin Endocrinol Metab. 1972 Feb;34(2):437–440. doi: 10.1210/jcem-34-2-437. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Henry H. The role of the kidney and vitamin D metabolism in health and disease. Clin Orthop Relat Res. 1974 Jan-Feb;(98):258–287. doi: 10.1097/00003086-197401000-00032. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Midgett R. J., Myrtle J. F., Nowicki H. G. Studies on calciferol metabolism. I. Production of vitamin D metabolite 4B from 25-OH-cholecalciferol by kidney homogenates. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1082–1087. doi: 10.1016/0006-291x(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Midgett R. J., Myrtle J. F., Nowicki H. G. Studies on calciferol metabolism. I. Production of vitamin D metabolite 4B from 25-OH-cholecalciferol by kidney homogenates. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1082–1087. doi: 10.1016/0006-291x(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., DeLuca H. F. Regulation of vitamin D metabolism and function. Physiol Rev. 1973 Apr;53(2):327–372. doi: 10.1152/physrev.1973.53.2.327. [DOI] [PubMed] [Google Scholar]
- Ponchon G., DeLuca H. F. The role of the liver in the metabolism of vitamin D. J Clin Invest. 1969 Jul;48(7):1273–1279. doi: 10.1172/JCI106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Holick M. F., DeLuca H. F. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972 Feb 18;175(4023):768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre G. V., Habener J. F., Powell D., Tregear G. W., Potts J. T. Parathyroid Hormone in Human Plasma: IMMUNOCHEMICAL CHARACTERIZATION AND BIOLOGICAL IMPLICATIONS. J Clin Invest. 1972 Dec;51(12):3163–3172. doi: 10.1172/JCI107143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood L. M., Rodman J. S., Lundberg W. B. Evidence for a precursor to circulating parathyroid hormone. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1631–1638. doi: 10.1073/pnas.67.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R., Yalow R. S. Heterogeneity of parathyroid hormone. Clinical and physiologic implications. J Clin Invest. 1973 Aug;52(8):1958–1971. doi: 10.1172/JCI107380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E., Goodman D. S. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971 Oct;50(10):2159–2167. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1040–1044. doi: 10.1073/pnas.71.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Frank H., DeLuca H. F. Biological activity of 1,25-dihydroxyvitamin D3 in the rat. Endocrinology. 1973 Feb;92(2):417–422. doi: 10.1210/endo-92-2-417. [DOI] [PubMed] [Google Scholar]
- Williams G. A., Hargis G. K., Bowser E. N., Henderson W. J., Martinez N. J. Evidence for a role of adenosine 3',5'-monophosphate in parathyroid hormone release. Endocrinology. 1973 Mar;92(3):687–691. doi: 10.1210/endo-92-3-687. [DOI] [PubMed] [Google Scholar]
- Wong R. G., Myrtle J. F., Tsai H. C., Norman A. W. Studies on calciferol metabolism. V. The occurrence and biological activity of 1,25-dihydroxy-vitamin D 3 in bone. J Biol Chem. 1972 Sep 25;247(18):5728–5735. [PubMed] [Google Scholar]
- Wong R. G., Norman A. W., Reddy C. R., Coburn J. W. Biologic effects of 1,25-dihydroxycholecalciferol (a highly active vitamin D metabolite) in acutely uremic rats. J Clin Invest. 1972 May;51(5):1287–1291. doi: 10.1172/JCI106923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


