Abstract
IL-27 is a pleiotropic cytokine with both activating and inhibitory functions on innate and acquired immunity. IL-27 is expressed at sites of inflammation in cytokine-driven autoimmune/inflammatory diseases, such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, and sarcoidosis. However, its role in modulating disease pathogenesis is still unknown. In this study, we found that IL-27 production is induced by TNF-α in human macrophages (Mϕ) and investigated the effects of IL-27 on the responses of primary human Mϕ to the endogenous inflammatory cytokines TNF-a and IL-1. In striking contrast to IL-27–mediated augmentation of TLR-induced cytokine production, we found that IL-27 suppressed Mϕ responses to TNF-α and IL-1β, thus identifying an anti-inflammatory function of IL-27. IL-27 blocked the proximal steps of TNF-α signaling by downregulating cell-surface expression of the signaling receptors p55 and p75. The mechanism of inhibition of IL-1 signaling was downregulation of the ligand-binding IL-1RI concomitant with increased expression of the receptor antagonist IL-1Ra and the decoy receptor IL-1RII. These findings provide a mechanism for suppressive effects of IL-27 on innate immune cells and suggest that IL-27 regulates inflammation by limiting activation of Mf by inflammatory cytokines while preserving initial steps in host defense by augmenting responses to microbial products.
Interleukin-27 is a heterodimeric cytokine composed of EBI3 and p28 subunits that share similarity with the p40 and p35 subunits of IL-12 (1). The IL-27R is a heterodimer composed of a WSX-1 subunit, which confers ligand specificity, and the gp130 signaling subunit, which is also used by the IL-6 family of cytokines (2). WSX-1 bears a STAT1-binding site, and gp130 has four STAT3-docking sites, two of which can also recruit STAT1. In lymphocytes, IL-27 activates STAT1, STAT3, STAT5, and low amounts of STAT4 (3). In myeloid cells, IL-27–induced phosphorylation of STAT1 and STAT3 has been observed (2). Our group has found that in human monocytes (Mo), IL-27 induces sustained activation of STAT1, resulting in potent induction of inflammatory STAT1 target genes and augmentation of TLR responses. In contrast, STAT3-mediated gene induction and suppressive functions were not readily apparent in IL-27–stimulated primary human Mo (4).
The major cellular source of IL-27 is APC: Mo, macrophages (Mϕ), and dendritic cells (DCs) (1). In the setting of infections, pathogen recognition by TLRs triggers the synthesis of IL-27. Downstream of TLRs, MyD88-dependent activation of NF-κB and TRIF-dependent activation of IFN regulatory factor (IRF) 3 drive the induction of EBI3 and p28 subunits of IL-27 (5). Type I and II IFNs are also involved in the induction of p28 by activating several members of the IRF family including IRF1, IRF3, IRF7, and IRF9 (6–9). Recently, it has been reported that type I IFNs trigger production of IL-27 by downregulating the expression of the intracellular isoform of osteopontin in murine DCs (10).
In humans, IL-27 is expressed at sites of chronic sterile inflammation, such as the pannus (inflammatory synovial tissue) of rheumatoid arthritis (RA) (11), psoriatic skin lesions (12), inflamed intestine in Crohn's disease, and sarcoid granulomas (13). IL-27 is a pleiotropic cytokine, and its role in these diseases remains enigmatic and elusive (14–17). Initially, IL-27 was discovered as a cytokine that promotes the early stages of Th1 differentiation by inducing expression of IL-12Rb2 and rendering T cells responsive to the Th1-promoting effects of IL-12 (3, 18, 19). More recently, the preponderance of evidence suggests that the dominant in vivo function of IL-27 is immunoregulatory by suppressing Th1, Th2, and Th17 cells (5). IL-27 also has both activating and suppressive effects on innate immune cells (16). Our group has described STAT1-mediated proinflammatory effects of IL-27 in human Mo including: 1) induction of chemokines, such as CXCL9 and CXCL10, that are well-known for recruiting inflammatory cells; and 2) augmented production of proinflammatory cytokines in response to TLR ligands (4). In contrast, our laboratory and others (20, 21) observed that IL-27 has tissue-protective capacities by inhibiting osteoclastogenesis. Other reports suggest that IL-27 can suppress inflammatory cytokine production in Mf and DCs (22, 23), although these suppressive effects in murine cells appeared modest relative to IL-10, the potent Mϕ-deactivating cytokine that strongly activates STAT3 (24). Overall, evidence from animal models supports the concept that IL-27, depending on the context, can be either proor anti-inflammatory (25). Along these lines, in adjuvant-induced arthritis, proteoglycan-induced arthritis, and animal models of diabetes mellitus, IL-27 is pathogenic (26–28), whereas in experimental autoimmune encephalomyelitis and in collagen-induced arthritis, IL-27 is protective and may represent a potential treatment (11, 29).
In our current study, we wished to investigate the potential role of IL-27 in the process of chronic sterile inflammation. We first observed that TNF-α, a key player in the synovial inflammation of RA, skin inflammation of psoriasis, and intestinal inflammation of inflammatory bowel disease, triggers the production of large amounts of IL-27 by human Mϕ (synovial fluid Mϕ of patients with RA or Mϕ generated from peripheral blood CD14+ Mo). In striking contrast to augmentation of inflammatory TLR responses (4), we found that IL-27 suppressed responses of human Mf to endogenous inflammatory factors TNF-α and IL-1β, thus identifying a potent anti-inflammatory function of IL-27. In contrast to IL-10, which suppresses both TLR and TNF-α/IL-1 responses by STAT3-mediated suppression of downstream cytokine gene transcription (24, 30), IL-27 worked by suppressing proximal TNF-α and IL-1 signaling. IL-27 suppressed TNF-α–mediated proinflammatory functions by downregulating cell-surface expression of the signaling receptors TNFRSF1A (p55) and TNFRSF1B (p75). IL-27 inhibited IL-1β–induced signaling in human Mϕ by downregulating the expression of the signaling receptor IL-1RI, inducing expression of the receptor antagonist IL-1Ra, and by upregulating the expression of the decoy receptor IL-1RII. These results identify a mechanism by which IL-27 suppresses Mϕ activation by endogenous inflammatory factors and provide insights about the basis of context-dependent activating versus suppressive functions of IL-27 in innate immune and chronic inflammatory responses.
Materials and Methods
Cell culture
PBMCs from whole blood of healthy volunteers and mononuclear cells from synovial fluids of 13 patients with RA (fulfilling the American College of Rheumatology criteria [31]) were isolated by density gradient centrifugation using Ficoll (Invitrogen Life Technologies, Carlsbad, CA). CD14+ cells were purified from fresh PBMCs and synovial fluid-derived mononuclear cells using anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA) as recommended by the manufacturer. Purity of CD14+ cells was >97% as verified by FACS. Cells were cultured in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% FBS (HyClone, Logan, UT) and 100 U/ml penicillin and streptomycin in the presence or absence of 10 ng/ml human M-CSF (hM-CSF) (PeproTech, Rocky Hill, NJ). The following human cytokines were used to stimulate cells as indicated: human (h)IL-27 (3–100 ng/ml; R&D Systems, Minneapolis, MN), hTNF-α (10 ng/ml; PeproTech), hIL-1β (10 ng/ml; R&D Systems), and hIL-10 (100 ng/ml; R&D Systems). In some experiments, etanercept (10 μg/ml; Amgen, Thousand Oaks, CA) was used to block the effects of TNF-α; hIgG1 (10 μg/ml; R&D Systems) was used as an isotype control. The following TLR ligands were used to stimulate cells as indicated: Pam3CysSer(Lys)4 (EMC Microcollections, Tübingen, Germany), LPS (InvivoGen, San Diego, CA), and CL097 (InvivoGen). In all of the experiments, the culture medium was not replenished, and cells were not washed before cytokine or TLR ligand stimulation. The cell density used in our cultures for immunoblotting, IL-27, IL-8, and IL-6 ELISA was 2 × 106 cells/ml, whereas for FACS, IL-1R antagonist (IL-1Ra) ELISA, and RNA extraction was 4.5 × 106 cells/ml. Experiments with human cells were approved by the Hospital for Special Surgery Institutional Review Board.
Immunoblotting, ELISA, and FACS
Whole-cell extracts were prepared by lysis of cells in buffer containing 20 mM HEPES (pH 7), 300 mM NaCl, 10 mM KCl, 1 mM MgCl2, 0.1% Triton X-100, 0.5 mM DTT, 20% glycerol, and 1× proteinase inhibitor mixture (Roche, Basel, Switzerland). A total of 5 or 10 μg whole-cell lysates were fractioned on polyacrylamide gels using SDS-PAGE, transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA), and incubated with specific Abs. ECL was used for detection. Abs to ERK, p-ERK, p-p38, p-IκBα, IκBα, and cleaved IL-1β were purchased from Cell Signaling Technology (Beverly, MA), and the Abs to p38 and c-Fos were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). For sandwich ELISA, paired capture and detection Abs to IL-27 (R&D Systems), IL-8 (R&D Systems), IL-6 (BD Pharmingen, San Diego, CA), and IL-1Ra (R&D Systems) were used according to the instructions of the manufacturer.
For flow cytometry, goat PE-conjugated Ab to human IL-1RI, goat PE-conjugated control Ab, mouse PE-conjugated Ab to human TNFRSF1A (p55), mouse PE-conjugated control Ab, mouse fluorescein-conjugated Ab to human TNFRSF1B (p75), and mouse fluorescein-conjugated control Ab (R&D Systems) were used according to the instructions of the manufacturer. FcRs on cell surface were blocked by human FcR Blocking Reagent (Miltenyi Biotec).
Real-time quantitative RT-PCR
Total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA), and 1 μg total RNA was reverse transcribed using a First Strand cDNA Synthesis kit (Fermentas, Glen Burnie, MD). Quantitative PCR (qPCR) was performed using iQ SYBR Green Supermix and iCycler iQ thermal cycler (Bio-Rad, Hercules, CA) following the manufacturer's protocols, and triplicate reactions were run for each sample. The oligonucleotide primers used were: hGAPDH: 5′-ATCAAGAAGGTGGTGAAGCA-3′ and 5′-GTCGCTGTTGAAGTCAGAGGA-3′; hIL-8: 5′-TCTGTTAAATCTGGCAACCC-3′ and 5′-TAAAGGAGAAACCAAGGCAC-3′; hIL-1β: 5′-TTCTTCGACACATTGGATAACG-3′ and 5′-TGGAGAACACCACTTGTTGCT-3′; hIL-6: 5′-TAATGGGCATTCCTTCTTCT-3′ and 5′-TGTCCTAACGCTCATACTTTT-3′; hTNFRSF1A: 5′-CCCCCGGTGACTGTCCCAACTTT-3′ and 5′-GGCGCTGTCCTCCCACTTCTGA-3′; hTNFRSF-1B: 5′-TGAGCTTGGCAGCTGAACTA-3′ and 5′-AGCCAGCCAGTCTGACATCT-3′; hIL-1RI: 5′-AGCTGGCTGGGTGGTTCAT-3′ and 5′-CGATTCTGGCATTTTCTCATAGTC-3′; hIL-1RII: 5′-GGGGGAAATGATCACAGGAATGGT-3′ and 5′-CCCATGAAGGCCAGCAATACAACA-3′; hIL-1Ra: 5′-CGCTCAGGTCAGTGATGTTAA-3′ and 5′-GAAGATGTGCCTGTCCTGTGT-3′; and hIL-1AcP: 5′-CCTCTCGGGGCAACATCAAC-3′ and 5′-GACCGCCTGGGACTTTTCTTC-3′.
Results
Human Mϕ derived from synovial fluid of patients with RA and from peripheral blood of healthy donors produce IL-27 in response to TNF-α
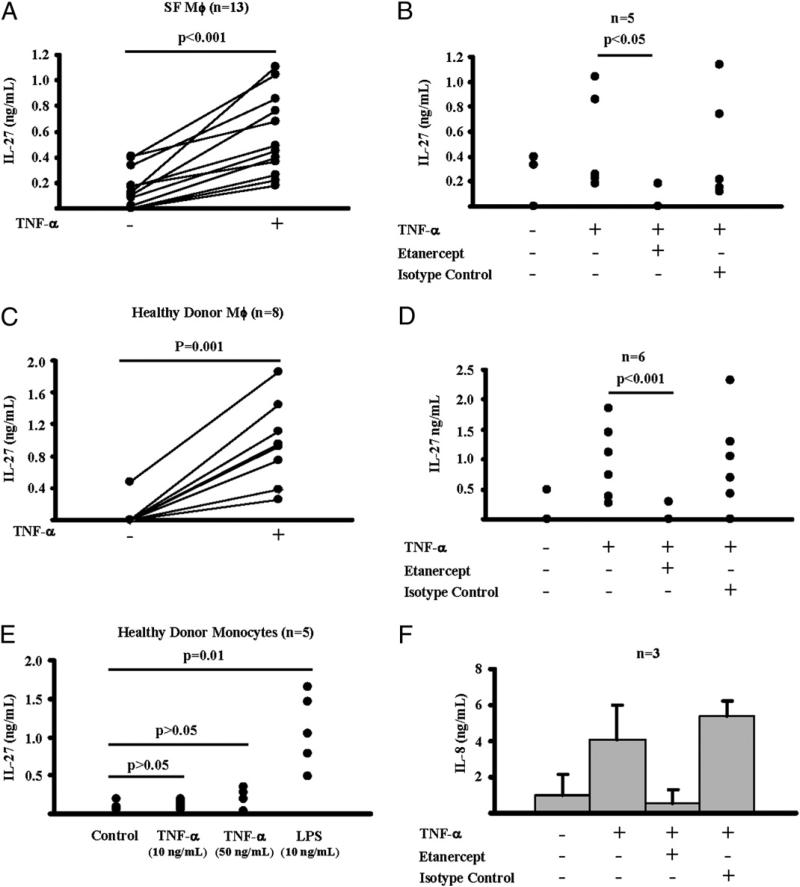
Recently, it has been reported that IL-27 is expressed in the pannus of patients with RA (11), but the stimulus for IL-27 production during synovial inflammation has not yet been identified. TLR-mediated stimulation of APCs is a well-characterized inducer of IL-27 (5–9). Although TLR ligands have been implicated in synovial inflammation (32), we wished to investigate whether endogenous inflammatory factors, such as TNF-α, which has a more established role in RA pathogenesis and is abundant in the inflamed synovium (33), can trigger production of IL-27. In this context, we isolated Mϕ (CD14+ cells) from synovial fluid of 13 patients with active RA and cultured them overnight (12 h) in the presence or absence of TNF-α (10 ng/ml). Interestingly, we observed that TNF-α stimulation of patients’ Mϕ led to a significant production of IL-27 protein (n = 13; p < 0.001, paired Student t test) (Fig. 1A). It is of note that the amount of the TNF-α–induced IL-27 was substantial; the mean concentration of IL-27 in culture supernatants was 0.5 ng/ml (ranging from 0.18–1.11 ng/ml), and induction of IL-27 by TNF-α was observed in all 13 independent experiments (Fig. 1A). When TNF-α function was blocked using the soluble TNFR etanercept, TNF-α–induced production of IL-27 by patients’ Mϕ was abrogated (n = 5; p < 0.05, paired Student t test) (Fig. 1B), supporting the conclusion that, in our experimental system, TNF-α triggered the production of IL-27 by RA patient-derived synovial Mϕ.
FIGURE 1.
Human Mϕ produce IL-27 in response to TNF-α. A and B, CD14+ cells isolated from synovial fluids of patients with active RA were cultured overnight (12 h) in the presence or absence of hTNF-α (10 ng/ml). For B, the effect of TNF-α was blocked by etanercept (10 μg/ml); human IgG1 (10 μg/ml) was used as an isotype control. C and D, CD14+ cells isolated from PBMCs of healthy donors were differentiated for 2 d in the presence of M-CSF (10 ng/ml) and then were cultured overnight as in A and B. B, Fresh Mo (CD14+ cells isolated from PBMCs of healthy donors) were cultured overnight (12 h) in the presence or absence of TNF-α or LPS. For F, Mo were cultured as described in B. Production of IL-27 (A–E) and IL-8 (F) in response to TNF-α was measured in culture supernatant with ELISA. Paired Student t test was used for statistical analysis.
We next wished to explore whether TNF-α stimulation induces IL-27 production by human Mϕ and Mo obtained from peripheral blood of healthy individuals. CD14+ cells from PBMCs of healthy donors cultured for 2 d with M-CSF to differentiate them into Mϕ-like cells were stimulated overnight with TNF-α. In all eight independent experiments, there was a robust (0.26–1.85 ng/ml) and significant (p = 0.001, paired Student t test) induction of IL-27 protein post stimulation of cells with 10 ng/ml TNF-α (Fig. 1C) that was prevented by etanercept (Fig. 1D). Freshly isolated peripheral blood Mo produced, as expected, high amounts of IL-27 in response to overnight stimulation with 10 ng/ml LPS (Fig. 1E). TNF-α stimulation of Mo induced a robust production of IL-8 (Fig. 1F), but surprisingly failed to induce IL-27 production even when TNF-α was used at the high concentration of 50 ng/ml (n = 5; p > 0.05, paired Student t test) (Fig. 1E). Overall, these results indicate that human Mϕ (derived from synovial fluid of RA patients or peripheral blood of healthy donors), but not Mo, produce substantial amounts of IL-27 in response to TNF-α stimulation; the amounts of TNF-α–induced IL-27 in Mϕ were comparable to those induced in Mo by LPS.
IL-27 suppresses TNF-α responses by downregulating the expression of the signaling receptors p55 and p75 and by inhibiting proximal TNF-α–induced signaling in human Mϕ
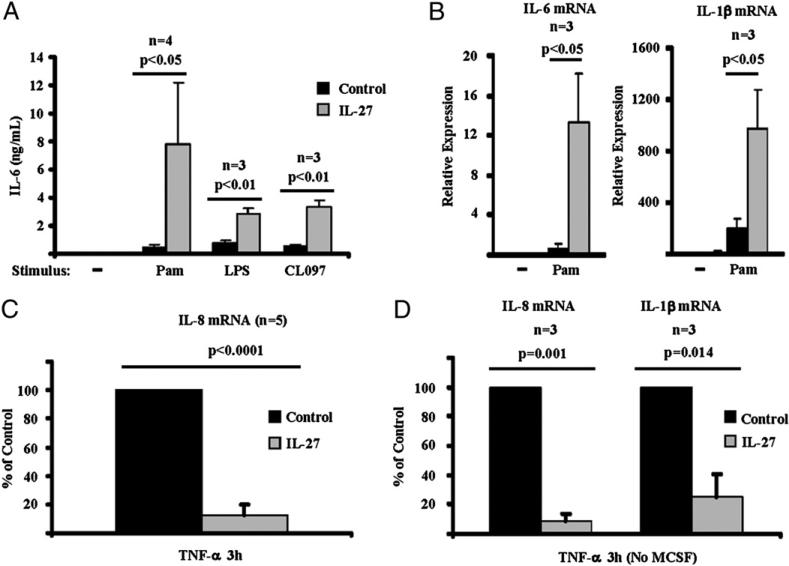
We have recently reported that IL-27 enhances responses of human Mo/Mϕ to TLR ligands (4). Cells exposed to IL-27 produce significantly higher amounts of protein and mRNA of the proinflammatory cytokines IL-6, IL-1b, and TNF-α in response to various TLR ligands including Pam3CysSer(Lys)4 (TLR2 ligand), LPS (TLR4 ligand), and CL097 (TLR7/8 ligand) (Fig. 2A, 2B) (4). In our current study, we wished to explore whether IL-27 also modulates responsiveness of human Mϕ to mediators that drive synovial inflammation including TNF-α and IL-1β. CD14+ cells, derived from healthy donors’ PBMCs, were cultured for 2 d with M-CSF in the presence or absence of IL-27 and were then stimulated with TNF-α. As expected, M-CSF–generated Mϕ responded to TNF-α stimulation with a robust induction of IL-8 mRNA (Fig. 2C, black bar). Surprisingly, in cells exposed to 100 ng/ml IL-27, the induction of IL-8 mRNA following TNF-α stimulation was attenuated (Fig. 2C, gray bar; n = 5; p < 0.0001, paired Student t test). A significant suppressive effect of IL-27 on TNF-α–mediated induction of IL-8 and IL-1β mRNA was also observed in the absence of M-CSF in our experimental system (Fig. 2D; n = 3; p = 0.001 for the attenuation of IL-8 mRNA expression and p = 0.014 for the suppression of IL-1β mRNA expression, paired Student t test).
FIGURE 2.
IL-27 primes human Mϕ for enhanced TLR responses and suppresses responses of human Mϕ to TNF-α. A and B, CD14+ cells isolated from healthy donors’ peripheral blood were cultured with M-CSF (10 ng/ml) in the presence or absence of IL-27 (100 ng/ml) for 24 h and then were stimulated with various TLR ligands including Pam3CysSer(Lys)4 (TLR2 ligand), LPS (TLR4 ligand), and CL097 (TLR7/8 ligand). For A, ELISA was used to measure production of IL-6 protein in culture supernatants following 6 h stimulation with Pam3CysSer(Lys)4 (10 ng/ml), LPS (1 ng/ml), or CL097 (1 μg/ml). For B, qPCR was used to measure the mRNA expression of IL-6 and IL-1β mRNA following 3 h stimulation with Pam3CysSer(Lys)4 (10 ng/ml), and results are depicted as expression relative to GAPDH. For A and B, paired Student t test was used for statistical analysis. CD14+ cells isolated from healthy donor PBMCs were cultured for 2 d with M-CSF (10 ng/ml) (C) or without M-CSF (D) in the presence or absence of IL-27 (100 ng/ml) and then were stimulated with TNF-α (10 ng/ml) for 3 h. Induction of IL-8 and IL-1β mRNA was measured by qPCR, and the results of the independent experiments were pooled (n = 5 for C and n = 3 for D). The mean levels of TNF-α–induced IL-8 mRNA were 238% relative to GAPDH mRNA levels (C) and 240% of GAPDH (D); IL-1β mRNA was induced to 22% of GAPDH levels (D). For comparison of pooled data from different donors, the induction of IL-8 and IL-1β mRNA in response to TNF-α in control cells (cells cultured without IL-27 pretreatment) was set as 100. Induction of IL-8 and IL-1β mRNA in cells pretreated with IL-27 is depicted as percentage relative to induction in control cells. For statistical analysis, paired Student t test was used.
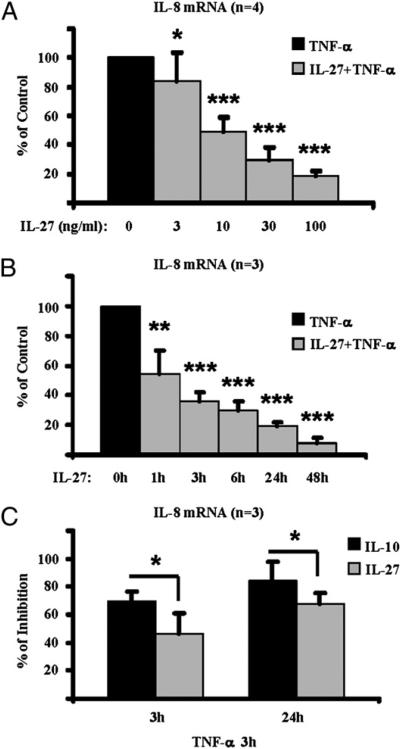
The above-mentioned suppressive effects of IL-27 on human Mϕ were dose dependent. Whereas 3 ng/ml IL-27 was only moderately suppressive, pre-exposure of cells to higher doses of IL-27 (10, 30, and 100 ng/ml) significantly suppressed the mRNA expression of the TNF-α target IL-8 (Fig. 3A; n =4; P < 0.01) in a dose-dependent manner. Interestingly, the inhibitory effects of 100 ng/ml IL-27 on the TNF-α–mediated expression of IL-8 mRNA were significant even when the cells were pre-exposed to IL-27 for only 1 h (Fig. 3B, first gray bar on the left; n = 3; p < 0.05, paired Student t test). The potency of inhibition increased with increased duration of IL-27 exposure, with an average inhibition of 46.1% after 1 h (Fig. 3B) increasing to 91.7% after 48 h of IL-27 pretreatment (Fig. 3B, last gray bar on the right; n = 3; p < 0.01, paired Student t test). To further address the potency of the anti-inflammatory effects of IL-27 on human Mϕ at the earlier time points, a head-to-head comparison of IL-27 with the powerful anti-inflammatory cytokine IL-10 was performed. Interestingly, at both time points tested (3 h and 24 h pretreatment with 100 ng/ml IL-27 or 100 ng/ml IL-10), IL-27 displayed a suppressive effect comparable to that of IL-10 on the TNF-α–mediated induction of IL-8 mRNA expression in human Mϕ (Fig. 3C; n = 3; no statistically significant difference was found by paired Student t test).
FIGURE 3.
IL-27 suppresses responses of human Mϕ to TNF-α in a dose- and time-dependent manner, and these suppressive effects of IL-27 are comparable to the effects of IL-10. CD14+ cells isolated from healthy donor PBMCs were cultured for 48 h with M-CSF (10 ng/ml) in the presence or absence of IL-27 (A–C) or IL-10 (C) and then were stimulated with TNF-α (10 ng/ml) for 3 h. Induction of IL-8 mRNA was measured by qPCR and the results of the independent experiments were pooled. A, Cells were pretreated for 48 h with different doses of IL-27 (3–100 ng/ml) and then were stimulated with TNF-α. B, Cells were pretreated with 100 ng/ml IL-27 for 1, 3, 6, 24, or 48 h and then were stimulated with TNF-α. The induction of IL-8 mRNA in response to TNF-α in control cells (cells cultured without IL-27 pretreatment) was set as 100. Induction of IL-8 mRNA in cells pretreated with IL-27 is depicted as percentage relative to induction in control cells (A, B). C, Cells were pretreated for 3 or 24 h with IL-27 (100 ng/ml) or IL-10 (100 ng/ml) and then stimulated with TNF-α. Results are depicted as percentage of inhibition relative to control cells (cells stimulated with TNF-α without IL-27 or IL-10 pretreatment). For statistical analysis, paired Student t test was used. *p > 0.05; **p < 0.05; ***p < 0.01.
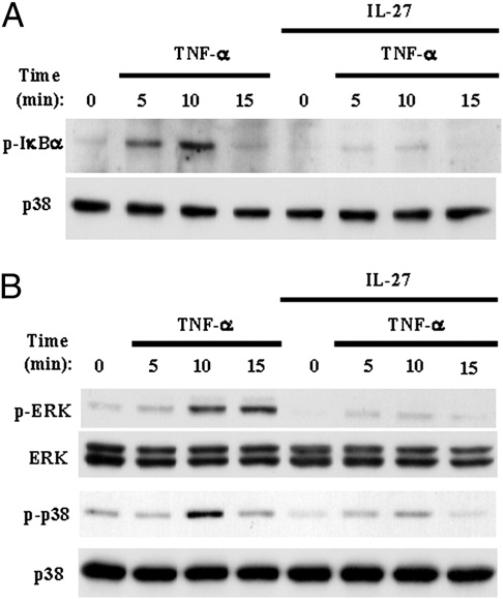
The induction of TNF-α target genes, including induction of IL-8, is dependent upon TNF-α–induced activation of NF-κB and MAPK pathways (34), and thus we investigated whether IL-27 inhibited TNF-α signaling. In agreement with the literature, we found that stimulation of human Mϕ with TNF-α (10 ng/ml) induced rapid phosphorylation of IκBα (Fig. 4A, upper panel, lanes 2–4) and rapid phosphorylation of ERK and p38 MAPKs (Fig. 4B, first and third panel, lanes 2–4). Notably, we observed strong inhibition of TNF-α–induced IκBa, ERK, and p38 phosphorylation by IL-27 (Fig. 4A, 4B, lanes 6–8). In summary, these results demonstrate that IL-27 attenuates responses of human Mϕ to TNF-α by inhibiting TNF-α–induced signaling.
FIGURE 4.
IL-27 blocks TNF-α–induced activation of signaling pathways in human Mϕ. CD14+ cells isolated from healthy donor PBMCs were cultured for 2 d with M-CSF (10 ng/ml) in the presence or absence of IL-27 (100 ng/ml) and then were stimulated with TNF-α (10 ng/ml) for 5, 10, and 15 min. Immunoblotting was used to measure Ser32 phosphorylation of IκBα (A), Thr202/Tyr204 phosphorylation of Erk1/Erk2, and Thr180/Tyr182 phoshorylation of p38 (B). Representative results of at least three independent experiments are shown.
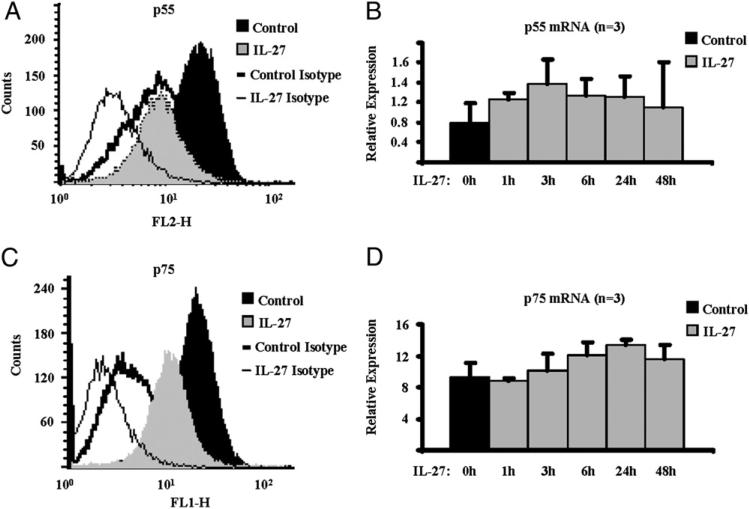
We next investigated the effects of IL-27 on the expression of the two signaling receptors for TNF-α. In the presence of IL-27 (100 ng/ml) for 48 h, we observed a significant downregulation of the p55 receptor on the cell surface of human Mϕ, measured by FACS (Fig. 5A; n = 5; average percentage of inhibition, 50; p = 0.001, paired Student t test). Interestingly, exposure of Mϕ to IL-27 for 1, 3, 6, 24, and 48 h had no effect on the expression level of p55 mRNA (Fig. 5B). Similarly, cell-surface expression of the second receptor for TNF-α, p75, was also significantly reduced by IL-27 (Fig. 5C; n = 5; average percentage of inhibition, 29.5; p = 0.019, paired Student t test), whereas the mRNA expression of p75 was not inhibited (Fig. 5D). Culture of Mϕ with IL-27 resulted in shifts in isotype control staining, most likely secondary to changes in autofluorescence as FcRs were blocked as described in Materials and Methods, and in each experimental condition, the appropriate background staining was subtracted to calculate IL-27–induced changes specific receptor staining. These results suggest that IL-27–mediated downregulation of cell-surface expression of TNFRs may contribute to suppression of downstream signal transduction.
FIGURE 5.
IL-27 downregulates the cell-surface protein levels of the TNF-α signaling receptors p55 and p75 on human Mϕ. CD14+ cells isolated from healthy donors’ peripheral blood were cultured with M-CSF (10 ng/ml) in the presence or absence of IL-27 (100 ng/ml) for 48 h. A, Expression level of p55 protein on the cell surface was measured by FACS. B, qPCR was used to measure the mRNA expression of p55, and results are depicted relative to GAPDH. C, Expression level of p75 protein on the cell surface was measured by FACS. Panel labels same as defined in A. D, qPCR was used to measure the mRNA expression of p75, and results are depicted relative to GAPDH. For A and C, representative results of five independent experiments are shown. For B and D, pooled results of three independent experiments are shown. Control, control Mϕ stained for p55; IL-27, IL-27–treated Mϕ stained for p55; Control Isotype, control Mϕ stained with isotype control; IL-27 Isotype, IL-27–treated Mϕ stained with isotype control.
IL-27 abrogates responses of human Mϕ to IL-1β
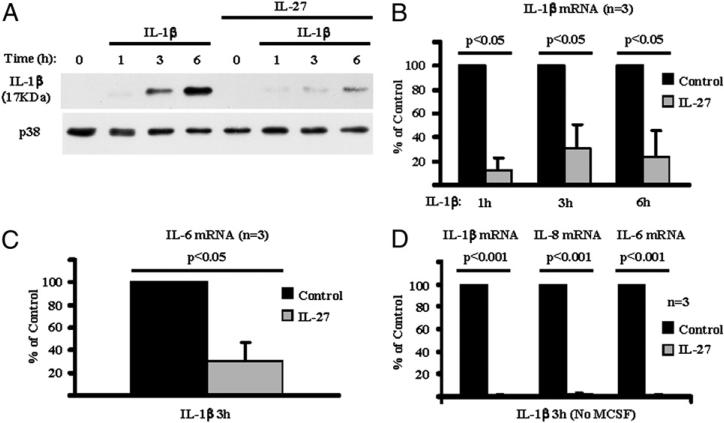
IL-1β is an important inflammatory cytokine that utilizes MyD88-dependent signaling pathways similar to TLR-induced MyD88-dependent pathways (35). We then investigated whether IL-27 enhances (similar to LPS and other TLR ligands) or suppresses (similar to TNF-α) responses of human Mϕ to IL-1β. In agreement with the literature, human Mϕ stimulated with IL-1β produced mature IL-1β protein (cleaved form with molecular mass of 17 kDa) (Fig. 6A, upper panel, lanes 3 and 4). In the presence of IL-27, production of the cleaved form of IL-1β protein in response to IL-1β stimulation was strongly attenuated (Fig. 6A, upper panel, lanes 6–8). We also used qPCR to measure expression of IL-1β target genes. IL-27 significantly suppressed the expression of IL-1β, IL-6, and IL-8 mRNA in response to IL-1β stimulation (data not shown and Fig. 6B, 6C; n = 3; p < 0.05, paired Student t test). Interestingly, in the absence of M-CSF in our system, the IL-1β–mediated induction of IL-1β, IL-8, and IL-6 mRNA was almost completely inhibited (average inhibition >97%) by pre-exposure of cells to 100 ng/ml IL-27 (Fig. 6D; n = 3; p < 0.001, paired Student t test).
FIGURE 6.
IL-27 suppresses responses of human Mϕ to IL-1β. CD14+ cells isolated from healthy donor PBMCs were cultured for 1 d with M-CSF (10 ng/ml) (A–C) or without M-CSF (D) in the presence or absence of IL-27 (100 ng/ml) and then were stimulated with IL-1β (10 ng/ml) for 1, 3, or 6 h. For A, immunoblotting was used to measure production of the cleaved mature form of IL-1β protein, and representative results of three independent experiments are shown. For B–D, qPCR was used to measure induction of IL-1β, IL-6, and IL-8 mRNA, and the results of three independent experiments were pooled. In B, the mean levels of IL-1β–induced IL-1β mRNA relative to GAPDH mRNA levels were 670%, 144%, and 176% at the 1 h, 3 h, and 6 h time points, respectively. In C, the mean levels of IL-1β–induced IL-6 mRNA were 1.0% of GAPDH. In D, the mean levels of IL-1β–induced mRNA relative to GAPDH were 510% (IL-1β), 470% (IL-8), and 4.5% (IL-6). For comparison of pooled data from different donors, induction of IL-1β, IL-6, and IL-8 mRNA in response to IL-1β in control cells (cells cultured without IL-27 pretreatment) was set as 100. Induction of IL-1β, IL-6, and IL-8 mRNA in cells pretreated with IL-27 was depicted as percentage relative to induction in control cells. For statistical analysis, paired Student t test was used.
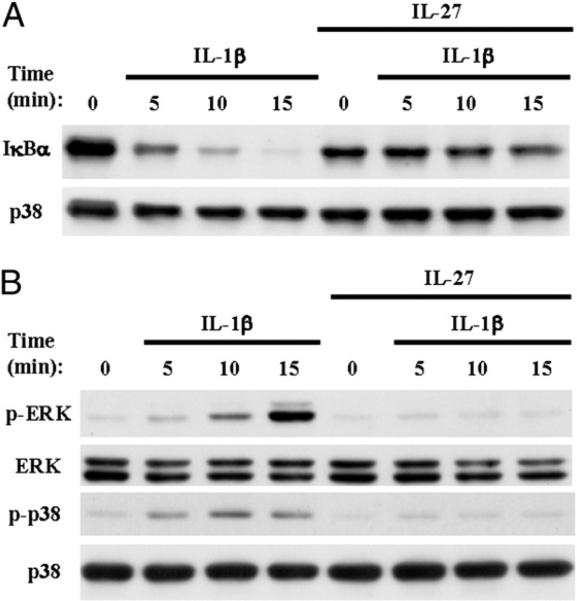
We next investigated whether IL-27 suppresses IL-1β responses by inhibiting IL-1β–induced signaling. After stimulating human Mϕ with 10 ng/ml IL-1β, we observed the expected activation of the classical NF-κB pathway as manifested by rapid degradation of the IκBα protein (Fig. 7A, upper panel, lanes 1–4). We also observed rapid phosphorylation of ERK and p38 (Fig. 7B, first and third panel, lanes 1–4), indicating activation of MAPK pathways. IL-1β–mediated degradation of IκBα and phosphorylation of ERK and p38 were prevented in the presence of IL-27 (Fig. 7A, 7B, lanes 5–8), suggesting that IL-27 suppresses the effects of IL-1β on human Mϕ by blocking IL-1β–induced signaling.
FIGURE 7.
IL-27 inhibits IL-1β–induced activation of NF-κB and MAPK signaling pathways in human Mϕ. CD14+ cells isolated from healthy donors’ PBMCs were cultured for 1 d with M-CSF (10 ng/ml) in the presence or absence of IL-27 (100 ng/ml) and then were stimulated for 5, 10, and 15 min with IL-1β (10 ng/ml). Immunoblotting was used to measure total IκBα (A), Thr202/Tyr204 phosphorylation of Erk1/Erk2, and Thr180/Tyr182 phoshorylation of p38 (B). Representative results of at least three independent experiments are shown.
IL-27 downregulates the expression of the signaling receptor IL-1RI and induces IL-1Ra and the decoy receptor IL-1RII
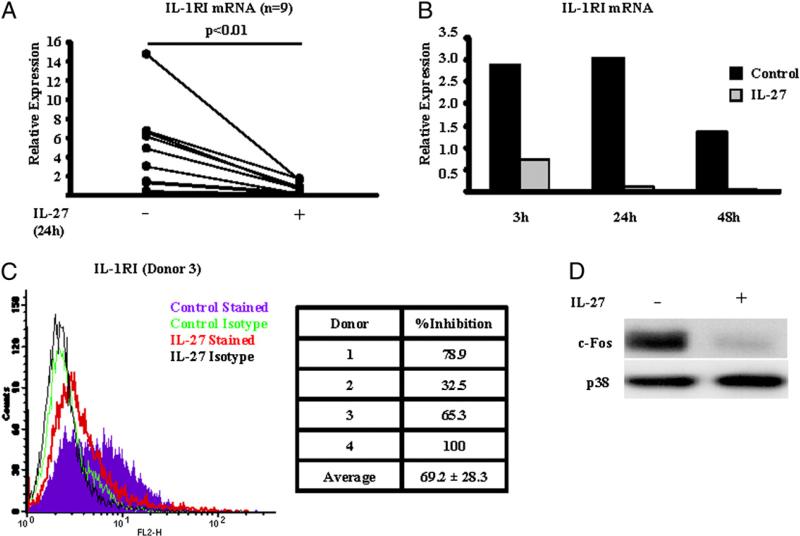
IL-1β first binds to the IL-1RI receptor subunit on the surface of target cells, and subsequently, IL-1 accessory protein (IL-1AcP) is recruited, thus forming a trimolecular signaling complex that activates NF-κB and MAPK signaling pathways (35). Because IL-1β has potent proinflammatory and tissue-destructive effects, its function is tightly regulated by the decoy receptor IL-1RII that binds and sequesters IL-1β but does not elicit any signal and by IL-1Ra that competes with IL-1β for binding with IL-1RI (35). In this context, we wished to investigate whether the inhibitory effect of IL-27 on IL-1β signaling and function was due to an effect on the above-mentioned molecules. Interestingly, we observed that in human CD14+ cells purified from PBMCs of healthy individuals, IL-27 significantly suppressed the mRNA expression of the signaling receptor IL-1RI (Fig. 8A; n = 9; p < 0.01, paired Student t test). This suppressive effect on IL-1RI mRNA expression was readily observed within 3 h following stimulation of cells with IL-27 and was sustained for at least 48 h (Fig. 8B). A similarly significant suppressive effect of IL-27 on the IL-1RI mRNA expression was observed in our system in the absence of M-CSF (average inhibition of IL-1RI mRNA, 77.5%; n = 5; p < 0.001, paired Student t test, data not shown). In agreement with our observations at the mRNA level, the expression of IL-1RI protein on the cell surface was also significantly reduced by IL-27 in all four donors tested (Fig. 8C, average percentage of inhibition, 69.2). IL-1RI expression has recently been shown to depend on basal expression of the transcription factor c-Fos (36). IL-27 downregulated the expression of c-Fos protein in human Mϕ (Fig. 8D), suggesting that IL-27 suppresses IL-1RI expression by targeting its transcriptional regulator c-Fos.
FIGURE 8.
IL-27 downregulates IL-1RI and suppresses the expression of c-Fos. CD14+ cells isolated from healthy donors’ peripheral blood were cultured with M-CSF (10 ng/ml) in the presence or absence of IL-27 (100 ng/ml) for 24h (A, C, D) or for 3, 24, and 48 h (B). A and B, qPCR was used to measure the mRNA expression of IL-1RI, and results are depicted relative to GAPDH. Paired Student t test was used for statistical analysis. For B, representative results of three independent experiments are shown. C, Expression level of IL-1RI protein on the cell surface was measured by FACS. Left panel shows representative results of four independent experiments, and right panel depicts the percentage of inhibition of IL-1RI expression by IL-27 in four donors that were tested. D, Immunoblotting was used to measure the expression of the transcription factor c-Fos (representative results of at least five independent experiments are shown). Control Stained, control Mϕ stained for IL-1RI; Control Isotype, control Mϕ stained with isotype control; IL-27 Stained, IL-27–treated Mϕ stained for IL-1RI; IL-27 Isotype, IL-27–treated Mϕ stained with isotype control.
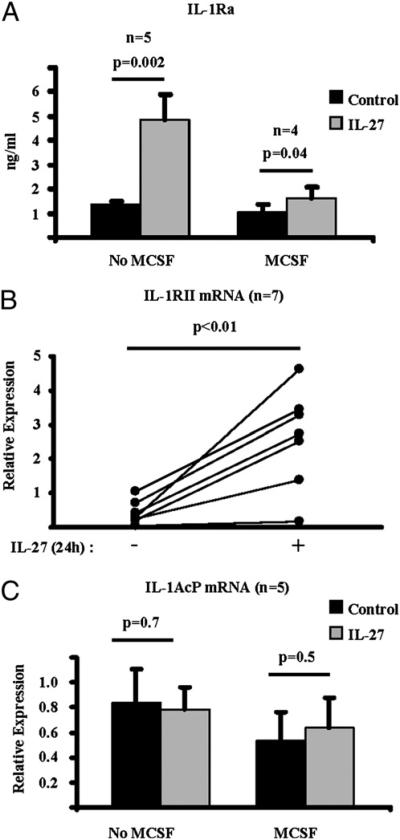
In contrast to the suppression of IL-1RI expression, IL-27 significantly increased the production of the receptor antagonist IL-1Ra by human Mϕ (Fig. 9A). Interestingly, the induction of IL-1Ra protein by IL-27 was more robust in the absence of M-CSF in our system. In supernatants collected from 24-h cultures, the average amount of the IL-27–induced IL-1Ra protein measured by ELISA was 4.87 ng/ml and 1.64 ng/ml, in the absence and presence of M-CSF, respectively (Fig. 9A). The decoy receptor IL-1RII was also induced in human Mϕ by IL-27 in the presence of M-CSF (Fig. 9B; n = 7; p < 0.01, paired Student t test). Finally, the expression of IL-1AcP was not modulated by IL-27 (Fig. 9C). Overall, IL-27 coordinately suppressed expression of an activating IL-1R component while inducing inhibitors of the IL-1R and thereby suppressed human Mϕ responses to IL-1β.
FIGURE 9.
IL-27 induces the expression of IL-1Ra and upregulates the decoy receptor IL-1RII. CD14+ cells isolated from healthy donors’ peripheral blood were cultured with M-CSF (10 ng/ml) (A–C) or without M-CSF (A, C) in the presence or absence of IL-27 (100 ng/ml) for 24 h. A, Production of IL-1Ra protein was measured in culture supernatants with ELISA. qPCR was used to measure the mRNA expression of IL-1RII (B) and IL-1AcP (C). Results are depicted relative to GAPDH, and paired Student t test was used for statistical analysis.
Discussion
IL-27 is a highly pleiotropic cytokine that can promote or suppress immune and inflammatory responses depending on context (15–17, 25). One of the challenges in understanding IL-27 function has been to elucidate molecular mechanisms that underlie its activating versus suppressive functions. In this study, we have identified a new suppressive function of IL-27, namely, inhibition of Mϕ responses to the key endogenous inflammatory cytokines TNF-α and IL-1β. IL-27 suppressed Mϕ responses to TNF-α and IL-1β by blocking proximal steps in signaling by TNFRs and IL-1Rs. IL-27 downregulated the protein expression of the signaling receptors p55 and p75 on the surface of human Mϕ. The mechanism of inhibition of IL-1 signaling was downregulation of the ligand-binding IL-1RI concomitant with increased expression of the IL-1Ra protein and the decoy receptor IL-1RII. These findings provide a mechanism for suppressive effects of IL-27 on innate immune cells and suggest that IL-27 regulates innate immune and inflammatory responses by attenuating Mϕ activation in response to endogenous inflammatory cytokines.
Our findings that IL-27 inhibits TNF and IL-1 responses stand in striking contrast to IL-27–mediated augmentation of TLR-induced inflammatory cytokine production in human Mϕ (Fig. 2A, 2B) (4). This dual regulation suggests that IL-27 has a different function in the context of an infection due to TLR stimulation than its function during chronic sterile inflammation that is driven primarily by endogenous cytokines, such as TNF-α and IL-1. In the first case, IL-27 augments innate immunity to eliminate pathogens by increasing responses of Mϕ to pathogen-associated molecular patterns (TLR ligands) (4). In the latter case, IL-27 functions as a homeostatic cytokine by suppressing responses of Mϕ to TNF-α and IL-1β. A similar pleiotropic and context-dependent function of IL-27 has been described in adaptive immunity (5, 14–17). In the early phase of the adaptive immune response, IL-27 promotes Th1 polarization of naive lymphocytes to promote a robust response to weak infectious stimuli (37). When an immune response is established, the function of IL-27 transitions to immunosuppressive aiming to restrict inflammation by inhibiting polarization of T cells (5).
IL-27 is expressed at sites of TNF-α–driven inflammation, such as the RA synovium (11), the psoriatic skin (12), and the intestine of patients with Crohn's disease (13). Our finding that TNF-α induces production of IL-27 by synovial fluid Mϕ supports the hypothesis that TNF-α stimulation of Mϕ in the joint microenvironment may contribute to the observed expression IL-27 in the pannus of RA patients. It is not yet clear whether IL-27 expressed in TNF-α–driven diseases is predominantly pathogenic or represents a homeostatic attempt to control inflammation and restrain tissue destruction. In a recent report, we observed that IL-27 is tissue protective by inhibiting osteoclastogenesis (20). Our current study further supports a homeostatic function of IL-27 in cytokine-driven inflammation by suppressing Mϕ activation. Another group of investigators that described the expression of IL-27 in psoriatic skin lesions also observed anti-inflammatory effects on human keratinocytes (12). In addition, several studies indicate that IL-27 suppresses Th1 and Th17 cells (10, 29, 38–41), two subsets of T cells that have been implicated in the pathogenesis of RA (42), psoriasis, and Crohn's disease (43, 44). These results are consistent with reports of suppressive properties of IL-27 in several mouse models of autoimmune and inflammatory diseases (10, 11, 22, 29, 38, 40, 41, 45–51). However, the contribution of IL-27–mediated suppression of Mϕ function, as described in this study, to suppressing inflammation in vivo is difficult to assess in animal models, as murine Mϕ are minimally responsive to IL-27 (4). The alternative approach of ex vivo analysis of human arthritic Mϕ suggests that the in vivo functions of IL-27 are regulated by the synovial microenvironment (20), and definitive resolution of this question would require a clinical trial of IL-27 in human diseases with concomitant measurement of Mϕ function and cytokine production.
The anti-inflammatory effects of IL-27 on human Mϕ are more circumscribed than the effects of the prototypic Mϕ-deactivating cytokine IL-10. IL-10 potently blocks inflammatory cytokine production in response to multiple Mϕ-activating stimuli, including TNF, IL-1, and multiple TLRs (24). The broad suppressive activity of IL-10 is explained by its targeting of inflammatory cytokine gene transcription; inhibition of transcription appears to occur via STAT3-mediated induction of as yet unknown transcriptional repressors (30). In contrast, IL-27 exhibits receptor-specific inhibitory effects that are explained by selective targeting of IL-1 and TNFRs and proximal signaling components, whereas TLR-induced cytokine responses are actually augmented (4). Consistent with this, we found that although STAT3 is activated by IL-27, IL-27 is a weak inducer of STAT3 target genes relative to IL-10 (4). This difference in downstream gene expression and mechanism of inhibition between IL-27 and IL-10 suggests that, in contrast to IL-10, IL-27–mediated suppressive effects may not be predominantly STAT3 dependent. Indeed, IL-27 inhibits IL-1 responses at least in part by downregulating Fos expression and thus expression of IL-1RI. As Fos is also downregulated by IFNs acting via STAT1 (52, 53), it is possible that STAT1 contributes to the homeostatic functions of IL-27 that we have described.
TLR ligands, TNF-α, and IL-1β are potent activators of Mϕ, inducing production of proinflammatory mediators that are crucial for an effective immune response to pathogens. Activators of Mϕ also trigger homeostatic mechanisms that restrain inflammation and limit associated tissue damage. One key homeostatic mechanism that TLR ligands activate is the production of IL-10 (54), a potent anti-inflammatory cytokine that limits inflammatory cytokine production and controls the duration and intensity of immune responses (24). Interestingly, TNF-α does not induce IL-10 production in Mϕ (55), but according to our findings, induces the synthesis of IL-27 that suppresses responses of Mϕ to TNF-α and IL-1β. In this context, our observations suggest that TNF-α–stimulated production of IL-27 may represent a selective homeostatic mechanism that limits activation of Mϕ by inflammatory cytokines, but preserves responses to microbial products that are sensed by TLRs.
Acknowledgments
We thank the physicians of the Hospital for Special Surgery (Drs. Sergio Schwartzman, Susan Goodman, Joseph Markenson, Theodore Fields, Ronald MacKenzie, and Dalit Ashany) for providing synovial fluids from patients with RA. We also thank Drs. Baohong Zhao and Kyung-Hyun Park-Min for critical review of the manuscript.
This work was supported by the Stavros S. Niarchos International Fellowship Exchange Program (to G.D.K.) and by a National Institutes of Health grant (to L.B.I.).
Abbreviations used in this paper
- Control
control macrophage stained for p55
- Control Isotype
control macrophage stained with isotype control
- Control Stained
control macrophage stained for IL-1RI
- DC
dendritic cell
- h
human
- IL-1AcP
IL-1 accessory protein
- IL-1Ra
IL-1R antagonist
- IL-27
IL-27–treated macrophage stained for p55
- IL-27 Isotype
IL-27–treated macrophage stained with isotype control
- IL-27 Stained
IL-27–treated macrophage stained for IL-1RI
- IRF
IFN regulatory factor
- Mϕ
macrophage
- Mo
monocyte
- qPCR
quantitative PCR
- RA
rheumatoid arthritis
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 3.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J. Immunol. 2008;180:6325–6333. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- 5.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J. Exp. Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirhonen J, Sirén J, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukoc. Biol. 2007;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 8.Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J. Immunol. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 9.Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J. Immunol. 2010;184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- 10.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin 27 attenuates collagen-induced arthritis. Ann. Rheum. Dis. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 12.Shibata S, Tada Y, Kanda N, Nashiro K, Kamata M, Karakawa M, Miyagaki T, Kai H, Saeki H, Shirakata Y, et al. Possible roles of IL-27 in the pathogenesis of psoriasis. J. Invest. Dermatol. 2010;130:1034–1039. doi: 10.1038/jid.2009.349. [DOI] [PubMed] [Google Scholar]
- 13.Larousserie F, Pflanz S, Coulomb-L'Herminé A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J. Pathol. 2004;202:164–171. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 14.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J. Leukoc. Biol. 2009;86:1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida H, Miyazaki Y, Yoshiyuki M. Regulation of immune responses by interleukin-27. Immunol. Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [Published erratum appears in 2009 Immunol. Rev. 227: 283.] [DOI] [PubMed] [Google Scholar]
- 17.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J. Mol. Med. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 19.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 20.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–413. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa M, Takaishi H, Takito J, Yoda M, Sakai S, Hikata T, Hakozaki A, Uchikawa S, Matsumoto M, Chiba K, et al. IL-27 abrogates receptor activator of NF-kappa B ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J. Immunol. 2009;183:2397–2406. doi: 10.4049/jimmunol.0802091. [DOI] [PubMed] [Google Scholar]
- 22.Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1 (IL-27R) deficiency. J. Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J. Immunol. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J. Immunol. 2004;173:1171–1178. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J. Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Han G, Wang J, Chen G, Xu R, Wang L, Li X, Shen B, Li Y. The pathogenic role of interleukin-27 in autoimmune diabetes. Cell. Mol. Life Sci. 2008;65:3851–3860. doi: 10.1007/s00018-008-8540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 30.El Kasmi KC, Smith AM, Williams L, Neale G, Panopoulos AD, Panopolous A, Watowich SS, Häcker H, Foxwell BM, Murray PJ. Cutting edge: A transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J. Immunol. 2007;179:7215–7219. doi: 10.4049/jimmunol.179.11.7215. [Published erratum appears in 2008. J. Immunol. 180: 3612.] [DOI] [PubMed] [Google Scholar]
- 31.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill LA. Primer: Toll-like receptor signaling pathways—what do rheumatologists need to know? Nat. Clin. Pract. Rheumatol. 2008;4:319–327. doi: 10.1038/ncprheum0802. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol. Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, Kim N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009;183:1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 37.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol. Rev. 2004;202:106–114. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 38.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 39.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 40.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 41.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubberts E. Th17 cytokines and arthritis. Semin. Immunopathol. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 44.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol. Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J. Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, Hamano S, Yoshimura A, Yoshida H. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J. Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 47.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am.J.Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 50.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 51.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 52.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 53.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 54.Berg DJ, Kühn R, Rajewsky K, Müller W, Menon S, Davidson N, Grünig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]