Abstract
A small subset of basic helix–loop–helix transcription factors called PIFs [phytochrome (phy)-interacting factors] act to repress seed germination, promote seedling skotomorphogenesis and promote shade-avoidance through regulated expression of over a thousand genes. Light-activated phy molecules directly reverse these activities by inducing rapid degradation of the PIF proteins. Here, we review recent advances in dissecting this signaling pathway and examine emerging evidence that indicates that other pathways also converge to regulate PIF activity, including the gibberellin pathway, the circadian clock and high temperature. The PIFs thus have broader roles than previously appreciated, functioning as a cellular signaling hub that integrates multiple signals to orchestrate regulation of the transcriptional network that drives multiple facets of downstream morphogenesis. The relative contributions of the individual PIFs to this spectrum of regulatory functions ranges from quantitatively redundant to qualitatively distinct.
Phytochrome signal perception and transduction
The perception of light signals by the phytochrome (phy) family of sensory photoreceptors [phyA through phyE in Arabidopsis (Arabidopsis thaliana)] initiates an intracellular transduction process that culminates in the altered expression of nuclear genes that direct growth and developmental responses, termed photomorphogenesis, appropriate to the prevailing environment, throughout the plant life cycle [1, 2]. Current data indicate that the transduction process involves rapid translocation of the light-activated photoreceptor molecule (the Pfr conformer) from the cytoplasm into the nucleus, where it induces transcriptional responses in target genes [3]. The pathway by which the signaling information is propagated to the transcriptional network involves direct, physical interaction of the translocated Pfr conformer with a small subset of constitutively nuclear, basic helix–loop–helix (bHLH) transcription factors, designated Phytochrome-Interacting Factors (PIFs) [4, 5]. Several comprehensive articles have examined various aspects of this overall process in recent years [6–11]. This review focuses predominantly on recent advances in defining the phy-PIF signaling mechanism and the function of the PIFs in regulating the primary, light-responsive transcriptional network. Also examined, is growing evidence that the PIFs serve a broader function, as a hub that integrates signaling information from multiple cellular signaling pathways, in addition to the phy pathway.
PIF molecular characteristics and dynamics in the cell
The PIF proteins belong to the fifteen-member Subfamily 15 of the Arabidopsis bHLH superfamily [12–14]. Figure 1 provides an overview of the molecular phylogeny, domain structure and phy-binding activity of this subfamily. PIF3, the foundation member of the PIF subset, was initially identified in a yeast two-hybrid screen for phyB-interacting proteins, and subsequently shown to bind conformer-specifically, in photoreversible fashion, to the Pfr form of both phyA and phyB [15, 16]. All other PIFs similarly bind Pfr-specifically to phyB [17–21] (Y. Oka and P.H. Quail, unpublished). PIF1, like PIF3, also binds to phyA; PIF1 strongly, and PIF3 more weakly [17, 18, 21, 22]. All PIF members of Subfamily 15 contain a conserved N-terminal sequence, called the Active Phytochrome B-binding (APB) motif, which is necessary and sufficient for phyB-specific binding [19]. PIF1 and PIF3 also each contain a separate domain, called the Active Phytochrome A-binding (APA) region, which is necessary for phyA binding, but the actual binding sequence does not appear to be conserved between these two bHLH proteins [23, 24]. Of the remaining non-PIF subfamily members, LONG HYPOCOTYL IN FAR-RED 1 (HFR1) and PHYTOCHROME INTERACTING FACTOR 3-LIKE 1 (PIL1) have photomorphogenesis-related functions, and SPATULA (SPT) functions in regulating seed germination (see below), whereas the other five members either have no apparent photomorphogenic activity (ALCATRAZ (ALC)) and BHLH23, which carries a natural APB mutation that blocks phyB binding), or are yet to be fully characterized (BHLH56, BHLH119 and BHLH127).
Figure 1.
The PIF-subfamily of phy-interacting basic helix–loop–helix (bHLH) transcription factors. (a) PIF3 domain structure. Schematic of the PIF3 polypeptide showing the location of the consensus bHLH domain, which defines this class of transcription factors, as well as the binding sites for photoactivated phyB (APB) and phyA (APA). The bHLH domain is responsible for dimerization and DNA-binding of the protein. The Arabidopsis PIFs bind to the conserved G-box DNA-sequence motif shown. (b) Subfamily 15 of the Arabidopsis bHLH family showing the phylogeny, nomenclature, domain structures, phy-interaction activity and in vivo functional activity of the PIF proteins. Pfr-specific interaction with phyA and/or phyB is indicated as A and/or B, and lack of interaction as (−). The asterisk indicates evidence of PIF8 binding to phyB (Y. Oka and P.H. Quail, unpublished). Evidence of functional involvement in phy signaling in vivo is indicated as (+), and lack of such evidence as (−). ND = not determined. Modified from [5, 10].
Where examined (PIF1, PIF3, PIF4, PIF5 and PIF7), the PIFs have been found to bind sequence-specifically to a core DNA G-box motif (CACGTG) [18, 20, 25–30], suggesting a direct signaling pathway from photoactivated phy molecules to their target genes. Potentially consistent with this notion, Bauer et al. [31] showed that phyB rapidly (within minutes) colocalizes with PIF3 in subnuclear bodies called speckles upon light-activated translocation. However, these authors also made the pivotal observation that whereas cytologically visible PIF3:GFP was initially present at high levels in dark-grown seedlings, light exposure induced a rapid decline in abundance, thus establishing that the phys negatively regulate PIF3 activity in vivo (Figure 2). Subsequent studies by the Quail laboratory showed that this degradation is initiated by the direct binding of either phyA or phyB (and minimally phyD) to their respective APA or APB interaction sites in PIF3 in the nucleus [23, 32]. The data also showed that phy induces rapid (within minutes) phosphorylation of PIF3 as a result of this interaction [23] and that this leads to apparent ubiquitylation and degradation (t1/2, ~20 min) via the ubiquitin–proteasome system (UPS) [23, 33]. Similar data have also been obtained for PIF1, PIF4 and PIF5, with half-times of 5–20 min for degradation [24, 34–38]. Therefore, these data suggest that the transphosphorylation of these transcription factors might represent the primary biochemical mechanism of signal transfer from the photoactivated phy molecule to its signaling partners in the cell, and that this post-translational modification might flag the proteins for ubiquitylation and degradation via the UPS system. A recent report that an Arabidopsis protein called HEMERA (HMR), that is similar to the yeast multiubiquitin-binding protein, RAD23, is necessary for light-induced PIF1 and PIF3 proteolysis in nuclear speckles [39] is consistent with this scenario. RAD23 functions to shuttle polyubiquitylated proteins to the proteasome for degradation. However, despite these advances, neither the protein kinase nor the presumptive E3 ubiquitin-ligase responsible for the upstream signaling events have been definitively identified [3].
Figure 2.
Simplified schematic summary of PIF-mediated signaling pathways. phy photoactivation (Pfr formation) triggers rapid nuclear-localized proteolysis of the PIF proteins. Under long-term light exposure, a direct, mutually negative feedback loop from the PIFs to phyB emerges, whereby the PIFs modulate phyB abundance concomitantly with the converse, by inducing COP1-catalyzed ubiquitylation and degradation of phyB. In addition to this direct regulation of PIF protein abundance, photoactivated phys regulate PIF activity indirectly (i) at the transcriptional level via control of the circadian clock, which in turn induces circadian oscillations in PIF4 and PIF5 gene expression in the light, (ii) at the post-translational level via modulation of the GA signaling pathway, which controls the abundance of DELLA proteins, which, in turn, act negatively on PIF transcriptional activity through binding to the bHLH domain, and, potentially, (iii) by an unknown mechanism via the COP1–SPA regulatory pathway (broken line). PIF4 and PIF5 are also subject to negative feedback regulation via inhibitory heterodimer formation with HFR1, whose gene expression level is promoted by PIF4 and PIF5 in response to vegetational shade. These alterations in abundance or activity result in activation or repression of PIF target-genes, leading in turn to propagation of the cascade of transcriptional changes that drive the overt morphogenic responses. High temperature acts specifically through PIF4 by an unknown mechanism to induce enhanced elongation growth.
The initial rapid, light-induced degradation of these PIFs does not lead to the total disappearance of the protein, but rather results in a new, lower steady- state level of the protein in sustained light, such as that experienced during the day of a normal day night cycle [35, 37, 40]. Moreover, degradation ceases upon Pfr removal and the return of plants to darkness, with the result that the PIF proteins rapidly reaccumulate to high levels over the dark period. Subsequent re-exposure to light once again induces rapid degradation. This rapidly reversible, phy-induced, dynamic regulation of PIF levels indicates that rather than acting only briefly and transiently during the initial phases of seedling de-etiolation, the PIFs remain potentially functionally important in fully green seedlings. A notable exception to this dynamic behavior is PIF7, which despite interacting with, and colocalizing in nuclear speckles with photoactivated phyB, shows no detectable light-induced phosphorylation or degradation [20, 41].
Where examined (PIF1, PIF3, PIF4, PIF5 and PIF7), the PIFs have been shown to possess intrinsic transcriptional activation activity in transfection or heterologous systems [17, 20, 24–26, 32], indicating that they have the capacity to function as positive regulators of gene expression in planta. Interestingly, PIF7 has also been reported to function as a transcriptional repressor [41], suggesting possible dual activity depending on promoter context.
PIF function in regulating seed germination
Dormant Arabidopsis seeds require both light activation of the phytochrome system and cold treatment (stratification) to induce efficient germination [21, 36, 42, 43]. PIF1 and the related non-phy-interacting, Subfamily-15, bHLH factor SPT (Figure 1b), function to repress germination in the dark and in the light, respectively. PIF1 exerts this function, at least in part, by repressing the expression of the key gibberellin (GA)-biosynthetic genes GA3ox1 and GA3ox2 and promoting the expression of the GA catabolic gene GA2ox2 [42], thereby maintaining low GA levels. Conversely, PIF1 promotes the expression of the abscisic acid (ABA)-biosynthetic genes ABA1, NCED6 and NCED9, and represses the expression of the ABA catabolic gene CYP707A2, resulting in high ABA levels [42]. In addition, PIF1 promotes the expression of two GA-repressor (DELLA) genes, GAI and RGA, which decreases the sensitivity of the seed to the GA present [42]. Targeted chromatin immunoprecipitation (ChIP) analyses detected in vivo interaction of PIF1 with the GAI and RGA promoters, but not the other biosynthetic genes, suggesting that the two DELLA genes might be directly regulated by PIF1, whereas this and other evidence indicates that the biosynthetic genes are indirectly regulated [42]. Exposure of stratified seeds to phy-activating light induces a reversal of all these activities through phy-induced degradation of PIF1, resulting in increased GA levels, decreased ABA levels, and increased sensitivity to GA, leading in turn to release from dormancy and seed germination [6, 21, 42–44]. pif1-mutant seed germinate fully without the need for any light treatment, indicating that none of the other PIFs act redundantly with PIF1 in this capacity, a point confirmed recently using higher order combinations of pif1, pif3, pif4 and pif5 mutant loci [45]. A role in regulating seed dormancy for a putative splice-variant-encoded PIF6 protein, lacking the bHLH DNA-binding domain, was recently reported [46]. However, no evidence of participation in phy-regulated germination was obtained.
Recent microarray-based expression profiling has expanded the analysis to examine the genome-wide transcriptional changes induced by photoactivated phy and regulated by PIF1 in germinating seeds, and this has been coupled with ChIP analysis directed at identifying direct PIF1 gene-targets in these seeds [29]. The data show that 70% of the 2000 genes that respond >1.5-fold to light in wild-type seed within 12 h of exposure respond similarly to the absence of PIF1 in pif1 mutant seeds kept in darkness [29]. This demonstrates the dominant role of PIF1 in mediating the phy-induced transcriptional changes accompanying light-induced germination, and the minimal, if any, role of the other PIF-family members. Although no examination of early gene expression changes potentially matching the rapid light-induced decline in PIF1 levels was presented, genome-wide ChIp-chip analysis identified 166 (14%) of these phy-PIF1-regulated genes as containing promoter-bound PIF1, suggesting that they are direct targets of PIF1 [29]. These genes include transcription factors involved in hormone signaling and enzymes involved in cell wall metabolism, consistent with the pleiotropic changes that drive seed germination.
SPT, which is light-stable, functions to suppress seed germination in the absence of a cold pretreatment, regardless of light exposure [43]. Like PIF1, it performs this function, at least in part, by repressing expression of the GA-biosynthetic genes GA3ox1 and GA3ox2 [43]. Cold stratification reverses this repression, leading, upon exposure to light, to increased expression of both genes and germination. Taken together, these studies show that the dual repressive actions of two closely related transcription factors, on overlapping pathways, provide a convergence point for the signaling pathways from two major environmental factors that regulate a crucial transition in the plant life cycle.
PIF function in promoting seedling skotomorphogenesis and regulating de-etiolation
Early studies directed at defining the functional role of the PIF proteins in seedling de-etiolation, and the relevance of phy-induced PIF degradation to this function, provided a complex and sometimes superficially contradictory picture [9]. Genetic analysis showed that monogenic pif3, pif4, pif5 and pif7 null mutants exhibit light-hypersensitive seedling phenotypes, that is, shorter hypocotyls and larger cotyledons than wild type, at the completion of de-etiolation after several days of exposure to light [18, 20, 25, 34, 35, 40, 45, 47–50]. This observation has been broadly interpreted to indicate that the PIFs act negatively on phy signal transduction in the light, and was extrapolated to suggest that these factors act in the dark to autonomously repress photomorphogenesis [4–6]. However, the absence of any comparably robust de-etiolation phenotypes in rigorously dark-grown (“true-dark” [51]), monogenic pif-mutant seedlings [17, 45, 50, 52] did not support the postulated autonomous role of individual PIFs in repressing constitutive photomorphogenesis. Subsequent studies using various single and double pif-mutant combinations revealed that phyB levels are significantly elevated, in apparently additive fashion, by the absence of increasing numbers of PIF-protein species in prolonged, continuous R light (Rc), and that this is correlated with increasing seedling light-hypersensitivity [20, 32, 48]. Moreover, targeted site-specific mutagenesis of PIF3 showed that, whereas direct, intracellular phyB–PIF3 interaction is necessary for this activity in the light, PIF3 binding to its DNA target site is not [32]. Recent experiments with PIF6 support this conclusion [46]. Collectively, these data thus suggested that a major role of the PIFs in modulating the extent of seedling de-etiolation in prolonged Rc, is to modulate phyB abundance directly, and thereby overall seedling photosensory sensitivity, via a direct negative feedback loop (Figure 2), rather than participating as phy-activated signaling intermediates in this process [20, 32, 48]. There is now evidence that the PIFs facilitate this feedback process by stimulating CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1)-catalyzed ubiquitylation and degradation of phyB (Figure 2) [53].
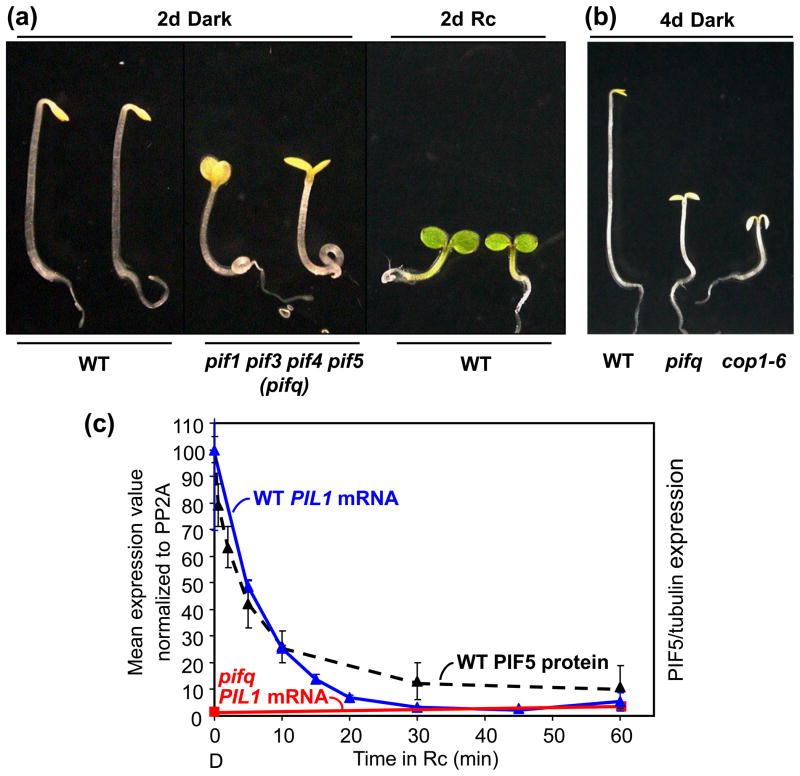
However, recent examination of the phenotypes of a series of monogenic, double, triple and quadruple (pifq) pif mutants has revealed various additive or synergistic effects between family members in the dark [45, 50–52], culminating in a striking constitutively photomorphogenic (cop)-like phenotype in rigorously dark-grown pifq seedlings [51] (Figure 3a and 3b). This phenotype is also evident at the cellular and subcellular levels in the dark-grown pifq mutant, where cytological parameters, such as chloroplast development, extensively phenocopy wild-type seedlings grown in light [54]. These findings provide compelling evidence that the PIFs do indeed function autonomously to repress photomorphogenesis and promote skotomorphogenesis in the dark in etiolated seedlings, and that the phys act to reverse this phenotypic repression–promotion upon light exposure, by inducing rapid degradation of the bHLH factors.
Figure 3.
Light reverses PIF-promoted skotomorphogenesis by phy-induced degradation of the PIF proteins. (a) PIFs promote skotomorphogenesis in dark-grown wild-type (WT) seedlings. This developmental state is reversed either in the dark by constitutive genetic removal of PIF1, PIF3, PIF4 and PIF5 in the quadruple pifq mutant, or by exposing the WT to light (2 days continuous R light). (b) The phenotype of dark-grown pifq-mutant seedlings resembles that of dark-grown cop1-mutant seedlings, such as the weak cop1-6 mutant allele shown in the picture [92]. (c) PIFs regulate expression of rapidly light-responsive genes such as PIL1. PIFs promote high-level expression of the PIL1 gene in dark-grown (D) WT seedlings (time zero). This expression is strongly repressed in the absence of the PIF quartet in the dark-grown pifq mutant (time zero). Light induces rapid repression of PIL1 gene expression in the WT, in parallel with similarly rapid photoactivated-phy-induced degradation of PIF5 (and other PIFs). Abbreviation: PP2A, SERINE/THREONINE PROTEIN PHOSPHATASE 2A. Modified from [38], [51].and [54].
However, the spectrum of phenotypes of the various pif-mutant combinations also indicates that there is considerable complexity in the system due to the varying degrees of apparent functional redundancy within the PIF family, as well as the likely involvement of additional family members (as suggested by the incomplete de-etiolation phenotype of the pifq mutant; Figure 3a and 3b) [35, 45, 50–52]. In addition, further intra-subfamily complexity is suggested by the evidence that non-phy-interacting HFR1 can form non-DNA-binding, transcriptionally unproductive heterodimers with PIF4 and PIF5 (and probably with PIF3) [26, 55]. Because light-induced repression of COP1 activity leads to increased HFR1 protein levels, it is possible that HFR1 negatively regulates PIF activity during seedling de-etiolation, as it does in shade-avoidance (see below) [26, 50, 56–59].
Genome-wide expression profiling of the pif mutants has provided valuable insight into the transcriptional network regulated by the PIF family during seedling de-etiolation, and the molecular mode of regulation exerted by these bHLH factors on target-gene expression [45, 50, 54]. Not unexpectedly, given the extent to which the unirradiated pifq mutant phenocopies the visible and cellular photomorphogenic development of the fully de-etiolated wild type (Figure 3a) [54], microarray-based transcriptome analysis shows that the majority of the gene expression changes elicited by the absence of the PIFs in dark-grown pifq seedlings are normally induced by prolonged light in wild-type seedlings. The functions of the bulk of these genes correlate strongly with the various morphogenic changes observed during de-etiolation, including numerous photosynthetic genes related to the biogenesis of active chloroplasts, various auxin, gibberellin, cytokinin and ethylene hormone pathway-related genes potentially mediating growth responses, and metabolic genes reflecting the transition from heterotrophic to autotrophic growth [45, 54]. These findings thus document the pleiotropic function of these PIFs in controlling the expression of phy-regulated genes during normal seedling development.
By comparing the genes that respond most rapidly to initial exposure of seedlings to light in the wild type (“early-response” genes) with those responding to the absence of the PIFs in darkness in the pifq mutant, we have identified an overlapping subset of genes that are potential direct targets of these bHLH transcription factors [54]. Because this subset includes both induced and repressed genes, it is possible that the PIFs have a dual capacity to function as either transcriptional activators or repressors of direct-target genes depending on promoter context. The simplest configuration consistent with present data appears to be that for the light-repressed genes. In this configuration, the PIFs would function as transcriptional activators, promoting target gene expression in the dark in the wild type, a capacity lacking in the pifq mutant, but would rapidly lose this capacity in the wild type upon light exposure, due to phy-induced PIF degradation. Consistent with this possibility, PIF1, PIF3, PIF4 and PIF5 all show intrinsic transcriptional activation activity in transfection assays, as mentioned above [17, 24–26, 32]. Early time-course analysis of the behavior of PIL1, one of the rapid-response genes, provides an example of a robust fit with this configuration, including a strong correlation in the kinetics of the rapid decline in PIF5 protein abundance and PIL1 transcript abundance in the wild type upon initial exposure to light (Figure 3c). This subset of repressed “early-response” genes is enriched in genes encoding transcription factors, suggesting a role as direct regulators of downstream genes in the phy-directed transcriptional network. Nevertheless, the converse configuration involving direct PIF-imposed dark-repression, coupled with light-induced derepression of the light-induced genes, also remains viable. Recent ChIP-PCR evidence that PIF1 binds to a G-box-containing region of the promoter of the induced early-response gene, PSY, in white-light grown, dark-adapted plants is potentially consistent with this alternative [60].
Expression analysis of single and double pif mutants has provided some initial insight into the potentially differential, as well as overlapping, contributions of the individual PIF family members to the overall molecular phenotype of the developing seedling. Microarray profiling of dark-grown monogenic pif1 [28] and pif3 [40, 54] mutant seedlings identified only a small number of genes (3 and 14, respectively) differentially expressed between mutant and wild type above the threshold commonly used in the field to define such differences (i.e. Statistically Significant and Two-Fold (SSTF) [61]). These findings are consistent with the weak or absent visible morphogenic phenotypes in these mutants in the dark [45, 51, 52, 54]. However, these and other relatively moderate changes in expression in the mutants in the dark are responsible for another insidious visible phenotype that only becomes manifest upon transfer of the seedlings to light. It was found that increasing durations of post-germinative growth in darkness beyond about two days results in the accumulation of protochlorophyllide in the pif1, pif3 and pif5 (but not pif4) mutants that ultimately reaches levels that cause lethal bleaching upon initial seedling exposure to light [17, 45, 52, 54]. This phenomenon is variable between experiments but becomes increasingly more severe in the various double, triple and quadruple mutants. The evidence indicates that the hyperaccumulation of protochlorophyllide is the result of misregulation of key chlorophyll-biosynthetic pathway genes in the mutants [17, 45, 52, 54]. The pif1, pif3 and pif5 mutants were found to express enhanced levels in the dark of genes such as HEMA1, which encodes the enzyme for the first committed step in tetrapyrrole synthesis, CHLH, which encodes a subunit of Mg chelatase, the first enzyme in the chlorophyll branch of the pathway, and GUN4, a regulator of the chelatase [45, 52]. In addition, pif1 was found to express reduced levels of PORC, resulting in sustained higher levels of photooxidizing protochlorophyllide in the mutant upon light exposure because of reduced efficiency of conversion to chlorophyllide [28]. Microarray profiling of a pif4 pif5 double mutant grown in the dark identified 113 genes that were differentially expressed in the mutant compared with the wild type [50]. A greater number of these genes than for the monogenic pif1 and pif3 mutants, overlap with those misregulated in the dark-grown pifq mutant. This result suggests that PIF4 and PIF5 together, additively or redundantly, contribute significantly to the combined regulatory activities of the PIF1, PIF3, PIF4, PIF5 quartet (although the necessary direct comparison with the pif4 and pif5 monogenic mutants has not been reported thus far). It would be interesting to extend this analysis to determine whether any differences in expression-pattern regulation between the PIFs represent qualitatively different contributions via differential target-gene selection by the different family members, or quantitatively additive contributions across the full spectrum of PIF-regulated genes.
Although rapid changes in expression in response to phy-induced degradation of the PIF proteins (Figure 3c) suggest that such “early-response” genes might be direct targets of PIF regulation, additional analysis, using strategies such as chromatin immunoprecipitation (ChIP), are necessary to determine whether the PIFs interact with the promoters of such genes in vivo. A few studies have used ChIP analysis of pre-selected target genes to assay for in vivo binding of PIF1, PIF3 and PIF4 to the promoters of these genes in developing seedlings. Moon et al. [28] detected the in vivo binding of PIF1 to a G-box-containing PORC-promoter region in dark-grown seedlings, consistent with the reduction in expression of this gene observed in pif1 mutants. Toledo et al., (2010) [60] showed PIF1 binding also to a G-box region of a PSY promoter in dark-adapted, light-grown plants, supporting a possible transcriptional repressor function for this PIF. Shin et al. [30] reported PIF3 binding to several anthocyanin-biosynthetic-gene-promoters in continuous far-red light (FRc)-grown seedlings. However, the functional relevance of these interactions is not clear because no FRc-induced reduction in PIF3 abundance or promoter-binding was observed. Feng et al. [62] reported the in vivo binding of PIF3 to five putative target genes. However, none of the promoters of these genes contain G-box (or E-box) sequence motifs in the fragments reported, raising the issue of how the protein would bind to the DNA in a functionally relevant manner in the absence of an established binding site. de Lucas et al. [25] have provided evidence of in vivo binding of PIF4 to the promoters of four of nine selected G-box-containing genes in dark-adapted, fully green seedlings, consistent with these being directly regulated by PIF4. Wider use of ChIP-chip and ChIP-seq technology should provide a more comprehensive picture of the network of direct targets of the PIF family.
PIF function in regulating shade avoidance
Franklin [63] has provided an excellent recent review of the Shade Avoidance Syndrome (SAS). PIF4 and PIF5 have been reported to function in the SAS in fully de-etiolated seedlings [34]. The abundance of these proteins increases rapidly in wild type upon transfer of white-light (WL)-grown seedlings to simulated shade, and pif4, pif5 and pif4 pif5 mutants have reduced hypocotyl-elongation and marker-gene responsiveness to this signal compared with wild type. Conversely, PIF4- and PIF5-overexpressors have the opposite phenotype, approaching constitutively long hypocotyls and petioles, and high marker-gene expression, with a concomitantly reduced residual capacity for shade-avoidance. Together, the data indicate that PIF4 and PIF5 act positively to promote the SAS [34]. Consistent with this conclusion, ChIP analysis indicates that PIF5 binds in vivo to the G-box-containing regions of the promoters of the marker genes PIL1, XTR7 and HFR1 [26]. A confounding factor for this straightforward interpretation is that there is evidence that the widely reported light-hypersensitivity of the various pif mutants (including pif4 and pif5) is due, at least in part, to elevated phyB levels, as a result of the direct negative feedback loop from PIF to phyB described above (Figure 2) [20, 32, 48]. These elevated phyB levels will produce higher residual, photoactive Pfr levels in the mutant than in the wild type in response to the FR-rich light of the simulated shade treatment, and these levels can be expected to attenuate the extent of the shade-response in the mutant. This suppression of the SAS by elevated phyB levels has already been demonstrated for Arabidopsis engineered to overexpress phyB [64]. Arguing against this possibility is the observation that the pif4 and pif5 mutations suppress the long-hypocotyl and high marker-gene phenotypes of the phyB mutant [34]. Thus, although some ambiguity remains as to the relative quantitative contributions of these two interwoven sources of SAS suppression, it does appear that PIF4 and PIF5 contribute significantly to the direct promotion of the SAS.
The participation of several rapidly shade-responsive genes in the SAS has been well documented [34, 64–69]. Of particular interest here are the two non-phy-interacting, PIF-related, bHLH genes HFR1 and PIL1 (Figure 1b). Although without effect initially, sustained HFR1 accumulation inhibits the SAS after longer-term exposure to vegetative shade, thus providing a negative feedback loop that prevents over-responsiveness (Figure 2). Hornitschek et al. [26] have provided evidence that HFR1 exerts this effect by forming transcriptionally unproductive, non-DNA-binding heterodimers with PIF4 and PIF5, thereby antagonizing the promotive effects of these two factors on elongation growth and shade marker-gene induction. PIL1 has been reported to be required for the normal growth responses to transient [66] and long-term [64] shade, but a mechanism has not yet been defined. Regardless, these data reveal an intricate, intra-subfamily signaling network involving cross-talk at transcriptional and post-translational levels.
PIF function in flowering
A recent association study implicates PIF4 in the regulation of flowering time [70]. However, no difference in flowering time was detected between wild type and the pifq mutant, or any of the monogenic null pif1, pif3, pif4 or pif5 mutants [45]. Further investigation of this apparent disparity might provide insights into the molecular basis of PIF4 signaling activity.
Intersection of other signaling pathways with the PIFs
Recent evidence indicates that the phys regulate the PIFs via three, possibly four, pathways in response to light: one direct pathway by induced proteolysis, as described above, and three indirect pathways – the first via the gibberellin signaling pathway, the second via the COP1–SPA pathway, and the third via the circadian clock (Figure 2). In addition, further endogenous, environmental and developmental signaling pathways intersect with PIF activity in regulating various morphogenic responses.
Gibberellins
Light-activated phys trigger rapid repression of GA-biosynthetic genes (such as GA3ox1, GA20ox1, GA20ox2 and GA20ox3) and induction of GA-catabolic genes (such as GA2ox1 and GA2ox2) [54, 71, 72], leading to declines in bioactive GAs upon exposure of dark-grown Arabidopsis seedlings to irradiation [71–75]. These responses are both PIF-mediated (GA3ox1) and PIF-independent (GA20ox and GA2ox genes) [54]. The decline in GA levels causes increased DELLA protein abundance, due to relief of activated-GA-receptor-imposed DELLA degradation [71, 76]. The DELLA proteins GAI and RGA interact with PIF3 and PIF4, inhibiting their DNA-binding activities, and thus their transcriptional regulatory activities [25, 62]. The DELLAs thus appear to function as negative modulators of PIF protein transcriptional regulatory activity in light-grown plants, partly enhancing the effects of the quantitatively more robust reduction in activity directly triggered by the light-induced reduction in PIF protein abundance. The absence of this function of DELLAs is observed in quadruple and pentuple della mutants as partial reversal of the full light-imposed inhibition of hypocotyl elongation seen in wild-type seedlings [62, 71]. However, no effect of these della mutations on the etiolated state is observed in dark-grown seedlings. The naturally high GA levels in etiolated seedlings thus appear to be permissive for maximum PIF activity in promoting skotomorphogenesis, rather than regulatory of this activity. At steady state, in fully de-etiolated, light-grown seedlings, other light-independent regulators of GA activity could presumably affect growth and development via DELLA-mediated alterations in PIF3 and PIF4 activity.
Investigation of the role of the GA pathway in shade avoidance has shown that shade induces a reduction in DELLA protein abundance, relieving the constraint on growth imposed by these proteins [77]. However, genetic evidence indicates that this reduction is necessary but not sufficient for a full shade-induced elongation response. A possible mechanism that would explain these data is that full shade responsiveness requires both release from phyB Pfr-imposed repression of PIF4 and/or PIF5 protein accumulation [34] and concomitant GA-induced removal of DELLA-imposed inhibition of PIF transcriptional activity [25, 62].
COP1 and SPAs
Both cop1 and spa1 spa2 spa3 triple mutants have been shown to express reduced levels of PIF3 in dark-grown seedlings [31, 51]. This result is the opposite to that expected if COP1 were the ubiquitin E3 ligase responsible for PIF degradation. Nevertheless, this phenotype suggests that reduced levels of PIF3, and possibly other PIFs, might partly explain the constitutively photomorphogenic phenotype of these mutants, and that light-repression of COP and SPA activity might contribute to seedling de-etiolation by indirectly reducing PIF levels (Figure 2), in addition to the well-established effect of enhancing HY5 accumulation [78–80]. However, there is currently no direct evidence for the light-regulated segment of this potential pathway. By contrast, the recent evidence that the converse, PIF-induced negative-feedback regulation of phyB levels (Figure 2) is directly mediated by COP1-catalyzed degradation of the photoreceptor molecule [53] suggests yet another level of complexity in this signaling pathway.
Circadian clock
In addition to direct negative regulation of PIF protein abundance at the post-translational level via targeted proteolysis as described above, photoactivated phys regulate PIF4, PIF5 and PIF7 (but not PIF1 or PIF3) expression indirectly, at the transcriptional level, via control of the circadian clock [41, 81] (Figure 2). The net product of these two levels of regulation determines the temporal pattern of PIF4 and PIF5 protein abundance and, therefore, the profile of hypocotyl elongation rates, across light–dark diurnal cycles [35]. These rates are maximal when PIF4 and PIF5 levels are high as a result of the temporal coincidence of high transcription rates and low degradation rates. This period occurs at the end of the night and marks the point of coincidence between an external cue (light) and an internal (albeit light-regulated or -phased) cue (the clock), providing a mechanism for observed diurnal growth rhythms [35]. A recent report concludes that this concept can be extended to explain the photoperiodic control of hypocotyl elongation [82].
Blue light
Not unexpectedly, blue-light activated phy has been shown to induce PIF1 degradation [83]. No evidence of blue-light-triggered, CRYPTOCHROME (cry)-induced degradation, or of physical cry-PIF1 interaction was obtained. Interestingly, however, the absence of both CRY1 and CRY2 in a cry1 cry2 double mutant accelerated the blue-induced, phy-mediated degradation of PIF1. Unclear at present is whether the CRYs normally have a regulatory function antagonizing phy-induced PIF1 degradation and whether this function requires CRY photoactivation, or whether the observed effect is an indirect result of the genetic removal of the CRY proteins.
High temperature
A striking, selective function of PIF4 in high-temperature-induced growth responses in de-etiolated Arabidopsis seedlings, similar to those induced by vegetative shade, has recently been reported [84, 85] (Figure 2). pif4, but not pif1, pif3 or pif5, mutants completely lack the robust enhancement of hypocotyl and epicotyl elongation and leaf hyponasty exhibited by the wild type upon transfer of seedlings from 22°C to 28°C. In addition, this temperature shift induces a rapid elevation of PIF4 transcript levels, suggesting that a temperature-sensing signaling pathway, independent of the phy pathway, might regulate this response via selective control of PIF4 abundance.
Auxin
The necessity of auxin to the SAS response was highlighted by the recent discovery of a new biosynthetic pathway for the hormone [86]. However, because no shade-induced increase in the expression of the key biosynthetic-enzyme-encoding gene, TAA, in the pathway was observed, it is possible that this auxin-production mechanism might be permissive rather than regulatory of the SAS response. The possible convergence of the shade-avoidance [65, 86] and high-temperature [84, 85, 87] signaling pathways on auxin mediation of the induced elongation response has recently been reviewed [88, 89]. However, whereas PIF4 is the dominant, if not exclusive player in the high-temperature response [84, 85], PIF5, and potentially other factors, also have a role in the SAS response [34, 64–66, 68, 69].
Ethylene
Recent evidence shows that the ethylene signaling pathway acts in parallel with PIF1 in promoting skotomorphogenesis in dark-grown seedlings by suppressing protochlorophyllide hyperaccumulation and inducing PORA and PORB gene expression [90]. Dark-grown ein3 eil1 double mutants, defective in ethylene signaling, show subsequent light-induced bleaching, similar to the pif mutants, and this effect is additive in the pif1 ein3 eil1 triple mutant. Although there is no evidence of cross-regulation in these loss-of-function mutants, overexpression of PIF5 induces overexpression of ethylene and a triple-response-like phenotype in dark-grown seedlings, and pif5 mutants have a subtle hook-unbending and cotyledon separation phenotype [48].
Cell fate
PIF4 (but not PIF3, PIF5 or PIF6) has recently been reported to function in light-regulated, phyB-mediated stomatal development in mature green leaves [91]. Notably, both the photoreceptor and the bHLH factor act positively in this developmental response, in contrast to their antagonistic activities in the de-etiolation and shade-avoidance responses. A possible mechanism of action involving PIF4 heterodimerization with the other non-PIF bHLH factors, well-known to control stomatal development, has been suggested [91].
PIF functional diversity
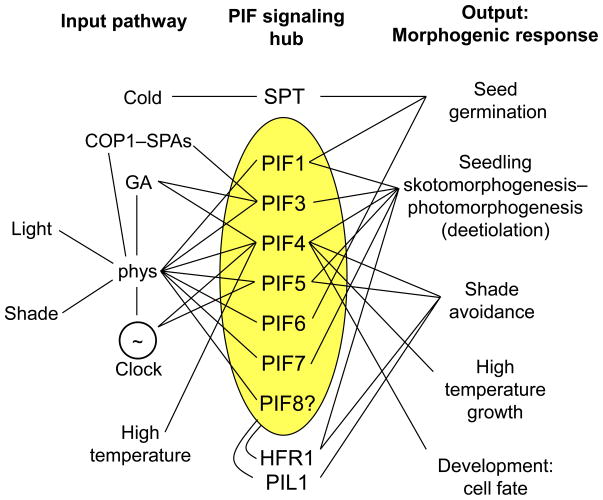
As outlined above, an increasing number of studies in recent years have identified PIF-family members as key components of other signaling pathways, besides the well-characterized photoregulated pathway. These pathways converge on the PIFs and their close relatives to regulate an array of developmental responses from seed germination to vegetational architecture and cell fate. The PIFs thus function as a cellular signaling hub, integrating multiple signals to coordinate regulation of the transcriptional network that drives multiple morphogenic responses (Figure 4). A degree of functional diversity among family members is also becoming increasingly apparent. Although there is considerable quantitative redundancy or additivity in the regulation of early seedling development by PIF1, PIF3, PIF4 and PIF5, there is also strong qualitative differential activity, such as in the dominance of PIF1 in controlling seed germination, the dominant, if not exclusive, role of PIF4 in high-temperature responsiveness, and the central roles that PIF4 and PIF5 appear to have in regulating shade avoidance. In addition, the possibility remains that other family members, such as PIF6 and PIF8, participate in at least some of these responses. The incomplete constitutively photomorphogenic phenotype of the dark-grown pifq mutant (Figure 3a), for example, suggests that factors additional to PIF1, PIF3, PIF4 and PIF5 play a significant role in promoting seedling skotomorphogenesis. Finally, there is emerging evidence of additional layers of complexity involving selective intra-family cross-talk at the transcriptional and post-translational levels, as exemplified by the heterodimer-mediated feedback inhibition by HFR1 of PIF4 and PIF5 transcriptional activation of the HFR1 gene.
Figure 4.
The PIFs function redundantly and differentially in a cellular signaling hub at the convergence of multiple pathways. Schematic summary of the network of known overlapping and differential activities of the PIF and related non-PIF Subfamily-15 members in integrating responses to both environmental and endogenous signaling pathways.
Acknowledgments
We thank the many colleagues who contributed to the work from this laboratory cited here, Elena Monte for her support and discussions, Yoshito Oka for unpublished evidence of PIF8 binding to phyB, Christian Fankhauser for stimulating discussions on the shade-avoidance syndrome and Jim Tepperman for discussions and figure preparation. This work was supported by the “Comissionat per a Universitats i Recerca del Departament d’Innovació, Universitats i Empresa”, of the Generalitat de Catalunya (Beatriu de Pinós program) and by Marie Curie International Reintegration Grant PIRG06-GA-2009-256420 to P.Leivar, and by National Institutes of Health Grant GM-47475, Department of Energy Grant DEFG03-87ER13742, and USDA Agricultural Research Service Current Research Information System Grant 5335-21000-027-00D to P.H.Quail.
Footnotes
Disclosures
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rockwell NC, et al. Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol. 2006;57:837–858. doi: 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schafer E, et al. Photomorphogenesis in Plants and Bacteria. Springer; 2006. [Google Scholar]
- 3.Quail PH. Phytochromes. Curr Biol. 2010;20:R504–507. doi: 10.1016/j.cub.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillon A, et al. Phytochrome Interacting Factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007;12:514–521. doi: 10.1016/j.tplants.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Duek PD, Fankhauser C. bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 2005;10:51–54. doi: 10.1016/j.tplants.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Bae G, Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- 7.Jiao Y, et al. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 8.Josse EM, et al. Paths through the phytochrome network. Plant Cell Environ. 2008;31:667–678. doi: 10.1111/j.1365-3040.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 9.Monte E, et al. Out of the dark: how the PIFs are unmasking a dual temporal mechanism of phytochrome signalling. J Exp Bot. 2007;58:3125–3133. doi: 10.1093/jxb/erm186. [DOI] [PubMed] [Google Scholar]
- 10.Quail PH. Phytochrome interacting factors. In: Whitelam GC, Halliday KJ, editors. Light and Plant Development. Blackwell Publishing; 2007. pp. 81–105. [Google Scholar]
- 11.Quail PH. Phytochrome-regulated Gene Expression. Journal of Integrative Plant Biology. 2007;49:11–20. [Google Scholar]
- 12.Bailey PC, et al. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell. 2003;15:2497–2502. doi: 10.1105/tpc.151140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heim MA, et al. The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol. 2003;20:735–747. doi: 10.1093/molbev/msg088. [DOI] [PubMed] [Google Scholar]
- 14.Toledo-Ortiz G, et al. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni M, et al. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature. 1999;400:781–784. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu-Sato S, et al. A light-switchable gene promoter system. Nat Biotechnol. 2002;20:1041–1044. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- 17.Huq E, et al. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305:1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- 18.Huq E, Quail PH. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 2002;21:2441–2450. doi: 10.1093/emboj/21.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna R, et al. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell. 2004;16:3033–3044. doi: 10.1105/tpc.104.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leivar P, et al. The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell. 2008;20:337–352. doi: 10.1105/tpc.107.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh E, et al. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell. 2004;16:3045–3058. doi: 10.1105/tpc.104.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, et al. Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc Natl Acad Sci U S A. 2000;97:13419–13424. doi: 10.1073/pnas.230433797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Sady B, et al. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell. 2006;23:439–446. doi: 10.1016/j.molcel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, et al. Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell. 2008;20:1586–1602. doi: 10.1105/tpc.108.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lucas M, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 26.Hornitschek P, et al. Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 2009;28:3893–3902. doi: 10.1038/emboj.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Garcia JF, et al. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288:859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- 28.Moon J, et al. PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc Natl Acad Sci U S A. 2008;105:9433–9438. doi: 10.1073/pnas.0803611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh E, et al. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21:403–419. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin J, et al. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007;49:981–994. doi: 10.1111/j.1365-313X.2006.03021.x. [DOI] [PubMed] [Google Scholar]
- 31.Bauer D, et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Sady B, et al. Mechanistic duality of transcription factor function in phytochrome signaling. Proc Natl Acad Sci U S A. 2008;105:2232–2237. doi: 10.1073/pnas.0711675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park E, et al. Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 2004;45:968–975. doi: 10.1093/pcp/pch125. [DOI] [PubMed] [Google Scholar]
- 34.Lorrain S, et al. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 35.Nozue K, et al. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 36.Oh E, et al. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 2006;47:124–139. doi: 10.1111/j.1365-313X.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- 37.Shen H, et al. PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 2005;44:1023–1035. doi: 10.1111/j.1365-313X.2005.02606.x. [DOI] [PubMed] [Google Scholar]
- 38.Shen Y, et al. Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 2007;145:1043–1051. doi: 10.1104/pp.107.105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, et al. Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell. 2010;141:1230–1240. doi: 10.1016/j.cell.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monte E, et al. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc Natl Acad Sci U S A. 2004;101:16091–16098. doi: 10.1073/pnas.0407107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidokoro S, et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009;151:2046–2057. doi: 10.1104/pp.109.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh E, et al. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19:1192–1208. doi: 10.1105/tpc.107.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penfield S, et al. Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr Biol. 2005;15:1998–2006. doi: 10.1016/j.cub.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Seo M, et al. Interaction of light and hormone signals in germinating seeds. Plant Mol Biol. 2009;69:463–472. doi: 10.1007/s11103-008-9429-y. [DOI] [PubMed] [Google Scholar]
- 45.Shin J, et al. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci U S A. 2009;106:7660–7665. doi: 10.1073/pnas.0812219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penfield S, et al. A role for an alternative splice variant of PIF6 in the control of Arabidopsis primary seed dormancy. Plant Mol Biol. 2010;73:89–95. doi: 10.1007/s11103-009-9571-1. [DOI] [PubMed] [Google Scholar]
- 47.Fujimori T, et al. Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2004;45:1078–1086. doi: 10.1093/pcp/pch124. [DOI] [PubMed] [Google Scholar]
- 48.Khanna R, et al. The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell. 2007;19:3915–3929. doi: 10.1105/tpc.107.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, et al. Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell. 2003;15:2399–2407. doi: 10.1105/tpc.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorrain S, et al. Phytochrome interacting factors 4 and 5 redundantly limit seedling de-etiolation in continuous far-red light. Plant J. 2009;60:449–461. doi: 10.1111/j.1365-313X.2009.03971.x. [DOI] [PubMed] [Google Scholar]
- 51.Leivar P, et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephenson PG, et al. PIF3 is a repressor of chloroplast development. Proc Natl Acad Sci U S A. 2009;106:7654–7659. doi: 10.1073/pnas.0811684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang IC, et al. Arabidopsis PHYTOCHROME INTERACTING FACTOR Proteins Promote Phytochrome B Polyubiquitination by COP1 E3 Ligase in the Nucleus. Plant Cell. 2010 doi: 10.1105/tpc.109.072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leivar P, et al. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell. 2009;21:3535–3553. doi: 10.1105/tpc.109.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fairchild CD, et al. HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 2000;14:2377–2391. [PMC free article] [PubMed] [Google Scholar]
- 56.Duek PD, et al. The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr Biol. 2004;14:2296–2301. doi: 10.1016/j.cub.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 57.Jang IC, et al. HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 2005;19:593–602. doi: 10.1101/gad.1247205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, et al. Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell. 2005;17:804–821. doi: 10.1105/tpc.104.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang KY, et al. Overexpression of a mutant basic helix-loop-helix protein HFR1, HFR1-deltaN105, activates a branch pathway of light signaling in Arabidopsis. Plant Physiol. 2003;133:1630–1642. doi: 10.1104/pp.103.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toledo-Ortiz G, et al. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc Natl Acad Sci U S A. 2010;107:11626–11631. doi: 10.1073/pnas.0914428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu W, et al. A light-independent allele of phytochrome B faithfully recapitulates photomorphogenic transcriptional networks. Mol Plant. 2009;2:166–182. doi: 10.1093/mp/ssn086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng S, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franklin KA. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 64.Roig-Villanova I, et al. Identification of primary target genes of phytochrome signaling. Early transcriptional control during shade avoidance responses in Arabidopsis. Plant Physiol. 2006;141:85–96. doi: 10.1104/pp.105.076331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roig-Villanova I, et al. Interaction of shade avoidance and auxin responses: a role for two novel atypical bHLH proteins. EMBO J. 2007;26:4756–4767. doi: 10.1038/sj.emboj.7601890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salter MG, et al. Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature. 2003;426:680–683. doi: 10.1038/nature02174. [DOI] [PubMed] [Google Scholar]
- 67.Sessa G, et al. A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev. 2005;19:2811–2815. doi: 10.1101/gad.364005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorin C, et al. ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant J. 2009;59:266–277. doi: 10.1111/j.1365-313X.2009.03866.x. [DOI] [PubMed] [Google Scholar]
- 69.Steindler C, et al. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- 70.Brock MT, et al. Genes underlying quantitative variation in ecologically important traits: PIF4 (phytochrome interacting factor 4) is associated with variation in internode length, flowering time, and fruit set in Arabidopsis thaliana. Mol Ecol. 2010;19:1187–1199. doi: 10.1111/j.1365-294X.2010.04538.x. [DOI] [PubMed] [Google Scholar]
- 71.Achard P, et al. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 2007;143:1163–1172. doi: 10.1104/pp.106.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alabadi D, et al. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 73.Alabadi D, Blazquez MA. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol Biol. 2009;69:409–417. doi: 10.1007/s11103-008-9400-y. [DOI] [PubMed] [Google Scholar]
- 74.Alabadi D, et al. Gibberellins repress photomorphogenesis in darkness. Plant Physiol. 2004;134:1050–1057. doi: 10.1104/pp.103.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Symons GM, et al. The hormonal regulation of de-etiolation. Planta. 2008;227:1115–1125. doi: 10.1007/s00425-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 76.Achard P, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 77.Djakovic-Petrovic T, et al. DELLA protein function in growth responses to canopy signals. Plant J. 2007;51:117–126. doi: 10.1111/j.1365-313X.2007.03122.x. [DOI] [PubMed] [Google Scholar]
- 78.Hoecker U. Regulated proteolysis in light signaling. Curr Opin Plant Biol. 2005;8:469–476. doi: 10.1016/j.pbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Yi C, Deng XW. COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Zhu D, et al. Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell. 2008;20:2307–2323. doi: 10.1105/tpc.107.056580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamashino T, et al. A Link between circadian-controlled bHLH factors and the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol. 2003;44:619–629. doi: 10.1093/pcp/pcg078. [DOI] [PubMed] [Google Scholar]
- 82.Niwa Y, et al. The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol. 2009;50:838–854. doi: 10.1093/pcp/pcp028. [DOI] [PubMed] [Google Scholar]
- 83.Castillon A, et al. Blue light induces degradation of the negative regulator phytochrome interacting factor 1 to promote photomorphogenic development of Arabidopsis seedlings. Genetics. 2009;182:161–171. doi: 10.1534/genetics.108.099887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koini MA, et al. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19:408–413. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 85.Stavang JA, et al. Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 2009;60:589–601. doi: 10.1111/j.1365-313X.2009.03983.x. [DOI] [PubMed] [Google Scholar]
- 86.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gray WM, et al. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci U S A. 1998;95:7197–7202. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franklin KA. Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol. 2009;12:63–68. doi: 10.1016/j.pbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Halliday KJ, et al. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol. 2009;1:a001586. doi: 10.1101/cshperspect.a001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong S, et al. EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci U S A. 2009;106:21431–21436. doi: 10.1073/pnas.0907670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Casson SA, et al. phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr Biol. 2009;19:229–234. doi: 10.1016/j.cub.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 92.McNellis TW, et al. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell. 1994;6:487–500. doi: 10.1105/tpc.6.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]