Abstract
Background
In a number of gram-positive bacteria, including Listeria, the general stress response is regulated by the alternative sigma factor B (SigB). Common stressors which lead to the activation of SigB and the SigB-dependent regulon are high osmolarity, acid and several more. Recently is has been shown that also blue and red light activates SigB in Bacillus subtilis.
Methodology/Principal Findings
By qRT-PCR we analyzed the transcriptional response of the pathogen L. monocytogenes to blue and red light in wild type bacteria and in isogenic deletion mutants for the putative blue-light receptor Lmo0799 and the stress sigma factor SigB. It was found that both blue (455 nm) and red (625 nm) light induced the transcription of sigB and SigB-dependent genes, this induction was completely abolished in the SigB mutant. The blue-light effect was largely dependent on Lmo0799, proving that this protein is a genuine blue-light receptor. The deletion of lmo0799 enhanced the red-light effect, the underlying mechanism as well as that of SigB activation by red light remains unknown. Blue light led to an increased transcription of the internalin A/B genes and of bacterial invasiveness for Caco-2 enterocytes. Exposure to blue light also strongly inhibited swimming motility of the bacteria in a Lmo0799- and SigB-dependent manner, red light had no effect there.
Conclusions/Significance
Our data established that visible, in particular blue light is an important environmental signal with an impact on gene expression and physiology of the non-phototrophic bacterium L. monocytogenes. In natural environments these effects will result in sometimes random but potentially also cyclic fluctuations of gene activity, depending on the light conditions prevailing in the respective habitat.
Introduction
Listeriae are gram-positive, non-sporulating rod-shaped bacteria. The genus comprises eight species, L. monocytogenes and L. ivanovii are pathogenic for humans and/or animals, L. seeligeri is considered as nonvirulent, L. innocua, L. welshimeri, L. grayi, L. marthii and L. rocourtiae are harmless saprophytes. Natural habitats of Listeriae are decaying plant material in soil and also the intestine of healthy animals and man [1], [2]. From there the bacteria gain access to sewage and water and may also contaminate food processing environments. Uptake of contaminated feed or food leads to the transmission of Listeria to mammalian hosts, including man [3], [4].
Listeriosis, a systemic disease caused in humans by L. monocytogenes, is rare but has a high mortality in severe manifestations, e.g. sepsis and meningoencephalitis. It mainly occurs in risk groups, such as children, pregnant, elderly and immunocompromised persons [5], [6]. The bacterium has also been implicated in a number of gastroenteritis cases [7]. L. monocytogenes is widely studied as model organism for facultative intracellular bacterial pathogens. It turned out that pathogenic Listeriae contain a chromosomal cluster of genes (Vcl) which are essential for virulence. The products of these virulence genes are involved in the escape of Listeria from the phagosome of the host cell, in actin-based intracellular movement of the bacteria and in their spreading to neighbouring cells [5], [8], [9]. The virulence gene cluster shows some features of a genomic island and therefore has been termed LIPI-1, for Listeria pathogenicity island 1 [10], [11]. A steadily growing number of other factors which are involved in the infection process has been identified, among them the internalins which trigger the bacterial uptake into non-phagocytic cells [12]. All virulence genes within LIPI-1 are under the transcriptional control of the Crp-like regulator PrfA [13], [14], [15].
The events during the transition of pathogenic Listeriae from the saprophytic lifestyle in the environment to that of an intracellular pathogen have been reviewed recently [16], [17].
L. monocytogenes is very robust, growing between pH 5–9, from 1–45°C and at salt concentrations up to 12% [5]. Listerial mechanisms counteracting environmental stress, e.g. high osmolarity, acid and bile, have been studied with respect to the survival in the environment and during the colonization of the intestinal tract [18]–[22]. The alternative stress sigma-factor B (SigB) of the RNA-polymerase holoenzyme was first discovered in the model organism for low G+C Firmicutes, Bacillus subtilis [23]. It is one of the key components in the general stress response of this group of microorganisms and controls the transcription of a large regulon of stress-related genes [24]. This also holds true for L. monocytogenes [19], [25], [26], [27], [28], in addition to SigB the stress gene regulators CtsR [29] and HrcA [30] play an important role. SigB of L. monocytogenes is also acting on the transcription of prfA and hence of PrfA-dependent genes, thus interconnecting stress response and virulence gene expression [31], [32], [33], [34], [35]. The activation of SigB by stress is a very complex process which has extensively been studied in B. subtilis, reviewed in [24]. There it involves, among other factors, the modulator protein RsbR [36]. B. subtilis RsbR and its paralogues [37], together with RsbS and RsbT, form a dynamic supramolecular complex, termed stressosome, which is supposed to integrate different environmental stress signals, such as high osmolarity, low pH etc. [38], [39], [40], [41], [42], [43], [44], [45]. Although the constituents of this complex are well characterized, it is not yet really clear which of the RsbR paralogues perceives which kind of environmental signal, with one notable exception. It has been shown recently that YvtA of B. subtilis activates SigB upon illumination with blue light [46], [47], [48]. Initially YtvA has been described as a positive regulator of SigB and as a RsbR paralogue with biochemical properties different from other RsbR proteins, i.e. not being phosphorylated by the RsbT kinase in vitro, showing a yellow color upon purification and bearing a N-terminal PAS domain [37]. Subsequent analyses identified YtvA as a member of the newly discovered and growing family of phototropin-related blue-light receptors in prokaryotes [49]. All these proteins carry a N-terminal LOV domain and a variety of C-terminal signalling domains [50]. LOV domains, found in blue-light receptors, oxygen-sensors and voltage-gated potassium channel proteins, are a subfamily of the PAS superfamily. Photoactive LOV domains non-covalently bind FMN (flavin mononucleotide), upon illumination with blue light a covalent photoadduct of a single molecule of FMN to a conserved cysteine in the LOV domain is formed and a photocycle is initiated which ultimately leads to a signal transduction process [51]. In YtvA the LOV domain is linked to a C-terminal STAS domain, the latter is characteristic for sulphate transporters and anti-sigma-factor antagonists [52] and is present in all RsbR paralogues and in RsbS of B. subtilis [37], [41]. The photochemistry and structure of YtvA has been studied in great detail [53], [54], [55], [56], [57] as well as its possible mechanism of action in SigB activation [58]. In these studies it has firmly been established that YtvA is a true flavin-based photoreceptor. Recently it has been described that the SigB-mediated general stress response of B. subtilis is also activated by red light, however, a receptor has not unambiguously been identified [59]. It has been noted early that proteins with the particular domain architecture of B. subtilis YtvA, i.e. N-terminal LOV linked to C-terminal STAS, are only found in other Bacilli and in Listeria [50], [51], however, nothing was known so far about the function of Lmo0799, the presumptive homologue of YtvA in the pathogen L. monocytogenes. Since a long time light-induced physiological responses and signalling processes are well known for photosynthetic microorganisms, but reports on such phenomena in non-phototrophic bacteria were rather rare, this has changed in the last decade [49], [50], [60], [61], [62], [63], [64], in particular since an exponentially growing number of fully sequenced bacterial genomes became available [65], [66], [67]. However, still very little is known about light effects on bacterial pathogenesis [68]. It has been shown that light influences the virulence of the plant pathogen Agrobacterium tumefaciens [69]. In the plant pathogen Pseudomonas syringae a blue light inducible two-component system has been characterized [70], it is not known if this system has a role in pathogenicity. With respect to bacteria pathogenic for mammalians it has been reported that infection of macrophages by Brucella abortus was stimulated by blue light, this effect was dependent on a photoreceptor which combines a LOV domain with a histidine-kinase signalling domain [71]. Here we demonstrate for the first time that the Lmo0799 protein of L. monocytogenes EGD-e is indeed a functional homologue of the photoreceptor YtvA from B. subtilis. We show that it is involved in the blue-light driven transcriptional activation of SigB-regulated genes in L. monocytogenes and that blue-light activation of Lmo0799 impaired the swimming motility of the bacteria. Among the light-induced genes were also those for the internalins A and B, resulting in an increase of bacterial invasiveness for enterocyte-like Caco-2 cells. Furthermore, we could demonstrate that blue light had a Lmo0799-independent effect on the transcription of stress- and virulence-related genes of L. monocytogenes and that transcription of these genes was also influenced by red light, the underlying mechanisms remain unknown. Light induction of SigB-dependent genes was completely abolished in a sigma B deletion mutant.
Results
Lmo0799 of Listeria monocytogenes EGD-e is highly similar to YtvA from Bacillus subtilis, homologues are present in all sequenced L. monocytogenes isolates and in other Listeria species.
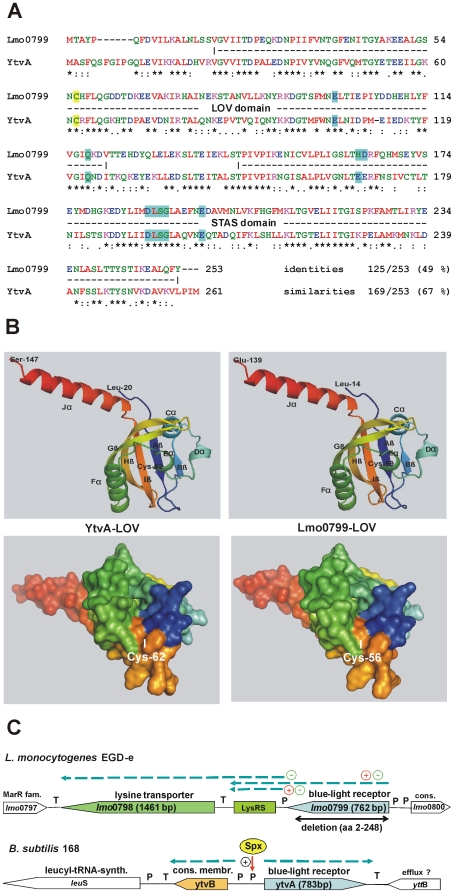
During a recent investigation into the thiol:disulfide redox metabolism of Listeria monocytogenes EGD-e we observed that, together with the genes for thioredoxin A (trxA) and thioredoxin reductase (trxB), the transcription of lmo0799 was highly up regulated when the bacteria were selectively depleted for the biological thiol glutathione (GSH) by a deletion in the glutathione synthetase gene gshF. Treatment of L. monocytogenes with diamide, an oxidant for biological thiols (not only GSH but also thiol-containing proteins), did not significantly induce lmo0799 (unpublished results). The gene product of lmo0799 has originally [72] been annotated as a hypothetical protein of 253 amino acids (http://genolist.pasteur.fr/ListiList/), however, a closer look revealed a significant relatedness to the blue-light photoreceptor YtvA of B. subtilis, this had already been noted earlier by others [50], [51]. Figure 1A shows that the two amino acid sequences are highly similar over their total length, this includes the cysteine (C62 in YtvA, C56 in Lmo0799) which is required for formation of the FMN photoadduct. As already mentioned in the introduction, YtvA of B. subtilis is a RsbR paralogue. However, it lacks the conserved phosphorylatable threonines found at positions 171 and 205 in RsbRA and at corresponding positions in RsbRB-D [37], [73]. In YtvA negatively charged glutamates (E168 and E202) are present at positions corresponding to T171/T205 of RsbRA. It has been suggested that these negatively charged amino acids mimick the phosphorylated state of threonine [47]. In Lmo0799 a histidine (H163) is found at the position of YtvA-E168, followed by a negatively charged aspartate (D164). The second glutamate, E202 in YtvA, is conserved in Lmo0799 (E197). Other amino acids which have been identified as being important for the function of YtvA, e.g. E105, Q123 and the DLSG motif [57], [58], [74] are conserved in Lmo0799. The 3-D structure of the LOV domain of YtvA from B. amyloliquefaciens has recently been solved [75], using this structure as a template we modeled the probable structure of Lmo0799-LOV at the SWISS-MODEL workspace. Figure 1B shows that the modeled 3-D structures of YtvA-LOV and Lmo0799-LOV are virtually identical, the coil connecting Hβ and Iβ of Lmo0799-LOV could not be modeled unambiguously. Modeling of the surface of both proteins showed that the crucial cysteines at the N-terminus of Eα are located in a pocket but are accessible in both proteins. Figure 1C depicts the transcriptional organization of the lmo0799/ytvA loci, as deduced from the literature and from genome data. For lmo0799 it has been shown by Toledo-Arana et al. [35] that a riboswitch (LysRS), regulating the transcription of the lysine transporter Lmo0798, is located downstream of lmo0799 in L. monocytogenes EGD-e. In the above mentioned study it was shown that both in the absence or presence of lysine in the medium, lmo0799 was transcribed from a promoter immediately upstream of the gene, this transcript extended into the riboswitch sequence. In addition, a short transcript comprising the riboswitch only was also found. The lysine transporter gene lmo0789 was only transcribed in the absence of lysine. The graphical representation of the B. subtilis 168 genome [76] which is available on a website at the Pasteur Institute Paris (http://genolist.pasteur.fr/SubtiList) clearly indicated that ytvA is transcribed monocistronically, its promoter has been mapped by Gaidenko et al. [47].
Figure 1. Structure and genetic organization of Lmo0799 and YtvA.
Detailed explanations in the text. (A) Amino acid sequence alignment of Lmo0799 and YtvA, using ClustalW2 [106] (http://www.ebi.ac.uk/Tools/clustalw2/index.html). Asterisks below the sequence indicate identical, double points very similar amino acids. The boundaries of the LOV and STAS domains are according to [56]. Amino acids which have been shown to be particularly important for the function of YtvA are highlighted in yellow (C62/C56) or blue. (B) 3-D models of YtvA-LOV and Lmo0799-LOV, generated with the SWISS-MODEL server (http://swissmodel.expasy.org/workspace/). Upper panel: Ribbon models, the designations of beta-sheets and alpha-helices were from [56]. Lower panel: Surface models. (C) Genomic context and transcriptional organization of lmo0799 and ytvA. The data for lmo0799 were from [35], for ytvA from SubtiList (http://genolist.pasteur.fr/SubtiList) and from [47]. P: promoter, T: transcriptional terminator. The GenBank accession nos. for the respective genome sequences are AL591824 (L.m.) and AL009126 (B.s.).
When we performed a BLAST search, using Lmo0799 as the query sequence, in 1299 bacterial genomes in the NCBI database, our results confirmed previous report, based on a smaller number of genomes [50], [51], that proteins with the particular domain architecture of YtvA/Lmo0799 can be found only in other Bacilli, including the extremely halotolerant and alkaliphilic deep-sea isolate Oceanobacillus iheyensis, and in Listeria. In this search and also using the Genolist website at the Pasteur Institute Paris we found identical or very similar proteins in all sequenced L. monocytogenes isolates and Listeria species (alignments not shown). The similarity values (identical/positive, in percent) were 99/99 for L. monocytogenes serovar 4b strains, 93/99 for L. innocua, 91/97 for L. welshimeri, 87/96 for L. ivanovii (searched at http://genolist.pasteur.fr/LivaList), 85/95 for L. seeligeri and 64/80 for L. grayi. These values are in agreement with the established phylogenetic tree of Listeria which classifies L. monocytogenes and L. innocua as belonging to one group, L. ivanovii and L. welshimeri to another and L. grayi as the most distantly related one to all other Listeria species [11], [77].
A deletion mutant for lmo0799 growths like wild type
By allel replacement a mutant (Δ0799) of L. monocytogenes EGD-e was constructed in which the coding sequence for the amino acids 2–248 of the 253 amino acids long Lmo0799 protein was deleted (Figure 1C). Such an in frame deletion should not influence the expression of the riboswitch LysRS and of the lysine transporter Lmo0798. When wildtype or the mutant Δ0799 were grown in BHI at 37°C no difference in their multiplication was observed (data not shown).
Blue and red light, in combination with salt stress, induce the transcription of the SigB-dependent ctc gene and of sigB
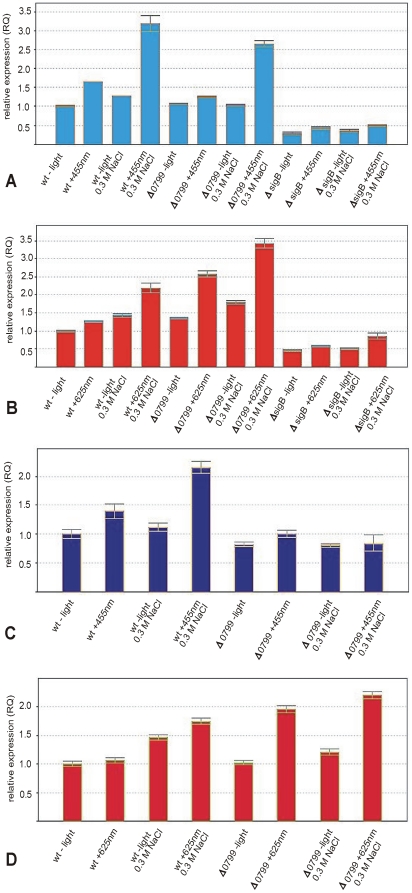
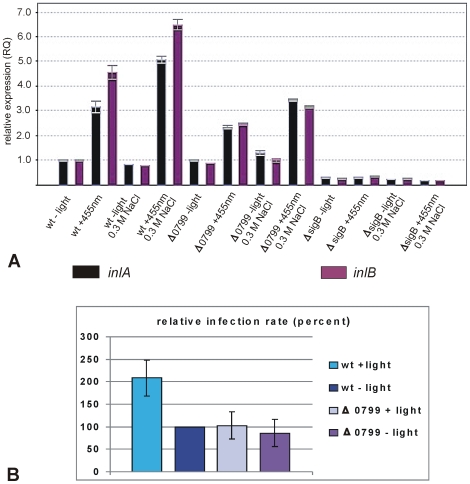
In the studies on YtvA of B. subtilis the ctc gene was used as a reporter gene [46], [47], [48]. The promoter of this general stress gene is the best studied SigB-dependent one in B. subtilis, a homologue (lmo0211) is also present in the genome of L. monocytogenes EGD-e. It has been shown that Ctc of L. monocytogenes is involved in osmotolerance [78], in almost all studies on the SigB regulon of L. monocytogenes the ctc gene appeared as being positively regulated by SigB [32], [79], [80], [81], [82]. In the initial study on the blue-light mediated effect of YtvA it was claimed that red light had no effect on the transcription of ctc in B. subtilis [46]. However, our preliminary experiments concerning effects of blue light on L. monocytogenes yielded highly variable and sometimes conflicting results when we used a red-light dark chamber lamp for the handling of dark controls (not shown). Therefore we used low-intensity, diffuse infrared (λ = 850 nm) illumination for our dark controls in all experiments reported here and night vision goggles for observation during the manipulations. Our assumption that red light might have an effect on transcription was later confirmed for B. subtilis too [59]. For YtvA is has been demonstrated that the blue-light effect was enhanced upon exposure of B. subtilis to salt stress (0.3 M NaCl) [46], [47], [48], therefore we performed all our transcription analyses after exposure of Listeria to all possible combinations of light and salt stress. Figure 2A shows that blue light alone moderately increased ctc transcription in the wild type (1.5-fold), the effect of salt stress alone was not significant here as well as in the Δ0799 receptor mutant, blue light alone had no effect on the mutant. The combination of blue light and 0.3 M NaCl resulted in a 3-fold increase for the wild type and 2.5-fold for the mutant. The response profile for red light was different (Figure 2B), light or salt stress alone resulting in no significant increase for the wild type, red light plus 0.3 M NaCl yielding a 2.2-fold increase here. Surprisingly, red light alone resulted in a 2.5-fold increase in the Δ0799 mutant, red light plus salt gave a 3.5-fold increase, i.e. significantly higher than in the wild type. Deletion of sigB (ΔsigB) decreased the transcription of ctc, under all conditions, to about half of the level observed for the wild type without light (Figures 2A and 2B). The transcription of sigB itself, which is positively autoregulated [26], [82] showed the same pattern as ctc, except for the Δ0779 mutant with blue salt plus salt, where no induction was seen (Figures 2C and 2D).
Figure 2. Effect of blue and red light on ctc expression.
Transcription analysis by qRT-PCR for wild type, Δ0799 and ΔsigB mutants. The strains were grown at 37°C in BHI. Cells were harvested in mid-log phase (OD600 ∼0.9) and exposed for 10 min to blue (455 nm) or red (625 nm) light as described in material and methods. The results from the qRT-PCR analysis, obtained with a StepOnePlus Real-Time PCR system (Applied Biosystems Inc.) were normalized using rpoB as an internal standard [103], [104] and expressed as fold change with the values for wild type without light set as 1.0. Calculations were performed with the StepOne Software v2.1 (Applied Biosystems Inc.). Means and standard deviations from three independent biological samples and four technical replicates per sample.
Other SigB-dependent genes respond to light in a manner similar to ctc, PrfA-regulated virulence genes and the thiol redox-related gene trxA behave differently
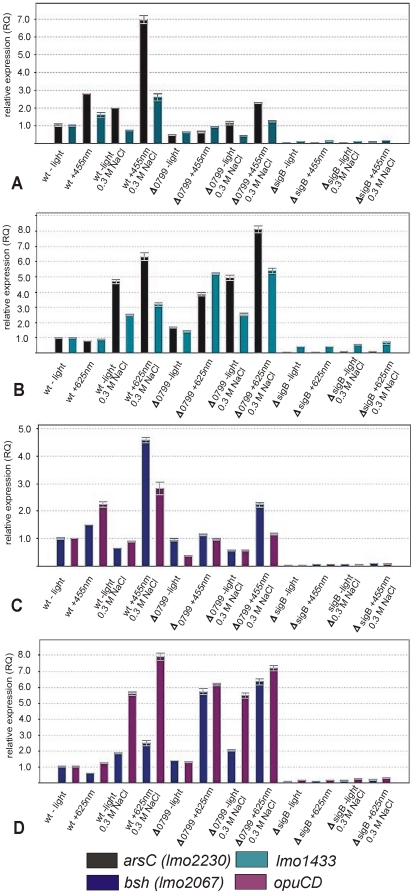
After having established the proper experimental conditions for dark controls, we next investigated the behavior of four other genes the transcription of which is commonly regarded as being SigB-dependent in L. monocytogenes [32], [79], [80], [81]. These were lmo2230 (arsC, enconding a protein with similarity to arsenate reductase), lmo1433 (putative glutathione reductase), lmo2067 (bsh, bile salt hydrolase) and lmo1425 (opuCD). The latter gene encodes one of the two membrane-spanning proteins of a carnitine-ABC-transporter involved in osmo- and bile-tolerance of L. monocytogenes [22]. Lmo1425 was chosen instead of lmo1428 (opuCA), the first gene in the opuC operon, because in this way we could better monitor the transcription of the entire operon. Figure 3 shows that the light- and salt-dependent transcription profiles of all these genes were similar to that observed for ctc. Induction by blue light was always strongest in combination with salt stress and was significantly reduced or abolished when Lmo0977 was lacking (Figures 3A and 3C). In wild type bacteria an induction by red light was again only observed in combination with salt stress whereas in the Δ0799 mutant all four genes were induced by red light also in the absence of salt stress (Figures 3B and 3D). In contrast to ctc the deletion of sigB decreased the transcription to almost zero under all conditions.
Figure 3. Effect of blue and red light on expression of arsC, lmo1433, bsh and opuCD.
Transcription analysis by qRT-PCR for wild type, Δ0799 and ΔsigB mutants. The strains were grown at 37°C in BHI. Cells were harvested in mid-log phase (OD600 ∼0.9) and exposed for 10 min to blue (455 nm) or red (625 nm) light as described in material and methods. The results from the qRT-PCR analysis, obtained with a StepOnePlus Real-Time PCR system (Applied Biosystems Inc.) were normalized using rpoB as an internal standard [103], [104] and expressed as fold change with the values for wild type without light set as 1.0. Calculations were performed with the StepOne Software v2.1 (Applied Biosystems Inc.). Means and standard deviations from three independent biological samples and four technical replicates per sample.
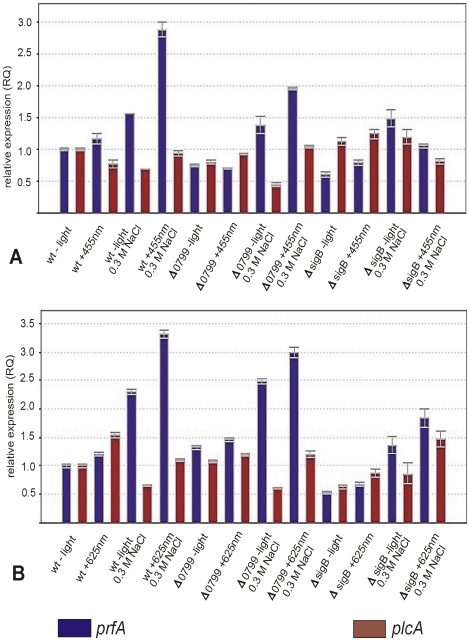
As figures 4A and 4B show the transcription profiles of prfA (encoding the master regulator of virulence in L. monocytogenes) and of the PrfA-regulated virulence gene plcA exhibited clear differences to canonical SigB-regulated genes. PrfA transcription was slightly induced by 0.3 M NaCl in wild type bacteria and in the Δ0799 mutant without light and was further increased under combined stress conditions (blue or red light plus salt). PrfA showed neither a significant induction nor repression in the ΔsigB mutant, the transcription of plcA was not significantly altered in all strains and under all conditions tested.
Figure 4. Transcription profiles of virulence genes.
Transcription analysis by qRT-PCR for wild type, Δ0799 and ΔsigB mutants. The strains were grown at 37°C in BHI. Cells were harvested in mid-log phase (OD600 ∼0.9) and exposed for 10 min to blue (455 nm) or red (625 nm) light as described in material and methods. The results from the qRT-PCR analysis, obtained with a StepOnePlus Real-Time PCR system (Applied Biosystems Inc.) were normalized using rpoB as an internal standard [103], [104] and expressed as fold change with the values for wild type without light set as 1.0. Calculations were performed with the StepOne Software v2.1 (Applied Biosystems Inc.). Means and standard deviations from three independent biological samples and four technical replicates per sample.
The transcription of the thiol redox-related gene trxA (lmo1233, thioredoxin A) was moderately induced by salt stress in the wild type and the Δ0799 mutant, no further increase was caused by blue or red light. For blue, but not for red light these effects were less in the ΔsigB strain (Figure S1).
Blue light induces the transcription of the internalin A and B genes and increases the invasiveness of L. monocytogenes EGD-e for Caco-2 enterocytes
Since long it has been established by others that the invasion of normally non-phagocytic eukaryotic host cells by L. monocytogenes depends on the leucine-rich repeat proteins of the internalin superfamily. Internalin A (InlA, Lmo0433) is required for the invasion of enterocytes, the inlA gene is co-transcribed with the downstream gene for internalin B (InlB, Lmo0434) which recognizes other host cell receptors and cell types, reviewed in [8], [9], [12] Figure 5A shows that the transcription of inlA and inlB was significantly induced (3 and 4.5-fold, respectively) by blue light in the case of the L. monocytogenes EGD-e wild type. When blue light was combined with salt stress, inlA transcription was further increased to 5-fold, that of inlB to 6.5-fold. In the Δ0799 receptor mutant the induction of both genes by light or light plus salt stress was only 2.5 and 3.5-fold, respectively. Deletion of sigB resulted in an almost undetectable transcription under all conditions. L. monocytogenes EGD-e wild type and its Δ0799 receptor mutant were grown at 37°C and in the dark until mid-log phase (OD600 = 0.9) with BHI as medium, then one aliquot was exposed to blue light plus 0.3 M NaCl for 10 min at 37°C, the other aliquot was also adjusted to 0.3 M NaCl but kept in the dark under otherwise identical conditions. Semi-confluent Caco-2 enterocyte-like human cells were infected (m.o.i. of 20) with the differently pretreated bacteria for 2 hours (1 hour attachment time in the dark without antibiotic, washing step, followed by one hour incubation with gentamicin). The absolute infection rates (c.f.u./ml lysate) obtained in independent experiments were rather variable, therefore the means and standard deviations of the relative infection rates were used for comparison, setting the values for the wild type without light as hundred percent. As Figure 5B shows an exposure of the bacteria to blue light and 0.3 M NaCl for 10 min resulted in a two-fold increased infectivity of the wild type, when compared to the dark control, whereas infectivity of the Δ0799 receptor mutant was not significantly changed by blue light. It has repeatedly shown by others that the invasiveness of a ΔsigB mutant is drastically reduced [19], [31], [94], therefore this mutant was not tested here. Since the effect of red light on inlA and inlB transcription basically was identical to that of blue light (results not shown), no infection experiments were performed with red light-exposed bacteria.
Figure 5. Effect of blue light on inlA and inlB transcription and on infection rate.
(A) Transcription analysis by qRT-PCR for wild type, Δ0799 and ΔsigB mutants. The strains were grown at 37°C in BHI. Cells were harvested in mid-log phase (OD600 ∼0.9) and exposed for 10 min to blue light (455 nm) as described in material and methods. The results from the qRT-PCR analysis, obtained with a StepOnePlus Real-Time PCR system (Applied Biosystems Inc.) were normalized using rpoB as an internal standard [103], [104] and expressed as fold change with the values for wild type without light set as 1.0. Calculations were performed with the StepOne Software v2.1 (Applied Biosystems Inc.). Means and standard deviations from three independent biological samples and four technical replicates per sample. (B) Caco-2 enterocyte-like cells were infected (m.o.i = 20) with wild type and its isogenic Δlmo0799 mutant. Prior to infection the bacteria were either exposed for 10 min to blue light or kept in the dark, in both cases at 37°C and with 0.3 M NaCl in the medium. For details see materials and methods. One hour after the addition of gentamicin the cells were lysed, CFU/ml were determined and the relative infection rates were calculated. Means and standard deviations from four independent experiments. The values for wild type without light were set as 100 percent, therefore no standard deviation is shown there.
Blue, but not red light inhibits swimming motility of L. monocytogenes EGD-e in a Lmo0799- and SigB-dependent manner
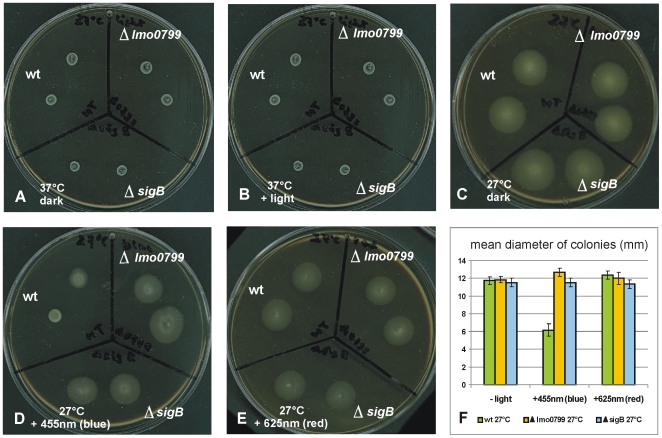
Assays for swimming motility on semi-solid (0.3 percent agar) BHI plates showed that L. monocytogenes EGD-e wild type and its Δ0799 or ΔsigB mutants were non-motile at 37°C, with a colony size of just 6 mm after 16 h incubation time (Figures 6A and 6B). When incubated for 18 h at 27°C in the dark, all strains were clearly motile, reaching colony diameters of about 12 mm (Figures 6C and 6F). Upon constant illumination for 18 h with blue light at an incubation temperature of 27°C, wild type bacteria were almost as non-motile as at 37°C, with a colony diameter of 6 mm and a very faint halo surrounding the colonies (Figures 6D and 6F). The inhibition of swimming motility by blue light was completely relieved in the Δ0799 blue-light receptor and in the ΔsigB mutant (Figures 6D and 6F), no inhibition was observed after exposure to red light of the same intensity (Figures 6E and 6F).
Figure 6. Effect of incubation temperature and light on swimming motility.
Semi-solid BHI agar (0.3%) plates were inoculated with 2 µl of mid-log cultures (OD600 ∼0.7) of wild type, Δlmo0799 and ΔsigB mutants. Incubation was for 18 h at temperatures and light conditions as indicated in the figure. (B) shows the effect of blue light, the result for red light was identical, (F) shows the means and standard deviations of the colony diameters from three independent experiments.
Discussion
As has already been noticed by others some time ago, Listeria monocytogenes, L. innocua [50], [51] and other Listeria species [57] contain a gene, lmo0799 in L. monocytogenes EGD-e, which encodes a protein with high similarity to the well characterized blue-light photoreceptor YtvA of B. subtilis. Figure 1A depicts the sequence similarities between the two proteins, see also results. Our modeling of the light-receiving domain Lmo0799-LOV, based on the experimentally determined structure of YtvA-LOV [75], revealed that Lmo0799-LOV most probably has the same three-dimensional structure as LOV in the Bacillus protein (Figure 1B).
In order to test experimentally if Lmo0799 is a functional blue-light receptor we constructed a strain (Δ0799) carrying an in-frame deletion, this mutant was not impaired in growth in BHI. The appropriate test conditions for light exposure experiments were based on the following considerations. For the photocycle of B. subtilis YtvA is has been shown that the time required for light activation (conversion of the dark state YtvA447 into the red-shifted intermediate YtvA660) and for photoadduct formation (YtvA390) is very short, 20 nsec and 2 µsec, respectively, whereas the dark recovery is unusually slow, with a Taurec of 2600 sec (ca. 43 min) [55]. We assumed that the kinetics of the postulated photocycle of Lmo0799 would be in the same time range, allowing to monitor effects after the inevitable interval (about 25 min) between exposure to light and experimental measurements. Since our preliminary experiments had indicated that even low-intensity red light had an effect on gene transcription our dark controls were handled without any visible light. Furthermore, all experiments were done in parallel with blue (λ = 455 nm) and red (λ = 625 nm) light.
Our qRT-PCR results showed that a 10 min exposure of L. monocytogenes wild type to blue light and 0.3 M NaCl lead to a three-fold induction of the transcription of ctc, the traditional sigB reporter gene in B. subtilis. This effect was only slightly reduced in the Δ0799 photoreceptor mutant and also the ΔsigB mutant still showed a substantial transcription of ctc (Figure 2A). This shows that in L. monocytogenes ctc is not strongly dependent on SigB, which is in line with a previous report by others [79]. The transcription of the autoregulated [26], [82] sigB gene itself was only induced by the combination of blue light and salt stress, this induction was dependent on the presence of Lmo0799 (Figure 2C). The transcription analysis of the four genes arsC, lmo1433, bsh and opuCD, which are representative for the SigB regulon of L. monocytogenes [32], [79], [80], [81], showed another patten (Figure 3). For blue light alone the induction was more than two-fold only for arsC and opuCD, 0.3 M NaCl alone had almost no effect on all four genes. The blue light plus salt effect was abolished in the Δ0799 deletion mutant in the case of lmo1433 and opuCD and significantly lowered for arsC and bsh. This is in line with previous reports which have demonstrated that arsC and bsh showed a very strong induction upon activation of the SigB system, whereas lmo1433 and opuCD were only moderately induced [32], [34], [79], [80], [81]. Deletion of sigB completely abolished the transcription of all four genes under all conditions (Figure 3). Together these results showed that i) blue light or salt alone had a stimulatory effect on those genes only which require a low level of active SigB, e.g. arsC and opuCD, ii) full induction required both blue light and salt stress and was dependent on Lmo0799, iii) the blue light and osmotic effects were strictly dependent on SigB. Thus we have firmly established that Lmo0799 is a genuine blue-light receptor and a functional homologue of YtvA.
Furthermore, our results demonstrated that red light too activated the transcription of SigB-regulated genes in L. monocytogenes. Such a red-light effect on SigB activity has recently also been reported for B. subtilis and the results presented there showed that the B. subtilis proteins RsbP/Q were required to transduce the red-light signal to the SigB cascade, it has been proposed that RsbP was the light-sensing protein [59]. In B. subtilis RsbP/Q are part of the energy stress pathway of SigB activation which responds to changes in the intracellular ATP level [24]. In L. monocytogenes the perception and transduction of the red-light signal must be different from the mechanism proposed for B. subtilis because no homologues of RsbP/Q can be found in Listeria [27], [82]. The prototype of red-light photoceptors are the phytochromes [83], also found in many prokaryotes [60], [61], [84] The bacteriophytochromes all share a domain required for the binding of a bilin chromophore [85]. However, proteins with a phytochrome-like structure could not be detected in Listeria (and Bacillus) genomes during expert bioinformatic analyses published by others [67], [85]. It should be noted, however, that acccording to its genome annotation [72] (http://genolist.pasteur.fr/ListiList) L. monocytogenes EGD-e contains all genes required for heme biosynthesis and also for heme oxygenase (ctaA, lmo2058), required for the conversion of heme into biliverdin, however, a bilin-binding photoreceptor protein could not be identified. Although bilins in their photo-excited singlet- or triplet state can react with molecular oxygen giving rise to superoxides [86], [87], [88], we have no evidence for the generation of substantial oxidative stress in L. monocytogenes by red (or blue) light. As figure S1 shows neither blue nor red light stimulated the transcription of the genes for superoxide dismutase (sod, lmo1439), catalase (kat; lmo2785) or of lmo0799 itself and although SigB is involved in oxidative stress resistance of L. monocytogenes [89], such stress is not a typical inducer of the SigB system. Therefore the mechanism of L. monocytogenes SigB acticvation by red light remains unknown.
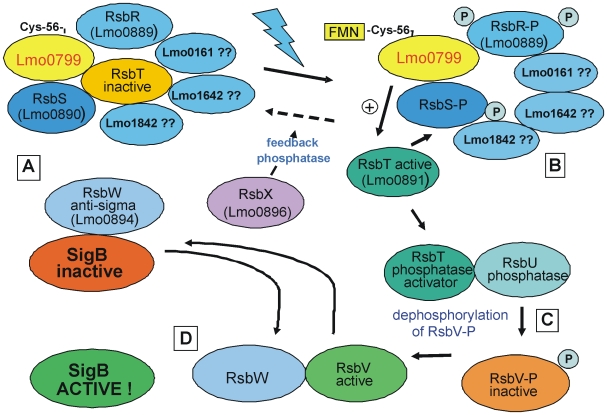
As has been mentioned in the introduction, in B. subtilis different environmental stresses, ultimately evoking the activation of SigB, are integrated by a dynamic supramolecular complex, the stressosome [38], [39], [42], [43], [44], [45]. For B. subtilis it has also firmly been established that YtvA is part of its stressosome [47], this raised the question if L. monoyctogenes could have a stressosome too. Homologues of the B. subtilis stressosome constituents RsbS, RsbT, RsbRA and for the downstream components RsbU, RsbV and RsbW as well as for the feedback phosphatase RsbX have been identified in L. monocytogenes and it has been shown that their genomic and transcriptional organization is identical to that of B. subtilis [27], [82]. We identified Lmo0799 as a YtvA homologue and hence as a RsbR paralogue in L. monocytogenes. A BLAST search in the complete genome of L. monocytogenes EGD-e with the protein sequences of B. subtilis RsbRA and the RsbRA paralogues RsbRB (YkoB), RsbRC (YojH) and RsbRD (YqhA) as query confirmed Lmo0889 as the RsbRA homologue. Some homology (about 25 percent identity/46 percent similarity) to RsbRB-D was only found for Lmo0161. When RsbR (Lmo0889, 278 amino acids) of L. monocytogenes itself was used as a query sequence, again homology to Lmo0161 was found, but now also to Lmo1642 (267 amino acids, 21/52 percent) and Lmo1842 (274 amino acids, 23/45 percent). These homologies extended over the whole length of all three proteins, which also in size were very similar to RsbR (Lmo0889). Domain analysis with SMART [90] and InterPro [91] revealed that all three proteins had a C-terminal STAS domain, typical for the RsbR protein family, but a canonical Rsbr-N domain could not be identified. In a comparative analysis of B. subtilis RsbRB-D also only a C-terminal STAS domain could be identified by SMART and InterPro (results not shown). Figure S2 shows the domain analyses for L. monocytogenes, together with a sequence alignment of B. subtilis RsbRA-D. Future experiments have to show if these proteins are functional RsbR paralogues. Figure 7 shows a hypothetical and simplified, with respect to the RsbR paralogues purely speculative model of the SigB activation via blue light and Lmo0799.
Figure 7. Hypothetical and simplified model of SigB activation by blue light.
(A) Hypothetical complex of Lmo0799, RsbR and its putative paralogues (Lmo0161, Lmo1642, Lmo1842), RsbS and RsbT in the dark state. SigB is sequestered by the anti-sigma factor RsbW, which also keeps RsbV inactive by phosphorylation. (B) complex after blue-light driven FMN-Lmo0799 photoadduct formation, activating the kinase function of RsbT, which phophorylates RsbS and RsbR and is released from the complex, binds and activates RsbU. (C) dephosphorylation of RsbV-P by activated RsbU, (D) dephosphorylated RsbV binds RsbW and active SigB is released. The feedback phosphatase RsbX is believed to limit the activation of the system. Further explanations in the text. The graph does not intend to reflect the stoichiometry of the individual proteins, which presumably are present in multiple copies, nor the true architecture of the hypothetical complex. Modified from the scheme proposed for B. subtilis YtvA [47].
Although the mechanism by which red light induces the SigB regulon in L. monocytogenes is unknown, there is obviously some crosstalk or interference with the Lmo0799-dependent blue-light triggered mechanism. As shown in figures 2BD and 3BD red light induced the transcription of SigB-dependent genes and of sigB in the wild type only when additional salt stress was imposed whereas in the absence of Lmo0799, i.e. in the Δ0799 mutant, red light alone lead to a significant up regulation. This shows that Lmo0799 or the hypothetical Lmo0799-containing complex inhibited the transduction of the red-light signal by an unknown mechanism. It will be interesting to see if such an effect also occurs in B. subtilis where the red-light signal obviously is transduced in a different way.
Figure 4 shows that the transcription profile of the virulence regulator prfA was very similar to that obtained for SigB-dependent genes, whereas light or dark had no effect on the expression of the PrfA-dependent gene plcA. PrfA can be transcribed as a bicistron together with the upstream plcA gene and monocistronically from two promoters directly upstream of prfA [14], [15], one of which (P2) is under SigB control [15], [16], [32]. Since no increase in plcA transcription was seen here, salt and light caused an increase solely in monocistronic prfA mRNA due to an activation of SigB, this is in line with previous reports on the impact of SigB on prfA [15], [16], [31], [32], [34]. However, elevated levels of prfA mRNA do not always lead to a corresponding increase in the transcription of PrfA-dependent virulence genes, e.g. plcA, rather this is also determined by the amount and activity of the PrfA protein which is subject to a very complicated and not completely understood regulation. It has been shown that two S-adenosylmethionine riboswitches control the translation of prfA mRNA [92]. Furthermore, there is evidence that an unknown cofactor regulates PrfA activity [15], [16], alternatively or in addition, the transport of sugars via phosphotransferase systems has a negative effect on PrfA activity, reviewed in [17]. So it seems that under the conditions used in our experiments the increase in prfA transcription does not translate into more and active PrfA protein.
The results were different for the internalins A and B. It has previously been reported that transcription of inlA and inlB was not only positively regulated by PrfA, but that it was also diminished in mutants lacking SigB [32], [33], [34], [79], [81] and increased when SigB was activated [93]. Furthermore, it has been shown that invasion of Caco-2 enterocytes is largely dependent on SigB [94]. Our results show (Figure 5A) that the transcription profiles of inlA and inlB in the dark or after exposure to blue light were identical to those observed by us for SigB-dependent genes. Invasion experiments with Caco-2 enterocytes showed that two hours after the addition of L. monocytogenes the number of intracellular bacteria was twice as high after pretreatment of the wild type with blue light and 0.3 M NaCl as without light or for the Δ0799 blue-light receptor mutant (Figure 5B). Potential differences in intracellular replication during this short time are not supposed to have a significant effect on the results. Also for the internalins A and B post-transcriptional control of the protein levels has been reported [95], [96], this could be a reason why the slight transcriptional induction in the Δ0799 mutant did not result in a corresponding invasiveness, however, experimental data supporting this assumption are lacking. If this light-induced invasiveness has a role in the natural infection process, preparing the bacteria for a potential ingestion by a host organism, will be difficult to test. It could be an accessory factor, contributing to the postulated major effect of the intestinal environment on invasiveness [22].
Light is one of the environmental factors which may influence flagella-mediated movement of bacteria [63]. We observed (Figure 6) a drastic reduction of swimming motility when L. monocytogenes wild type was exposed to blue light at 27°C, this inhibition was completely relieved in the Δ0799 blue-light receptor and ΔsigB mutants. Most L. monocytogenes strains, including EGD-e, are non-motile at temperatures around 37°C because at this temperature the flagella- and motility gene cluster [72], [97] is repressed by a system comprising several factors [97], [98]. There is no evidence that blue-light activated Lmo0799 can directly interfere with this regulatory system, however, it has previously been described that the flagella- and motility cluster was transcriptionally down regulated at 24°C in salt-stressed L. monocytogenes and that a ΔsigB strain showed increased swarming. From their data the authors concluded that SigB negatively regulates motility in an indirect way [80], a negative regulation of flagella- and motility-associated genes by SigB has also been reported by others [34], [79]. Here we could show that blue light activated SigB in a Lmo0799-dependent manner and that inhibition of motility by blue light was SigB-dependent. Therefore the observed inhibition of swimming motility by blue light in the wild type most presumably was also due to transcriptional silencing of the flagella- and motility cluster via Lmo0799 and SigB. An experiment-based explanation for the fact that red light had no inhibitory effect here, although it also induced SigB-dependent genes, is lacking. It could be that this effect was transient and faded away during the 18 h duration of the swimming motility assay, whereas the Lmo0799-mediated activation by blue light was persistent. A persistent SigB activation by blue light has been mentioned for YtvA of B. subtilis (59). Alternatively, one could assume that the inhibition of motility requires both SigB and blue light-activated Lmo0799, the latter one missing under red light illumination.
Intense blue and, to a lesser extent, red light can result in oxidative damage to cells by the light-driven formation of reactive oxygen intermediates [86], [87], [88]. Therefore the benefit of evolving light sensing systems also in non-phototrophic bacteria seems obvious, possibly enabling them to activate appropriate defense systems at an early time point, i.e. already at low light intensity before massive damage will occur [67]. However, in our experiments we did not obtain evidence for the up regulation of oxidative stress defense genes in L. monocytogenes by moderate illumination. Furthermore, light would be a suitable signal for upcoming osmotic stress, caused by water evaporation under sun light. The illuminance we used in our experiments, i.e. 30 microeinsteins/m2/s of blue or red light, cannot exactly be compared to day- or sunlight because natural light contains the whole visible spectrum with varying proportions of the different wavelengths and a maximum in the blue-green range [99]. The experimental conditions used here correspond to approximately the illuminance in the half-shade at noon on an overcast day or to about 1/30 of full sunlight at mid-latitude [99]. Our results show that visible light had an impact not only on osmotic stress defense factors of L. monocytogenes but on other functions too, e.g. invasiveness and swimming motility. Furthermore, it has previously been reported that the large SigB regulon of L. monocytogenes also comprises genes for transport, metabolism and protein synthesis [32], [79], [80], [81]. Therefore one can assume that in natural environments, depending on their exposure to light, all these processes may undergo cyclic fluctuations. These will not be genuinely circadian because prokaryotic endogenous clocks have so far been found in cyanobacteria only [100], rather their periodicity will be determined by day length and hence by season, weather and geographical latitude. Furthermore, it has been shown that the specific transcriptional regulation by SigB varies among L. monocytogenes strains [81], therefore also the dimension of light effects will vary accordingly.
Materials and Methods
General techniques
PCR amplifications, cloning procedures, isolation of chromosomal DNA, and DNA manipulations were carried out according to standard procedures [101]. DNA sequencing was carried out by Seqlab GmbH (Germany).
Bacterial strains, plasmid, and cell line
L. monocytogenes Sv1/2a EGD-e (ATCC BAA-679) was obtained from T. Chakraborty (University of Giessen, Germany) whom we also thank for the isogenic sigB-deletion mutant (ΔsigB) [79]. E. coli strain TG1 and plasmid pG+host4 [102] were kindly provided by E. Maguin (INRA Jouy en Josas, France). Human colon epithelial cells (Caco-2 cells) were obtained from the American Type Culture Collection (ATCC HTB-37) and were cultured at 37°C and 5% CO2 in RPMI 1640 (Gibco, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Biochrom KG, Germany).
Media and growth conditions
L. monocytogenes was grown in brain heart infusion (BHI, Difco, Germany) at 37°C. Cultivation of E. coli was carried out in Luria-Bertani (LB, 10 g/l peptone, 5 g/l yeast extract, 10 g/l NaCl) medium at 37°C. For transformation experiments media were supplemented with erythromycin to final concentrations of 300 µg/ml for E. coli or 10 µg/ml for L. monocytogenes. For growth tests of L. monocytogenes 300 µl of an overnight culture in BHI were diluted into 10 ml prewarmed BHI and shaken at 190 rpm and 37°C. The optical density of the cultures was recorded every hour with a photometer (Ultrospec, Amersham Biosciences, Germany) at 600 nm in 1 cm cuvettes.
Mutant construction
For construction of the in frame deletion mutant (Δlmo0799) PCR-amplified fragments of ∼300 bp from the 5′ and 3′ region of lmo0799 were cloned into the temperature-sensitive integration vector pG+host4 and transformed into E. coli TG1 [102]. L. monocytogenes EGD-e was transformed with the plasmid construct and plasmid integrants were selected at the non-permissive temperature of 42°C on erythromycin-containing BHI agar. To obtain the deletion, the mutant strains were subcultured twice for 24 h in BHI without antibiotic at 30°C. At this temperature the plasmid origin of replication is fully active which favors plasmid excision [102]. Serial dilutions of the subcultures were plated on BHI without antibiotic and erythromycin-sensitive clones were identified by replica-plating on erythromycin-containing medium. Plasmid loss and deletion was confirmed by PCR and DNA sequencing (data not shown). Oligonucleotides used for mutant construction are listed in table S1.
RNA isolation and preparation of samples for infection
For transcription analysis and infection experiments L. monocytogenes was precultured in BHI at 37°C under aerobic conditions without light, i.e. all culture vessels were wrapped in two layers of aluminium foil. For dark controls, all manipulations were carried out under low-intensity, diffuse infrared illumination (Osram Opto SFH4730 LED, 3 W, λ = 850 nm), using Dipol D2MVSL night vision goggles (Gross, Germany) for observation. Overnight precultures were diluted into fresh medium and grown in the dark at 37°C to an OD600 ∼0.9. For salt stress experiments 3.0 M sterile NaCl was added to the respective cultures to a final concentration of 0.3 M. Cultures were split into two 250 ml cell culture bottles, one wrapped twice in aluminium foil, the other exposed to blue (light-emitting diode, peak wavelength λ = 455 nm, Luxeon Star LXHL-MRRD, 1 W) or red (peak wavelength λ = 625 nm, Luxeon LXHL-MD1D, 1 W) light with an illuminance of 30 microeinstein/m2/s for 10 min, both samples were kept at 37°C. Samples for infection were washed twice with 1xPBS in the dark and stored in 1xPBS/20% glycerol (v/v) as 1 ml aliquots at −80°C in a light-tight container. Samples for RNA isolation (10 ml) were centrifuged in the dark for 10 min at 4°C and cell pellets were frozen in liquid nitrogen. RNA was prepared using the Seqlab RNA Mini Kit (Seqlab, Germany). Residual DNA was removed by Turbo DNAse treatment (Applied Biosystems/Ambion, USA). All RNA isolations were repeated at least three times.
Real-time qRT-PCR
Real-time quantitative reverse transcriptase PCR (qRT-PCR) was performed on total RNA isolated. The absence of DNA from RNA samples was verified by PCR prior to reverse transcription, using rpoB -specific primers. 5 µg of total RNA was reverse transcribed with random hexamers and SuperScript II™ Reverse Transcriptase (Invitrogen, USA) according to the manufacturer's instruction. qRT – PCRs were performed in a total volume of 25 µl using PerfeCTa™ SYBR® Green FastMix™ ROX (Quanta Biosciences, USA) and an Applied Biosystems StepOne™ Plus cycler. The housekeeping gene rpoB served as an internal standard [103], [104]. All transcription analyses were done on at least three independent biological samples and with four technical replicates each. The primers used are listed in Table S1.
Motility assays
Swimming motility of the wild type, of the Δlmo0799 and of the ΔsigB mutant strain was tested on semi-solid BHI agar (0.3%) plates, inoculated with 2 µl of mid-log bacterial cultures (OD600 ∼0.7) grown in BHI at 37°C. Plates were sealed and incubated for 18 h at the indicated temperature in the complete dark or under exposure to blue (λ = 455 nm) or red (λ = 625 nm) light, respectively, at an illuminance of 30 microeinstein/m2/s. Motility was quantified as the diameter of the swimming colony.
Infection assays with Caco-2 enterocytes
Infection assays were performed in triplicate using 24-well tissue culture plates (Greiner Bio, Germany), seeded with 3×105 Caco-2 cells per well and then incubated at 37°C and with 5% CO2. After 24 hours the wells were checked for a semi-confluent monolayer, the cells were washed with 1xPBS containing Ca/Mg and infected using low-intensity, diffuse infrared illumination (LED, λ = 850 nm) and Dipol D2MVSL night vision goggles (Gross, Germany) for observation. RPMI 1640 as medium and an m.o.i. of 20 for L. monocytogenes EGD-e wild type or its Δlmo0799 mutant were used, the bacteria had been preincubated at 37°C with 0.3 M NaCl either in the dark or under exposure to blue light (λ = 455 nm) for 10 min at an illuminance of 30 microeinstein/m2/s. The plates were wrapped in two layers of aluminium foil and incubated for 1 h at 37°C in a 5% CO2 atmosphere. After 1 h the infection medium was replaced by RPMI with 100 µg ml−1 gentamicin and incubation was continued for another hour. The experimental steps after the addition of gentamicin were carried out at ambient light. At the end of the infection period cells were washed twice with 1× PBS and lysed with distilled water for 30 min on ice. Serial dilutions of the bacteria-containing lysate were plated in triplicate on BHI plates which were incubated for 24 h at 37°C. Colonies were counted and absolute (CFU/ml) and relative (percent) infection rates were calculated. All infection experiments were repeated at least three times.
Sequence data acquisition, bioinformatics and data analysis
Protein sequences were obtained from the sequenced genomes of L. monocytogenes EGD-e [72] and B. subtilis 168 [76], respectively, using the Genolist website at the Pasteur Institute Paris (http://genolist.pasteur.fr/ListiList; http://genolist.pasteur.fr/SubtiList). Homology searches, using BLAST [105], were performed on these websites, for L. ivanovii at http://genolist.pasteur.fr/LivaList and for other listerial and bacterial genomes at NCBI (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi). Sequence alignments were done with ClustalW2 [106] at the European Bioinformatics Institute (EBI, UK) facility (http://www.ebi.ac.uk/Tools/clustalw2/index.html), using the default settings. For 3-D modelling of proteins the SWISS-MODEL server (http://swissmodel.expasy.org/workspace/) at the Swiss Institute of Bioinformatics (SIB) was used in the automated mode [107], [108]. Protein domain analysis was performed with SMART6 [90] at http://smart.embl-heidelberg.de and also using InterPro [91] at http://www.ebi.ac.uk/interpro/. For the analysis of the results from qRT-PCR experiments the Applied Biosystems' StepOne™ Software was used.
Supporting Information
Oligonucleotide primers used in this study.
(PDF)
Effect of blue and red light on trxA, lmo0799, kat and sod expression. (A, B) Transcription analysis by qRT-PCR of trxA for wild type, Δ0799 and ΔsigB mutants. (C, D) Transcription analysis by qRT-PCR of lmo0799, kat and sod for wild type. The strains were grown at 37°C in BHI. Cells were harvested in mid-log phase (OD600 ∼0.9) and exposed for 10 min to blue (455 nm) or red (625 nm) light as described in material and methods. The results from the qRT-PCR analysis, obtained with a StepOnePlus Real-Time PCR system (Applied Biosystems Inc.) were normalized using rpoB as an internal standard [103], [104] and expressed as fold change with the values for wild type without light set as 1.0. Calculations were performed with the StepOne Software v2.1 (Applied Biosystems Inc.). Means and standard deviations from three independent biological samples and four technical replicates per sample.
(PDF)
Domain analysis and alignment of RsbR paralogues. (A) Graphical representation of domains identified by SMART (http://smart.embl-heidelberg.de) [92] in RsbR (Lmo0889), Lmo0161, Lmo1642, Lmo1842, Lmo0799 and RsbS (Lmo0890) of L. monocytogenes EGD-e. The protein sequences were obtained from the ListiList website (http://genolist.pasteur.fr/ListiList). The LOV domain is not implemented in SMART, therefore this domain of Lmo0799 is depicted as PAS-PAC (Per-Arnt-Sim signal sensor domain/PAS-associated domain). LOV domains are a subfamily of the PAS superfamily [51]. (B) Amino acid alignment of RsbRA-D of B. subtilis, RsbR and putative paralogues of L. monocytogenes EGD-e, using ClustalW2 [106]. The prefix Bs denotes proteins from B. subtilis, Lm from L. monocytogenes. Asterisks below the sequence indicate identical, double points very similar amino acids. The C-terminal STAS domain is indicated, the crucial threonines (T171/T205 in B.s. RsbRA, T175/T209 in L.m. RsbR) are highlighted in yellow, negatively charged amino acids (aspartate D, glutamate E) in the putative RsbR paralogues in blue. Further explanation in the text. The B. subtilis protein sequences were from SubtiList (http://genolist.pasteur.fr/SubtiList), the L. monoytogenes sequences from ListiList (http://genolist.pasteur.fr/ListiList). The GenBank accession nos. for the respective genome sequences are AL591824 (L.m.) and AL009126 (B.s.).
(PDF)
Acknowledgments
The authors thank very much Georg Nagel, Dept. of Molecular Plant Physiology, University of Würzburg, for technical support, valuable advice and fruitful discussions and Thomas Rudel, Dept. of Microbiology, University of Würzburg, for helpful advice.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the German Federal Ministry of Education and Research via Project Management Jülich, ERA-NET PathoGenoMics (www.fz-juelich.de/ptj/pathogenomics) (grant no. 0313939C) and by the EU Network of Excellence EuroPathoGenomics (www.noe-epg.uni-wuerzburg.de/). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
References
- 1.Ivanek R, Gröhn YT, Wiedmann M. Listeria monocytogenes in multiple habitats and host populations: review of available data for mathematical modeling. Foodborne Pathog Dis. 2007;3:319–336. doi: 10.1089/fpd.2006.3.319. [DOI] [PubMed] [Google Scholar]
- 2.Saunders BM, Wiedmann M. Ecology of Listeria species and L. monocytogenes in the natural environment. In: Ryser ET, Marth EH, editors. Listeria, Listeriosis and Food Safety. Boca Raton: CRC Press; 2007. pp. 21–54. [Google Scholar]
- 3.Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9:1236–1243. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing EJ, Gregory SH. Listeria monocytogenes: clinical and experimental update. J Infect Dis. 2002;185(Suppl 1):S18–24. doi: 10.1086/338465. [DOI] [PubMed] [Google Scholar]
- 7.Ooi ST, Lorber B. Gastroenteritis due to Listeria monocytogenes. Clin Infect Dis. 2005;40:1327–1332. doi: 10.1086/429324. [DOI] [PubMed] [Google Scholar]
- 8.Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 9.Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes Infect. 2008;10:1041–1050. doi: 10.1016/j.micinf.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Kreft J, Vázquez-Boland JA, Altrock S, Dominguez-Bernal G, Goebel W. Pathogenicity islands and other virulence elements in Listeria. Curr Top Microbiol Immunol. 2002;264:109–125. [PubMed] [Google Scholar]
- 11.Schmid MW, Ng EYW, Lampidis R, Emmerth M, Walcher M, et al. Evolutionary history of the genus Listeria and its virulence genes. Syst Appl Microbiol. 2005;28:1–18. doi: 10.1016/j.syapm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Bierne H, Sabet C, Personnic N, Cossart P. Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect. 2007;9:1156–1166. doi: 10.1016/j.micinf.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Leimeister-Wächter M, Haffner C, Domann E, Goebel W, Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc Natl Acad Sci USA. 1990;87:8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreft J, Vázquez-Boland JA. Regulation of virulence genes in Listeria. Int J Med Microbiol. 2001;291:145–157. doi: 10.1078/1438-4221-00111. [DOI] [PubMed] [Google Scholar]
- 15.Scortti M, Monzó HJ, Lacharme-Lora L, Lewis DA, Vázquez-Boland JA. The PrfA virulence regulon. Microbes Infect. 2007;9:1196–1207. doi: 10.1016/j.micinf.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Gray MJ, Freitag NE, Boor KJ. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect Immun. 2006;74:2505–2512. doi: 10.1128/IAI.74.5.2505-2512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freitag NE, Port GC, Miner MD. Listeria monocytogenes - from saprophyte to intracellular pathogen. Nat Rev Microbiol. 2009;7:623–628. doi: 10.1038/nrmicro2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gahan CGM, Hill C. Gastrointestinal phase of Listeria monocytogenes infection. J Appl Microbiol. 2005;98:1345–1353. doi: 10.1111/j.1365-2672.2005.02559.x. [DOI] [PubMed] [Google Scholar]
- 19.Chaturongakul S, Raengpradub S, Wiedmann M, Boor KJ. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 2008;16:388–396. doi: 10.1016/j.tim.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturongakul S, Raengpradub S, Palmer ME, et al. Transcriptomic and phenotypic analysis identify co-regulated, overlapping regulons among PrfA, CtsR, HrcA and the alternative sigma factors sigma B, C, H and sigma L in Listeria monocytogenes. . Appl Environ Microbiol. 2010 doi: 10.1128/AEM.00952-10. doi: 10.1128/AEM.00952-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olesen I, Vogensen FK, Jespersen L. Gene transcription and virulence potential of Listeria monocytogenes strains after exposure to acidic and NaCl stress. Foodborne Pathog Dis. 2009;6:669–680. doi: 10.1089/fpd.2008.0243. [DOI] [PubMed] [Google Scholar]
- 22.Sleator RD, Watson D, Hill C, Gahan CGM. The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology. 2009;155:2463–2475. doi: 10.1099/mic.0.030205-0. [DOI] [PubMed] [Google Scholar]
- 23.Haldenwang WG, Losick R. Novel RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci USA. 1980;77:7000–7004. doi: 10.1073/pnas.77.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecker M, Pané-Farré J, Völker U. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu Rev Microbiol. 2007;61:215–236. doi: 10.1146/annurev.micro.61.080706.093445. [DOI] [PubMed] [Google Scholar]
- 25.Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. General stress transcription factor sigmaB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker LA, Cetin MS, Hutkins RW, Benson AK. Identification of the gene encoding the alternative sigma factor sigmaB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaturongakul S, Boor KJ. RsbT and RsbV contribute to sigmaB-dependent survival under environmental, energy, and intracellular stress conditions in Listeria monocytogenes. Appl Environ Microbiol. 2004;70:5349–5356. doi: 10.1128/AEM.70.9.5349-5356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaturongakul S, Boor KJ. SigmaB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl Environ Microbiol. 2006;72:5197–5203. doi: 10.1128/AEM.03058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Raengpradub S, Schwab U, Loss C, Orsi RH, et al. Phenotypic and transcriptomic analyses demonstrate interactions between the transcriptional regulators CtsR and Sigma B in Listeria monocytogenes. Appl Environ Microbiol. 2007;73:7967–7980. doi: 10.1128/AEM.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Oliver HF, Raengpradub S, Palmer ME, Orsi RH, et al. Transcriptomic and phenotypic analyses suggest a network between the transcriptional regulators HrcA and sigmaB in Listeria monocytogenes. Appl Environ Microbiol. 2007;73:7981–7991. doi: 10.1128/AEM.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadon CA, Bowen BM, Wiedmann M, Boor KJ. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect Immun. 2002;70:3948–3952. doi: 10.1128/IAI.70.7.3948-3952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. Listeria monocytogenes sigma B regulates stress response and virulence functions. J Bacteriol. 2003;185:5722–5734. doi: 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazmierczak MJ, Wiedmann M, Boor KJ. Contributions of Listeria monocytogenes sigmaB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth. Microbiology. 2006;152:1827–1838. doi: 10.1099/mic.0.28758-0. [DOI] [PubMed] [Google Scholar]
- 34.Ollinger J, Bowen B, Wiedmann M, Boor KJ, Bergholz TM. Listeria monocytogenes sigmaB modulates PrfA-mediated virulence factor expression. Infect Immun. 2009;77:2113–2124. doi: 10.1128/IAI.01205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459:950–956. doi: 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 36.Akbar S, Kang CM, Gaidenko TA, Price CW. Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 37.Akbar S, Gaidenko TA, Kang CM, O'Reilly M, Devine KM, et al. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor sigma(B) of Bacillus subtilis. J Bacteriol. 2001;183:1329–1338. doi: 10.1128/JB.183.4.1329-1338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C-C, Lewis RJ, Harris R, Yudkin MD, Delumeau O. A supramolecular complex in the environmental stress signalling pathway of Bacillus subtilis. Mol Microbiol. 2003;49:1657–1669. doi: 10.1046/j.1365-2958.2003.03663.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim T-J, Gaidenko TA, Price CW. A multicomponent protein complex mediates environmental stress signaling in Bacillus subtilis. J Mol Biol. 2004;341:135–150. doi: 10.1016/j.jmb.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 40.Kim T-J, Gaidenko TA, Price CW. In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis. J Bacteriol. 2004;186:6124–6132. doi: 10.1128/JB.186.18.6124-6132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pané-Farré J, Lewis RJ, Stülke J. The RsbRST stress module in bacteria: a signalling system that may interact with different output modules. J Mol Microbiol Biotechnol. 2005;9:65–76. doi: 10.1159/000088837. [DOI] [PubMed] [Google Scholar]
- 42.Delumeau O, Chen C-C, Murray JW, Yudkin MD, Lewis RJ. High-molecular-weight complexes of RsbR and paralogues in the environmental signaling pathway of Bacillus subtilis. J Bacteriol. 2006;188:7885–7892. doi: 10.1128/JB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardwick SW, Pané-Farré J, Delumeau O, Marles-Wright J, Murray JW, et al. Structural and functional characterization of partner switching regulating the environmental stress response in Bacillus subtilis. J Biol Chem. 2007;282:11562–11572. doi: 10.1074/jbc.M609733200. [DOI] [PubMed] [Google Scholar]
- 44.Marles-Wright J, Grant T, Delumeau O, van Duinen G, Firbank SJ, et al. Molecular architecture of the “stressosome,” a signal integration and transduction hub. Science. 2008;322:92–96. doi: 10.1126/science.1159572. [DOI] [PubMed] [Google Scholar]
- 45.Reeves A, Martinez L, Haldenwang W. Expression of, and in vivo stressosome formation by, single members of the RsbR protein family in Bacillus subtilis. Microbiology. 2010;156:990–998. doi: 10.1099/mic.0.036095-0. [DOI] [PubMed] [Google Scholar]
- 46.Avila-Pérez M, Hellingwerf KJ, Kort R. Blue light activates the sigmaB-dependent stress response of Bacillus subtilis via YtvA. J Bacteriol. 2006;188:6411–6414. doi: 10.1128/JB.00716-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaidenko TA, Kim T-J, Weigel AL, Brody MS, Price CW. The blue-light receptor YtvA acts in the environmental stress signaling pathway of Bacillus subtilis. J Bacteriol. 2006;188:6387–6395. doi: 10.1128/JB.00691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki N, Takaya N, Hoshino T, Nakamura A. Enhancement of a sigma(B)-dependent stress response in Bacillus subtilis by light via YtvA photoreceptor. J Gen Appl Microbiol. 2007;53:81–88. doi: 10.2323/jgam.53.81. [DOI] [PubMed] [Google Scholar]
- 49.Losi A, Polverini E, Quest B, Gärtner W. First evidence for phototropin-related blue-light receptors in prokaryotes. Biophysical J. 2002;82:2627–2634. doi: 10.1016/S0006-3495(02)75604-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Losi A. The bacterial counterparts of plant phototropins. Photochem Photobiol Sci. 2004;3:566–574. doi: 10.1039/b400728j. [DOI] [PubMed] [Google Scholar]
- 51.Crosson S, Rajagopal S, Moffat K. The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry. 2003;42:2–10. doi: 10.1021/bi026978l. [DOI] [PubMed] [Google Scholar]
- 52.Aravind L, Koonin EV. The STAS domain - a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10:R53–55. doi: 10.1016/s0960-9822(00)00335-3. [DOI] [PubMed] [Google Scholar]
- 53.Buttani V, Losi A, Eggert T, Krauss U, Jaeger K-E, et al. Conformational analysis of the blue-light sensing protein YtvA reveals a competitive interface for LOV-LOV dimerization and interdomain interactions. Photochem Photobiol Sci. 2007;6:41–49. doi: 10.1039/b610375h. [DOI] [PubMed] [Google Scholar]
- 54.Buttani V, Losi A, Polverini E, Gärtner W. Blue news: NTP binding properties of the blue-light sensitive YtvA protein from Bacillus subtilis. FEBS Lett. 2006;580:3818–3822. doi: 10.1016/j.febslet.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Losi A, Quest B, Gärtner W. Listening to the blue: the time-resolved thermodynamics of the bacterial blue-light receptor YtvA and its isolated LOV domain. Photochem Photobiol Sci. 2003;2:759–766. doi: 10.1039/b301782f. [DOI] [PubMed] [Google Scholar]
- 56.Möglich A, Moffat K. Structural basis for light-dependent signaling in the dimeric LOV domain of the photosensor YtvA. J Mol Biol. 2007;373:112–126. doi: 10.1016/j.jmb.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y, Cao Z, Livoti E, Krauss U, Jaeger K-E, et al. Interdomain signalling in the blue-light sensing and GTP-binding protein YtvA: a mutagenesis study uncovering the importance of specific protein sites. Photochem Photobiol Sci. 2010;9:47–56. doi: 10.1039/b9pp00075e. [DOI] [PubMed] [Google Scholar]
- 58.Avila-Pérez M, Vreede J, Tang Y, Bende O, Losi A, et al. In vivo mutational analysis of YtvA from Bacillus subtilis: mechanism of light activation of the general stress response. J Biol Chem. 2009;284:24958–24964. doi: 10.1074/jbc.M109.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avila-Pérez M, van der Steen JB, Kort R, Hellingwerf KJ. Red light activates the sigmaB-mediated general stress response of Bacillus subtilis via the energy branch of the upstream signaling cascade. J Bacteriol. 2010;192:755–762. doi: 10.1128/JB.00826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis SJ, Vener AV, Vierstra RD. Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science. 1999;286:2517–2520. doi: 10.1126/science.286.5449.2517. [DOI] [PubMed] [Google Scholar]
- 61.Vierstra RD, Davis SJ. Bacteriophytochromes: new tools for understanding phytochrome signal transduction. Semin Cell Dev Biol. 2000;11:511–521. doi: 10.1006/scdb.2000.0206. [DOI] [PubMed] [Google Scholar]
- 62.Hellingwerf KJ. The molecular basis of sensing and responding to light in microorganisms. Antonie Van Leeuwenhoek. 2002;81:51–59. doi: 10.1023/a:1020521424582. [DOI] [PubMed] [Google Scholar]
- 63.Purcell EB, Crosson S. Photoregulation in prokaryotes. Curr Opin Microbiol. 2008;11:168–178. doi: 10.1016/j.mib.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Braatsch S, Klug G. Blue light perception in bacteria. Photosyn Res. 2004;79:45–57. doi: 10.1023/B:PRES.0000011924.89742.f9. [DOI] [PubMed] [Google Scholar]
- 65.Takano H, Asker D, Beppu T, Ueda K. Genetic control for light-induced carotenoid production in non-phototrophic bacteria. J Ind Microbiol Biotechnol. 2006;33:88–93. doi: 10.1007/s10295-005-0005-z. [DOI] [PubMed] [Google Scholar]
- 66.van der Horst MA, Key J, Hellingwerf KJ. Photosensing in chemotrophic, non-phototrophic bacteria: let there be light sensing too. Trends Microbiol. 2007;15:554–562. doi: 10.1016/j.tim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Losi A, Gärtner W. Bacterial bilin- and flavin-binding photoreceptors. Photochem Photobiol Sci. 2008;7:1168–1178. doi: 10.1039/b802472c. [DOI] [PubMed] [Google Scholar]
- 68.Idnurm A, Crosson S. The photobiology of microbial pathogenesis. PLoS Pathog. 2009;5:e1000470. doi: 10.1371/journal.ppat.1000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberpichler I, Rosen R, Rasouly A, Vugman M, Ron EZ, et al. Light affects motility and infectivity of Agrobacterium tumefaciens. Environ Microbiol. 2008;10:2020–2029. doi: 10.1111/j.1462-2920.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- 70.Cao Z, Buttani V, Losi A, Gärtner W. A blue light inducible two-component signal transduction system in the plant pathogen Pseudomonas syringae pv. tomato. Biophys J. 2008;94:897–905. doi: 10.1529/biophysj.107.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swartz TE, Tseng T-S, Frederickson MA, Paris G, Comerci DJ, et al. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science. 2007;317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- 72.Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, et al. Comparative genomics of Listeria species. Science. 2001;294:849–852. doi: 10.1126/science.1063447. [DOI] [PubMed] [Google Scholar]
- 73.Gaidenko TA, Yang X, Lee YM, Price CW. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the environmental stress signaling pathway of Bacillus subtilis. J Mol Biol. 1999;288:29–39. doi: 10.1006/jmbi.1999.2665. [DOI] [PubMed] [Google Scholar]
- 74.Buttani V, Gärtner W, Losi A. NTP-binding properties of the blue-light receptor YtvA and effects of the E105L mutation. Eur Biophys J. 2007;36:831–839. doi: 10.1007/s00249-007-0155-1. [DOI] [PubMed] [Google Scholar]
- 75.Ogata H, Cao Z, Losi A, Gärtner W. Crystallization and preliminary X-ray analysis of the LOV domain of the blue-light receptor YtvA from Bacillus amyloliquefaciens FZB42. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:853–855. doi: 10.1107/S1744309109026670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 77.Buchrieser C. Biodiversity of the species Listeria monocytogenes and the genus Listeria. Microbes Infect. 2007;9:1147–1155. doi: 10.1016/j.micinf.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Gardan R, Duché O, Leroy-Sétrin S, Labadie J, Consortium ELG. Role of ctc from Listeria monocytogenes in osmotolerance. Appl Environ Microbiol. 2003;69:154–161. doi: 10.1128/AEM.69.1.154-161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hain T, Hossain H, Chatterjee SS, Machata S, Volk U, et al. Temporal transcriptomic analysis of the Listeria monocytogenes EGD-e sigmaB regulon. BMC Microbiol. 2008;8:20. doi: 10.1186/1471-2180-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raengpradub S, Wiedmann M, Boor KJ. Comparative analysis of the sigma B-dependent stress responses in Listeria monocytogenes and Listeria innocua strains exposed to selected stress conditions. Appl Environ Microbiol. 2008;74:158–171. doi: 10.1128/AEM.00951-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oliver HF, Orsi RH, Wiedmann M, Boor KJ. Listeria monocytogenes σB has a small core regulon and a conserved role in virulence but makes differential contributions to stress tolerance across a diverse collection of strains. Appl Environ Microbiol. 2010;76:4216–4232. doi: 10.1128/AEM.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferreira A, Gray M, Wiedmann M, Boor KJ. Comparative genomic analysis of the sigB operon in Listeria monocytogenes and in other Gram-positive bacteria. Curr Microbiol. 2004;48:39–46. doi: 10.1007/s00284-003-4020-x. [DOI] [PubMed] [Google Scholar]
- 83.Sharrock RA. The phytochrome red/far-red photoreceptor superfamily. Genome Biol. 2008;9:230. doi: 10.1186/gb-2008-9-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hughes J, Lamparter T, Mittmann F, Hartmann E, Gärtner W, et al. A prokaryotic phytochrome. Nature. 1997;386:663. doi: 10.1038/386663a0. [DOI] [PubMed] [Google Scholar]
- 85.Karniol B, Wagner JR, Walker JM, Vierstra RD. Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem J. 2005;392:103–116. doi: 10.1042/BJ20050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghetti F, Checcucci G, Lenci F. Photosensitized reactions as primary molecular events in photomovements of microorganisms. J Photochem Photobiol B. 1992;15:185–198. [Google Scholar]
- 87.Hellingwerf KJ, Hoff WD, Crielaard W. Photobiology of microorganisms: how photosensors catch a photon to initialize signalling. Mol Microbiol. 1996;21:683–693. doi: 10.1046/j.1365-2958.1996.411402.x. [DOI] [PubMed] [Google Scholar]
- 88.Redmond RW, Gamlin JN. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol. 1999;70:391–475. [PubMed] [Google Scholar]
- 89.Ferreira A, O'Byrne CP, Boor KJ. Role of sigmaB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl Environ Microbiol. 2001;67:4454–4457. doi: 10.1128/AEM.67.10.4454-4457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Letunic I, Doerks T, Bork P. SMART 6: recent updates and new developments. Nucleic Acids Res. 2009;37:D229–232. doi: 10.1093/nar/gkn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hunter S, Apweiler R, Attwood T, Bairoch A, Bateman A, et al. InterPro: the integrative protein signature database. Nucleic Acids. 2009;Res37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, et al. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009;139:770–779. doi: 10.1016/j.cell.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 93.McGann P, Wiedmann M, Boor KJ. The alternative sigma factor SigB and the virulence gene regulator PrfA both regulate transcription of Listeria monocytogenes internalins. Appl Environ Microbiol. 2007;73:2919–2930. doi: 10.1128/AEM.02664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim H, Marquis H, Boor KJ. SigmaB contributes to Listeria monocytogenes invasion by controlling expression of inlA and inlB. Microbiol. 2005;151:3215–3222. doi: 10.1099/mic.0.28070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stritzker J, Schoen C, Goebel W. Enhanced synthesis of internalin A in aro mutants of Listeria monocytogenes indicates posttranscriptional control of the inlAB mRNA. J Bacteriol. 2005;187:2836–2845. doi: 10.1128/JB.187.8.2836-2845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Personnic N, Bruck S, Nahori M-A, Toledo-Arana A, Nikitas G, et al. The stress-induced virulence protein InlH controls interleukin-6 production during murine listeriosis. Infect Immun. 2010;78:1979–1989. doi: 10.1128/IAI.01096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams T, Joseph B, Beier D, Goebel W, Kuhn M. Response regulator DegU of Listeria monocytogenes regulates the expression of flagella-specific genes. FEMS Microbiol Lett. 2005;252:287–298. doi: 10.1016/j.femsle.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Kamp H, Higgins DE. Transcriptional and post-transcriptional regulation of the GmaR antirepressor governs temperature-dependent control of flagellar motility in Listeria monocytogenes. Mol Microbiol. 2009;74:421–435. doi: 10.1111/j.1365-2958.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thorington L. Spectral, irradiance, and temporal aspects of natural and artificial light. Ann NY Acad Sci. 1985;453:28–54. doi: 10.1111/j.1749-6632.1985.tb11796.x. [DOI] [PubMed] [Google Scholar]
- 100.Kondo T, Ishiura M. The circadian clock of cyanobacteria. Bioessays. 2000;22:10–15. doi: 10.1002/(SICI)1521-1878(200001)22:1<10::AID-BIES4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 101.Sambrook J, William Russell D. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning: a laboratory manual, third edition. [Google Scholar]
- 102.Biswas I, Gruss A, Ehrlich SD, Maguin E. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol. 1993;175:3628–3635. doi: 10.1128/jb.175.11.3628-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sue D, Fink D, Wiedmann M, Boor KJ. SigmaB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150:3843–3855. doi: 10.1099/mic.0.27257-0. [DOI] [PubMed] [Google Scholar]
- 104.Mertins S, Joseph B, Goetz M, Ecke R, Seidel G, et al. Interference of components of the phosphoenolpyruvate phosphotransferase system with the central virulence gene regulator PrfA of Listeria monocytogenes. J Bacteriol. 2007;189:473–490. doi: 10.1128/JB.00972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 107.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 108.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oligonucleotide primers used in this study.
(PDF)
Effect of blue and red light on trxA, lmo0799, kat and sod expression. (A, B) Transcription analysis by qRT-PCR of trxA for wild type, Δ0799 and ΔsigB mutants. (C, D) Transcription analysis by qRT-PCR of lmo0799, kat and sod for wild type. The strains were grown at 37°C in BHI. Cells were harvested in mid-log phase (OD600 ∼0.9) and exposed for 10 min to blue (455 nm) or red (625 nm) light as described in material and methods. The results from the qRT-PCR analysis, obtained with a StepOnePlus Real-Time PCR system (Applied Biosystems Inc.) were normalized using rpoB as an internal standard [103], [104] and expressed as fold change with the values for wild type without light set as 1.0. Calculations were performed with the StepOne Software v2.1 (Applied Biosystems Inc.). Means and standard deviations from three independent biological samples and four technical replicates per sample.
(PDF)
Domain analysis and alignment of RsbR paralogues. (A) Graphical representation of domains identified by SMART (http://smart.embl-heidelberg.de) [92] in RsbR (Lmo0889), Lmo0161, Lmo1642, Lmo1842, Lmo0799 and RsbS (Lmo0890) of L. monocytogenes EGD-e. The protein sequences were obtained from the ListiList website (http://genolist.pasteur.fr/ListiList). The LOV domain is not implemented in SMART, therefore this domain of Lmo0799 is depicted as PAS-PAC (Per-Arnt-Sim signal sensor domain/PAS-associated domain). LOV domains are a subfamily of the PAS superfamily [51]. (B) Amino acid alignment of RsbRA-D of B. subtilis, RsbR and putative paralogues of L. monocytogenes EGD-e, using ClustalW2 [106]. The prefix Bs denotes proteins from B. subtilis, Lm from L. monocytogenes. Asterisks below the sequence indicate identical, double points very similar amino acids. The C-terminal STAS domain is indicated, the crucial threonines (T171/T205 in B.s. RsbRA, T175/T209 in L.m. RsbR) are highlighted in yellow, negatively charged amino acids (aspartate D, glutamate E) in the putative RsbR paralogues in blue. Further explanation in the text. The B. subtilis protein sequences were from SubtiList (http://genolist.pasteur.fr/SubtiList), the L. monoytogenes sequences from ListiList (http://genolist.pasteur.fr/ListiList). The GenBank accession nos. for the respective genome sequences are AL591824 (L.m.) and AL009126 (B.s.).
(PDF)