Abstract
Toll-like receptor 4 (TLR4) is expressed on a number of cells including neurons in the brain. However, it has yet to be determined if TLR4 is expressed on photoreceptor cells in the retina. In this report, we examined primary photoreceptor cells and an established photoreceptor cell line (661W). We found that functional TLR4 is constitutively expressed on photoreceptor cells, and can be activated by LPS. We conclude that TLR4 on photoreceptor cells could directly contribute to retinal inflammatory diseases and photoreceptor cell survival.
Keywords: retina, TLR4, photoreceptor, IL-6, CXCL1, inflammation, apoptosis
Introduction
Activation of Toll-like receptor 4 (TLR4) by either the exogenous ligand LPS (1), a major cell wall component of gram-negative bacteria, or endogenous ligands such as HSP70 (2) and high mobility group protein 1 (HMGB1) (3) stimulates the production of chemotactic and pro-inflammatory cytokines and regulates cell survival (4). TLR4 expression has been well characterized in macrophages, monocytes, and dendritic cells; however, this receptor is also expressed on a wide spectrum of cells including epithelial cells and endothelial cells. In the retina, TLR4 has been detected on retinal pigmented epithelial cells (RPEs) and the RPE-expressed TLR4 appears to be involved in the management of photoreceptor outer segments (5). However, expression of TLR4 on photoreceptor cells in the retina has not been established.
Materials and Methods
The Photoreceptor cell line and reagents
The photoreceptor cell line 661W was established and maintained in DMEM with supplements as described before (6). The murine IL-6 ELISA kit were purchased from eBioscience (San Diego, CA), and the murine chemokine CXCL1 ELISA kit was ordered from R&D Systems (Minneapolis, MN). Purified LPS was from Sigma (St. Louis, MO). The TLR4 antagonist Eritoran tetrasodium or placebo was obtained from Eisai Research Institute (Andover, MA) (7) and reconstituted in endotoxin-free water (Sigma, MO).
Laser microdissection of murine photoreceptor cells
Eyes were enucleated from 8–10 week old C57BL/6 mice and 10 micron cryosections were prepared in an RNase-free environment onto PEN (polyethylene-naphthalene) membrane glass slides. These slides were then loaded onto the Arcturus Veritas Laser Capture Microdissection System (Molecular Devices, Sunnyvale, CA) to capture the photoreceptor cell layer. Images of the sections were taken before and after microdissection.
RT-PCR detection of TLR4 transcripts in photoreceptor cells
Total RNA was extracted from laser microdissected photoreceptors or cultured 661W cells by Trizol (Invitrogen, CA). After reverse transcription with random hexamers using a first strand cDNA synthesis kit (Invitrogen, CA), the TLR4 transcripts were detected by PCR with primers specific for different TLR4 exons: 5′-CAAGAACATAGATCTGAGCTTCAACCC-3′ and 5′-GCTGTCCAATAGGGAAGCTTTCTAGAG-3′. Amplification conditions were as follows: 94°C 30″, 58°C 30″ and 72°C 60″ for 40 cycles on a PTC-200 thermocycler (MJ Research, MA).
Flow cytometry detection of TLR4 protein on primary photoreceptor cells
Detection of TLR4 protein on primary photoreceptor cells was performed as previously described (8). In brief, suspensions of primary retinal cells from 2 to 5 mice were pooled and incubated for 10 min with immunoglobulin constant fragment (Fc)-receptor blocking antibody anti-CD16/CD32, clone 2.4G2 (BD Biosciences, CA). After blocking, the cells were stained with APC-conjugated anti-mouse TLR4 or control mAb (eBiosciences, CA). Thereafter, cells were permeabilized and incubated with anti-rhodopsin mAb (clone 1D4) followed by FITC-conjugated anti-mouse IgG Ab (eBiosciences, CA) or appropriate isotype controls, and analyzed using a LSR II flow cytometer (Becton Dickinson, CA).
LPS treatments and IL-6, CXCL1 measurements
1×106 661W cells were cultured in 200 μl of complete DMEM with supplements in each well of a 48-well plate with different concentrations of LPS (0, 20 and 200 ng/ml). The culture supernatants were collected after 12, 24 and 48 hrs, and levels of IL-6 and CXCL1 were determined by ELISA. In some experiments, cells were pre-incubated with TLR4 antagonist Eritoran tetrasodium (E5564) (7) or placebos prior to stimulation with LPS.
Effect of TLR4 activation on oxidative stress - induced photoreceptor cell death
5×104 661W cells were cultured in 200 μl of complete DMEM with supplements in each well of 96-well plates with and without 50 ng/ml LPS. After 24 hr, different concentrations (0–0.09 mM) of SNP were added to induce apoptosis. The number of surviving cells after 48 hr was assessed by adding 10% v/v of AlarmaBlue, incubating for 3 hr at 37°C, and measuring the fluorescence with excitation at 530 nm and emission at 590 nm.
Statistics
All experiments were repeated at least twice. Results were compared using the Student’s t test. A p value <0.05 was considered significant.
Results
Primary photoreceptor cells constitutively express TLR4
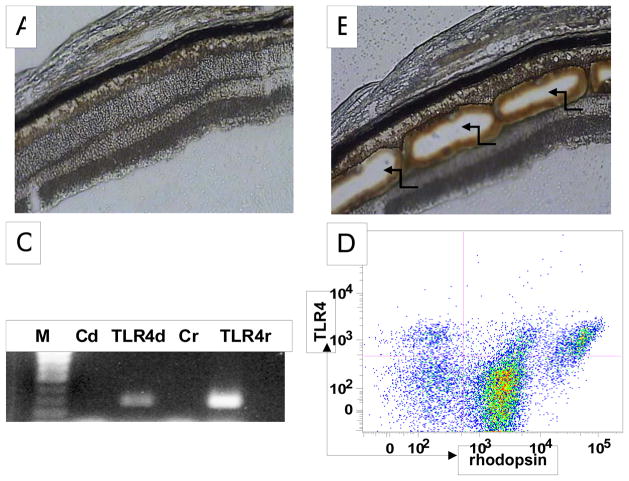
To examine whether TLR4 is constitutively expressed on photoreceptor cells, we dissected out the region of the outer nuclear layer by laser microdissection, and isolated RNA. We only collected the outer nuclear layers of the photoreceptor cells (Fig. 1A,B) to minimize possible contamination of other cells. After the isolation of total RNA from the collected primary photoreceptors, we amplified TLR4 transcripts by PCR. These assays showed that TLR4 transcripts are detectable by RT-PCR in primary photoreceptor cells (Fig. 1C).
Figure 1.
Primary photoreceptor cells express TLR4. A: A representative picture of mouse retinal cryosection before laser microdissection. B: The same retinal cryosection after laser microdissection, showing the outer nuclear layers (ONL) were dissected (arrows). C: Amplification of TLR4 transcript by RT-PCR in RNA isolated from dissected ONL (280 bp). M: 100 bp DNA ladder; Cd, control using non-reverse-transcripted dissected tissue RNA as PCR template; TLR4d, TLR4 amplification using reverse-transcribed dissected tissue RNA as PCR template; Cr, control using non-reverse-transcribed RAW 264.7 cell RNA as PCR template, TLR4r, positive control using reverse-transcribed RAW 264.7 cell RNA as PCR template. D: Detection of TLR4 protein on primary photoreceptors. Mouse retinal cells were prepared and stained for both rhodopsin and TLR4. Cells were analyzed by flow cytometry. Rhodopsinhi events represent outer segments while Rhodopsinlow events likely represent photoreceptor cells with outer segments sheared off in which only inner segments remain (8). Horizontal and vertical lines represent gates obtained with isotype control antibodies.
To examine if TLR4 protein is expressed on primary photoreceptors, we prepared single retinal cell suspension by collagenase and DNase I digestion using a protocol that we recently described (8), stained cells with anti-TLR4 and anti-rhodopsin mAbs, and analyzed TLR4 expression by flow cytometry. As shown in Fig. 1D, rhodopsin positive cells express TLR4, indicating that TLR4 protein is present on primary photoreceptor cells.
Photoreceptor cell line 661W constitutively express TLR4
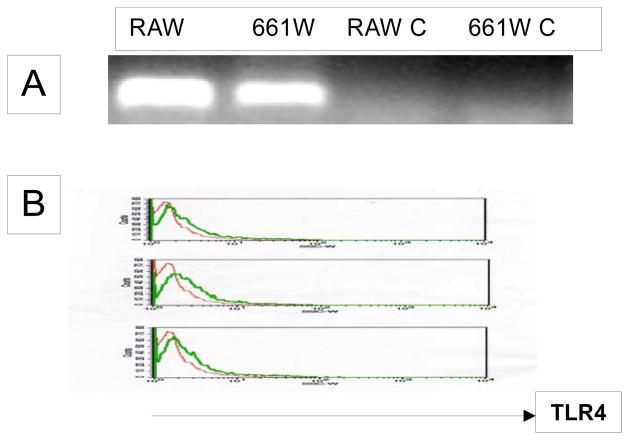
We next employed a well-established photoreceptor cell line 661W (6) to study the function of TLR4 on photoreceptor cells. To determine whether 661W cells express TLR4 as the primary photoreceptor cells, we analyzed the cells by RT-PCR and flow cytometry to detect TLR4 transcripts and proteins. Fig. 2 shows that 661W cells constitutively express TLR4 mRNA and protein.
Figure 2.
The photoreceptor cell line 661W expresses TLR4 as primary photoreceptor cells. A. Amplification of TLR4 transcript (280 bp) by RT-PCR from RNA RAW 624.7 and 661W RNAs. Non-reverse transcripted RNAs were used as negative control (RAW C and 661W C). B: Detection of TLR4 protein on 661W cells by flow cytometry. Thin line, isotype control, thick line, anti-TLR4 mAb staining.
LPS treatment stimulates IL-6 and CXCL1 production from photoreceptor cells
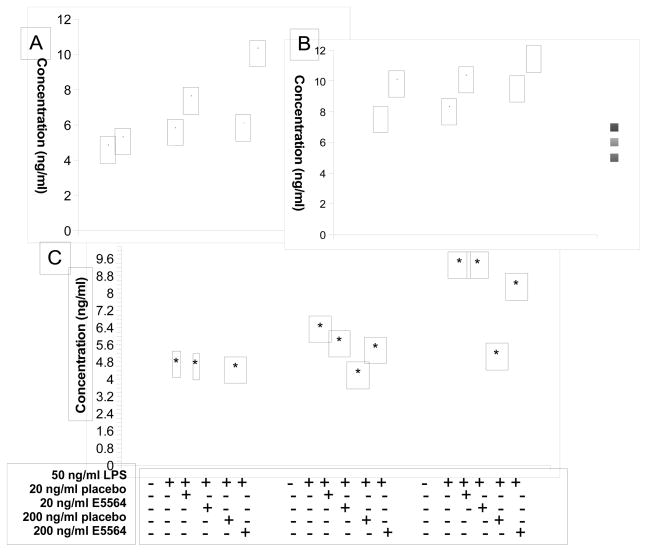
After verifying that 661W cells express TLR4 like primary photoreceptor cells, we next stimulated 661W cells with different concentrations of LPS, and collected culture supernatants at different time points to measure IL-6 and CXCL1 levels by ELISA. As shown in Fig. 3A&B, LPS stimulated IL-6 and CXCL1 production from 661W cells in a time (200 ng/ml LPS) and dose-dependent manner. As a second approach to examining whether LPS-induced IL-6 production from photoreceptors cells is mediated through TLR4, 661W cells were stimulated with LPS in the presence of TLR4 antagonist eritoran tetrasodium (7). Figure 3B shows that the TLR4 antagonist (E5564), but not vehicle control (placebo) inhibited LPS induced IL-6 production. Together, these findings demonstrate functional activity of TLR4 on this photoreceptor cell line.
Figure 3.
LPS induces IL-6 (A) and CXCL1(B) production from photoreceptor cells. Supernatants collected from 661W cells incubated with 0, 20 and 200 ng/ml LPS at 12, 24 and 48 hr, and IL-6 or CXCL1 levels were assessed by ELISA, respectively. C. TLR4 antagonist inhibited LPS-stimulated IL-6 production from 661W cells. 661W cells were incubated with 50 ng/ml LPS together with 0, 20 and 200 ng/ml TLR4 antagonist E5564 or placebo. Supernatants were collected at 12, 24 and 48 hr, and IL-6 levels were assessed by ELISA. (mean ± SD, representative results of three independent experiments. * p<0.05 compared to non-LPS treated group)
LPS- stimulated photoreceptor cells are protected from oxidative-stress induced cell death
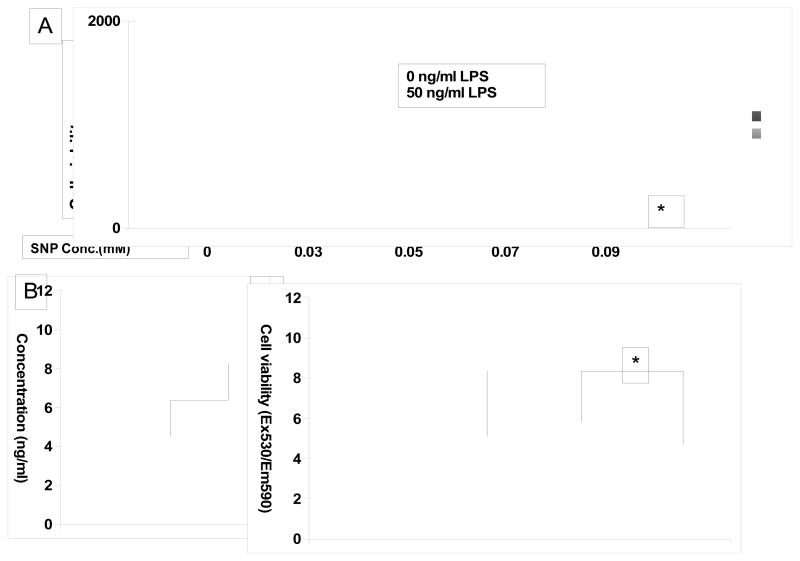
In light of previous reports that IL-6 inhibits apoptosis of brain neurons (9, 10) and photoreceptor cells (11, 12), we next examined the role of LPS stimulated IL-6 in photoreceptor cell viability. We induced cell death by adding the nitrous oxide (NO) donor sodium nitroprusside (SNP) in the presence or absence of LPS, and evaluated cell viability in 48 hrs by Alamar Blue. Consistent with previous reports, SNP induces photoreceptor cell death in a dose-dependent manner (13) (Fig. 4A); however, in the presence of LPS, viability of 661W cells was increased from 58% (at 0.05 mM SNP) to 140% (at 0.09 mM SNP) (Fig. 4A, p<0.05). Repeated experiments in the presence of 0.05 mM SNP, with and without 50 ng/ml LPS found that IL-6 levels were significantly augmented with LPS stimulation (Fig. 4B) in the presence of SNP. Moreover, neutralizing IL-6 abolished the protective effects of LPS on SNP-induced cell death (Fig. 4C). These data indicate that TLR4 has a protective effect in SNP induced oxidative damage of photoreceptor cells, which is mediated by IL-6.
Figure 4.
LPS stimulation protects photoreceptor cells from oxidative stress-induced damage by inducing IL-6 secretion. A. 661W cells were incubated with 0, 0.03, 0.05, 0.07 and 0.09 mM SNP with or without 50 ng/ml LPS. In 48 hr, cell survival was assessed by AlarmarBlue assays. (* p<0.05 compared to groups without LPS treatment). B. IL-6 concentration was assessed in the presence of 0.05 mM SNP with and without 50 ng/ml LPS. C. LPS protection effects were diminished after IL-6 was neutralized using 200 ng/ml of anti mouse IL-6 mAb (mean ± SD, representative results of three independent experiments. * p<0.05)
Discussion
In this study, we found that primary photoreceptor cells, as well as the photoreceptor cell line 661W, express TLR4 at both the mRNA and the protein levels. TLR4 appears to be functional, as stimulating the photoreceptor cells line with LPS induced IL-6 and CXCL1 production in a dose- and time-dependent manner. Furthermore, although we did not directly measure expression of the TLR4 co-receptor MD-2, we demonstrated that blocking TLR4 function using the TLR4/MD-2 antagonist eritoran tetrasodium significantly inhibited LPS-induced IL-6 production. In addition, LPS stimulation partially protected the photoreceptor cells from oxidative-stress induced cell death.
The conventional role of TLR4 on immune cells is to serve as the first defense against invading pathogens. Stimulation of TLR4 by pathogens not only activates the innate immunity to eliminate infection, but also facilitates later adoptive immunity against pathogens at least in part by promoting the maturation of dendritic cells (14). Recent studies indicated that TLR4 is also present on some brain neurons and may be involved in brain diseases and development [reviewed in (15)]. Our results that photoreceptor cells express functional TLR4 suggest that TLR4 expression is not restricted to neurons in the brain. The results that photoreceptor cells produce IL-6 and CXCL1 after LPS stimulation suggest that photoreceptor cells could be another local source of inflammatory cytokines/chemokines during retinal infections, which could directly contribute to the pathogenesis of many retinal infectious diseases such as infectious posterior uveitis.
Recent studies have demonstrated that IL-6 plays a critical role in protecting neurons from oxidative damage (9, 10). Our results that LPS stimulation significantly upregulated IL-6 production from photoreceptor cells and protected them from oxidative stress induced damage are consistent with these reports. To the best of our knowledge, this is the first report that shows the presence of functional TLR4 on photoreceptors. These new findings may be able to provide insight into developing novel reagents in promoting photoreceptor survival in age-related macular degeneration (AMD), in which oxidative stress induced photoreceptor degeneration has been implicated in the pathogenesis(16).
Acknowledgments
We thank Catherine Doller at the Vision Science Research Center (VSRC) imaging core for preparing the mouse ocular cryosections. This work was supported by National Institute of Health grants NS052471 (FL), EY018341 (CSS), EY019250 (CSS), EY14362 (EP) and EY018137 (MRA). The VSRC Core facilities are supported by NIH grant EY11373 (EP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 2.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- 3.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 49:1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindzelskii AL, Elner VM, Elner SG, Yang D, Hughes BA, Petty HR. Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments. J Gen Physiol. 2004;124:139–149. doi: 10.1085/jgp.200409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2009;50:1247–1254. doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portillo JA, Okenka G, Kern TS, Subauste CS. Identification of primary retinal cells and ex vivo detection of proinflammatory molecules using flow cytometry. Mol Vis. 2009;15:1383–1389. [PMC free article] [PubMed] [Google Scholar]
- 9.Bissonnette CJ, Klegeris A, McGeer PL, McGeer EG. Interleukin 1alpha and interleukin 6 protect human neuronal SH-SY5Y cells from oxidative damage. Neurosci Lett. 2004;361:40–43. doi: 10.1016/j.neulet.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Peng YP, Qiu YH, Lu JH, Wang JJ. Interleukin-6 protects cultured cerebellar granule neurons against glutamate-induced neurotoxicity. Neurosci Lett. 2005;374:192–196. doi: 10.1016/j.neulet.2004.10.069. [DOI] [PubMed] [Google Scholar]
- 11.Chong DY, Boehlke CS, Zheng QD, Zhang L, Han Y, Zacks DN. Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2008;49:3193–3200. doi: 10.1167/iovs.07-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueki Y, Wang J, Chollangi S, Ash JD. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008;105:784–796. doi: 10.1111/j.1471-4159.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanvicens N, Gomez-Vicente V, Masip I, Messeguer A, Cotter TG. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J Biol Chem. 2004;279:39268–39278. doi: 10.1074/jbc.M402202200. [DOI] [PubMed] [Google Scholar]
- 14.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47:4660–4664. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]