Abstract
To determine whether or not local, injury-induced aromatization and/orestrogen provision can affect cyto-or neuro-genesis following mechanical brain damage, two groups of adult male zebra finches sustained bilateral penetrating brain injuries. The first received contralateral injections of vehicle or the aromatase inhibitor fadrozole. The second group received contalateral injections of fadrozole, or fadrozole with 17β-estradiol. Subsequent to injury, birds were injected with the thymidine analog 5-Bromo-2′-deoxyuridine (BrdU). Two weeks following injury, the birds were perfused, and coronal sections were labeled using antibodies against BrdU and the neuronal proteins HuC/HuD. In a double blind fashion, BrdU positive cells and BrdU/Hu double-labeled cells in the subventricular zone (SVZ) and at the injury site (INJ) were imaged and sampled. The average numbers of cells per image were compared across brain regions and treatments using repeated measures ANOVAs and, where applicable, post-hoc, pairwise comparisons. Fadrozole administration had no detectable effect on cytogenesis or neurogenesis, however, fadrozole coupled with estradiol significantly increased both measures. The dorsal SVZ had the greatest proportion of new cells that differentiated into neurons, though the highest numbers of BrdU labeled and BrdU, Hu double-labeled cells were detected at the injury site. In the adult zebra finch brain, local estradiol provision can increase cytogenesis and neurogenesis, however, whether or not endogenous glial aromatization is sufficient to similarly affect these processes remains to be seen.
Keywords: neurogenesis, estrogens, neurosteroids, neuroplasticity, songbirds
INTRODUCTION
Estrogens and their precursors (progestins and androgens) are neuroprotective against numerous types of insult to the vertebrate brain (Brann et al., 2007; Saldanha et al., 2009; Suzuki et al., 2009). Interestingly, both brain-and gonadally-derived steroids are critical factors in promoting neuronal survival via the localized expression and the in situ actions of aromatase (estrogen synthase)(Saldanha et al., 2009). Indeed, aromatase knockout mice challenged with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or with a middle cerebral artery occlusion (MCAO) sustain significantly more brain damage than wild-type littermates (McCullough et al., 2003; Morale et al., 2008). Additionally, neuroprotective effects of progestins and androgens can be lessened via administration of the aromatase inhibitor fadrozole (Veiga et al., 2003) implicating the synthesis of estrogens as an important step in steroid mediated neuroprotection.
Estrogens can also have dramatic effects on neurogenesis (Brown et al., 2009). For example, in female rats, levels of neural cell proliferation differ across the phases of the estrous cycle, with highest levels occurring in proestrus, when circulating estrogens are maximal (Tanapat et al., 1999). Similarly, ovariectomy (OVX) in adult female rodents decreases hippocampal neurogenesis, while acute, peripheral treatment with 17β-estradiol (E2) increases the number of new neurons in the dentate gyrus (Pawluski et al., 2009). Taken together, these data strongly support a role for estrogen provision via aromatization as critical to many aspects of neuroplasticity in the adult vertebrate brain.
Songbirds have long been studied for their dramatic anatomical, physiological, and behavioral responses to estrogens and other steroids, particularly with respect to processes of neural plasticity (Alvarez-Buylla, 1992; Tramontin and Brenowitz, 2000; Ball et al., 2004; Brenowitz, 2004; Gahr, 2004). In several songbird species, seasonal differences in circulating steroids correlate to the volume of song nuclei, suggesting a relationship between the aromatization of androgens and neurogenesis (Ball et al., 2004). Correspondingly, exogenous E2 administration not only increases the volume of the song nucleus HVC (Soma et al., 2004), but also the incorporation of new neurons in this area (Hidalgo et al., 1995). However, the data describing natural and injury-induced cytogenesis in response to endogenous estrogens has been inconsistent, and even contradictory. For example, OVX or systemic aromatase-inhibition decrease neural cytogenesis in zebra finches (Taeniopygia guttata), (Lee et al., 2007)(Peterson et al., 2007), but in the canary (Serinus canarius) OVX actually increases cytogenesis in the nidopallium (Hidalgo et al., 1995). Additionally, in zebra finches, peripheral E2-treatment can rescue the effect of fadrozole or OVX on cytogenesis around a penetrating brain injury (Peterson et al., 2007), but it appears insufficient to rescue the effect of OVX on injury-induced cytogenesis when the SVZ is analyzed as well (Lee et al., 2007). These varied results suggest that the role of estrogens on cytogenesis (and neurogenesis) in songbirds may be complex and influenced by region within the brain, by the method used to manipulate hormone levels, and/or the source of the endogenous steroid.
While some studies suggest that exogenous E2, delivered peripherally, can enhance injury-induced neural cell proliferation and/or survival (Lee et al., 2007; Peterson et al., 2007; Suzuki et al., 2007), the role of acute, locally provided estrogens on injury induced neurogenesis has yet to be investigated. Hemorrhagic, ischemic, and cytotoxic brain injuries result in an upregulation of aromatase and presumably local estrogen synthesis in both birds and mammals (Saldanha et al., 2009). In the zebra finch, a songbird model for traumatic brain injury, it is readily apparent that neural aromatization is critical for neuroprotection. In response to traumatic insult, reactive astrocytes dramatically upregulate aromatase (Peterson et al., 2001; Wynne and Saldanha, 2004; Saldanha et al., 2005), the inhibition of which increases both lesion volume (Wynne and Saldanha, 2004) and the extent of apoptotic cell death in the surrounding neuropil (Saldanha et al., 2005). Conversely, the addition of exogenous E2 to the injury site restores neuroprotective processes, decreasing the number of apoptotic cells and the extent of degeneration (Saldanha et al., 2005; Wynne et al., 2008). However, whether or not this influence extends to neurogenesis or cytogenesis is unknown.
In the current report, we tested whether localized inhibition of aromatase or site specific replacement with E2 could influence telencephalic neurogenesis in the injured zebra finch brain. Local inhibition of endogenous aromatase did not have a significant effect on neurogenesis or cytogenesis when compared to vehicle injected controls. However, addition of E2 to local injections of the aromatase inhibitor fadrozole enhanced neurogenesis and cytogenesis when compared to hemispheres treated with fadrozole only. These data suggest that it may be beneficial to further examine the effects of local E2 treatment on brain injury in songbirds and in mammals, including humans, where such an effect might offer the potential to enhance regeneration or repair.
METHODS
Animals, Injury, and Drug Delivery
Adult male zebra finches (> 90 days post hatching) were obtained from Canary Bird Farm (Old Bridge, NJ) and were housed in single sex cages according to IACUC guidelines at Lehigh University. Birds were provided with food and water ad libitum and maintained under a 14:10 light:dark cycle.
Prior to injury, each bird was anesthetized with 0.03cc Nembutal (pentobarbital sodium salt from Sigma-Aldrich, St. Louis, MO, 25mg/mL in a 20% propylene glycol and 5% ethanol solution). Injuries were made as previously described (Wynne and Saldanha, 2004; Saldanha et al., 2005; Walters and Saldanha, 2008; Wynne et al., 2008). Briefly, animals were placed into a stereotaxic apparatus and given bilateral penetrating injuries using a 50μL-22S Hamilton syringe (Hamilton Co., Reno, NV) that also served as the drug delivery needle. With the heads angled at 45°, injuries were targeted to the entopallial nuclei (2mm anterior to the pineal, 3mm lateral to the midline, 3mm ventral to the dural surface) because they lack detectable aromatase expression in the absence of injury (Shen et al., 1995; Saldanha et al., 2000; Wynne et al., 2008). For experiment 1, (N=8) birds received bilateral injuries, where one telencephalic lobe was injected with 50μg of the aromatase inhibitor fadrozole (FAD, 10μg/μL)(Wade et al., 1994) and the contralateral hemisphere received 5μL of steroid suspension vehicle only (SSV; 9 mg NaCl, 5 mg sodium carboxymethylcellulose, 4 μL polysorbate-80, 9 μL benzyl alcohol in 1 ml distilled water). This dose of FAD has been used previously in finches, in an identical paradigm, and has been shown to affect gene expression, aromatase protein expression, and measures of gliosis, degeneration, and apoptotic cell death after injury when compared to contralateral hemispheres treated with vehicle only or hemispheres treated with fadrozole plus estradiol (Wynne and Saldanha, 2004; Saldanha et al., 2005; Walters and Saldanha, 2008; Wynne et al., 2008). For experiment 2, (N=8) animals received an injection of 50μg of FAD (5μL at 10μg/μL) into one hemisphere and 50μg of FAD plus 1μg of 17β-estradiol (E2) contralaterally (200ng/μL E2 and 10μg/μL FAD, in SSV, total volume 5μL). These doses of FAD and of FAD+E2 have also been used successfully to elicit localized, hemisphere specific effects on gliosis, degeneration, and apoptosis in previously published experiments (Saldanha et al., 2005; Wynne et al., 2008). All injuries were performed at a similar time of day (between 1300 and 1600 hrs.), and all injections were balanced to avoid laterality.
BrdU injections, Tissue Preparation, and Immunocytochemistry
Following injury, animals were given 3 single injections of 5-Bromo-2′-deoxyuridine (BrdU, Calbiochem, San Diego, CA). Injections were delivered intraperitoneally (i.p.) 24, 48, and 72 hrs. after injury, with each injection delivering 0.05 mg of BrdU per gram of body weight (see figure 1). Following the BrdU injections, birds were allowed to survive for eleven more days (2 weeks after injury, see figure 1) at which point they were perfused with 0.2% picric acid in 4% paraformaldehyde (in 0.1M PBS). Their brains were dissected out, post-fixed in 4% paraformaldehyde, embedded in gelatin (8%) and immersed in 30% sucrose before being sectioned on a cryostat (50μm coronal sections). Sections were then stored in antifreeze solution (Watson et al., 1986) at −20°C until use for immunohistochemistry (IHC). For each subject, 14 coronal sections were processed for IHC: one 50μm section taken every 250μm moving caudally from a point 4mm anterior to the pineal. Sections were washed, free floating, 6 × 15 min. in 0.1M PB, then immersed in 1N HCl for 30 min., washed 3 × 15 min. in 0.1% PBT (0.1M PB with 0.1% wbv triton X-100), immersed in 0.3% H2O2 for 10 min., washed 3 × 15 min. in 0.1% PBT, and then placed into 10% normal goat serum (Jackson Immunoresearch Laboratories Inc., West Grove, PA) for 60 minutes before being exposed to an antibody against BrdU (1:1000 in 0.3% PBT, mouse anti-BrdU clone Bu20a, Dako Corp., Carpinteria, CA) for 48 hrs. at 4°C. Sections were then washed 3 × 15 min. in 0.1% PBT, placed into a biotinylated goat anti-mouse secondary (pre-adsorbed for multiple labeling, Jackson Immunoresearch, West Grove, PA) for 60 minutes at room temp., then washed 3 × 15 min. in 0.1% PBT, placed in Vectastain ABC solution (Vector Labs, Burlingame, CA) for 60 minutes at room temp., then washed 3 × 15 min. in 0.1% PBT, and stained using DAB (diaminobenzidine tetrahydrochloride hydrate, Amresco, Solon, OH, 0.05% DAB, 0.003% H2O2, in 0.1M PBS). Sections were washed 6 × 15 min. in 0.1% PBT and then incubated in M.O.M. mouse IgG blocking reagent overnight at room temp. (diluted in PBS as recommended by the manufacturer: Vector Labs). Sections were then washed 3 × 15 min. in 0.1% PBT, and placed into 0.3% PBT containing mouse anti-HuC/HuD (2.5mg/mL, clone 16A11, Invitrogen -Molecular Probes, Eugene, OR ) for 48 hours at 4°C. This anti “Hu” antibody has been previously reported to label the cytoplasm of neurons and committed neuronal precursors in the songbird brain (Barami et al., 1995). After this incubation, the sections were washed 3 × 15 min. in 0.1% PBT, spent 60 min. in pre-adsorbed, biotinylated goat anti-mouse secondary (1:200 in 0.3% PBT, Jackson Immunoresearch Laboratories), were then washed again (3 × 15 min. 0.1% PBT), immersed in Vectastain ABC solution for 60 min. (Vector Labs), and then washed again (3 × 15 min. 0.1% PBT) before being exposed to Vector SG peroxidase substrate (Vector Labs). Sections were then washed 3 × 5 min. in 0.1% PBT before being mounted to gelatin subbed slides, dehydrated in ethanol, and coverslipped with Eukitt (Electron Microscopy Sciences, Hatfield, PA). The timepoints for BrdU administration were chosen based on previous data that suggested injury induced aromatase expression is maximal between 24 and 72 hours after injury (Wynne et al., 2008). Additionally, preliminary experiments in the injured zebra finch brain suggested that, using the current BrdU injection paradigm, the numbers of BrdU positive cells were greater at 2 weeks than at 72 hours or 1 week after injury, but not different from numbers obtained at 3 and 4 week timepoints (unpublished data). Additionally, aromatase immunoreactivity, as an indirect measure for fadrozole activity, suggests that the effects of identical acute FAD injections persist for at least two weeks, but dissipate at some point between 2 and 6 weeks (Wynne et al., 2008).
Figure 1.
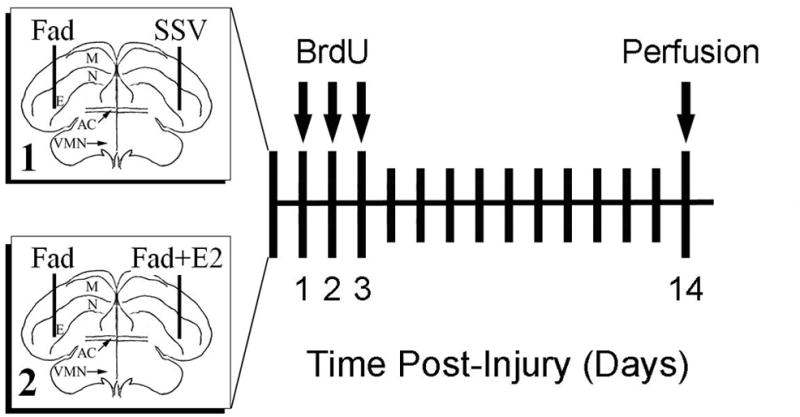
Experimental design. 1A. Two experiments were conducted to investigate the role of local, injury-induced aromatization (experiment 1) and intracerebral estradiol provision (experiment 2) on cytogenesis and neurogenesis in the adult zebra finch brain. Boxes at left are schematic representations of coronal sections of a zebra finch brain with vertical lines indicating the targeting of injury and drug delivery needles. Box 1 represents experiment 1 where the aromatase inhibitor Fadrozole (FAD) was injected unilaterally, and counterbalanced with injection of the steroid suspension vehicle (SSV) in the contralateral hemisphere. Box 2 represents experiment 2 where a similar paradigm pitted FAD injection against contralateral injections of steroid suspension vehicle containing fadrozole plus estradiol (FAD+E2). For both experiments, single injections of BrdU were given at 24, 48, and 72 hrs. after injury, and the animals were allowed to survive until 14 days after being injured, at which point they were perfused and tissue was prepared for immunocytochemistry.
Data Collection and Statistical Analyses
Slides with BrdU/Hu double labeled sections were coded, and photomicrographs were taken at 200X magnification by an experimenter blind to the treatment conditions. Images were collected bilaterally from the ventricular zone and grouped into dorsal subventricular zone (DSVZ) and ventral subventricular zone (VSVZ) by dividing the lateral ventricle at its dorsoventral midline (see figure 2). Images were also collected from the injury site at 200X. All of the images were taken so that the ventricle or the needle track was positioned in the mediolateral center of the field of view. These areas were chosen for investigation due to their cytogenic or neurogenic nature (Goldman and Nottebohm, 1983; Alvarez-Buylla and Kirn, 1997; Dewulf and Bottjer, 2005; Lee et al., 2007), and the SVZ was divided into dorsal and ventral portions because a previous report demonstrated differences in BrdU staining between these two portions (Lee et al., 2007). Photomicrographs were subsequently coded again and the total number of BrdU positive nuclei and the total number of double labeled cells were counted within each image by a separate experimenter who was blind to the experimental conditions as well as the brain areas represented within images. After these data were collected, the images were decoded and the final measures obtained were:the average number of BrdU positive nuclei per (0.038 mm2) image (cytogenesis) and the average number BrdU/Hu double-labeled cells per (0.038 mm2) image (neurogenesis). An additional measure for proportional neurogenesis (neuronal index) was derived from these measures by dividing the number of double labeled cells into the number of BrdU positive cells and similarly averaging these across images. In order to better control for unequal variance, the data for the BrdU and BrdU/Hu measures were log transformed before being analyzed. For experiment 1, a repeated measures ANOVA was conducted to compare the log of the mean number of BrdU cells per image across FAD treated and SSV treated hemiepheres (between subjects) as well as across the 3 brain regions sampled (DSVZ, VSVZ, and INJ, within-subjects). Two similar ANOVAs were conducted to compare between FAD and SSV hemispheres and across brain regions sampled for the log transformed BrdU/Hu means, and for the neuronal index measures. For experiment 2, a similar repeated measures ANOVA was conducted to compare the log transformed BrdU data across brain regions and between FAD and FAD+E2 treated hemispheres, and two similar ANOVAs were conducted for the neuronal index, and the log transformed BrdU/Hu data. If initial Mauchly’s tests were significant, Greenhouse-Geisser corrections to the degrees of freedom were applied, and for significant main effects of brain region (p ≤ 0.05), post-hoc Fisher’s LSD tests were used to make pairwise comparisons. All statistical tests were conducted using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL).
Figure 2.
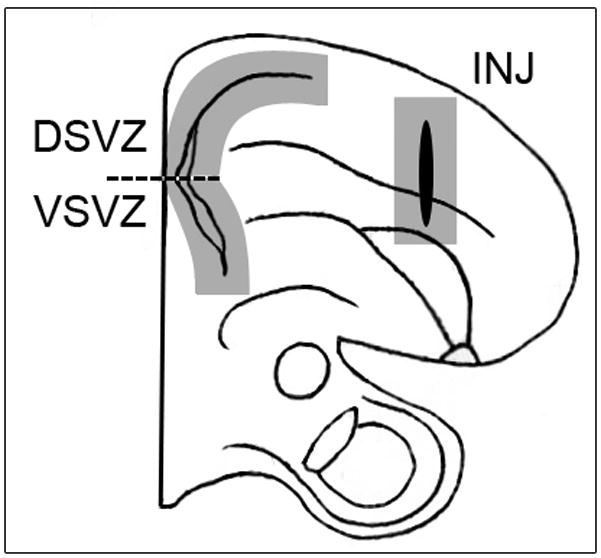
Schematic representation of one half of a coronal section (approx. 3mm anterior to the pineal) demonstrating the three cytogenic regions of the injured zebra finch brain. Shaded boxes demonstrate the approximate areas sampled for BrdU and Hu immunopositive cells and the dashed line represents the division point between the dorsal (DSVZ) and ventral (VSVZ) subventricular zones.
RESULTS
Experiment 1: Effect of local aromatase inhibition on cytogenesis, neurogenesis, and neuronal morphogenesis
Cytogenesis
Injury-induced cytogenesis was measured as the average number of BrdU positive cells per (0.038mm2) image. Measures were taken from 3 brain regions defined as the dorsal subventricular zone (DSVZ), ventral subventricular zone (VSVZ), and the site of injury (INJ) through the nido-, meso-, and ento-pallia (see Fig. 2). Comparison of the log transformed means for this measure of cytogenesis revealed no main effect of treatment (F(1, 14) = 0.440, p = 0.518), suggesting that the mean number of BrdU positive nuclei per image did not differ between the FAD treated (38.78 ± 5.61) and (SSV) vehicle-treated (31.77 ± 5.61) hemispheres. There was, however, a significant effect of brain region on cytogenesis (F(2, 28) = 74.67, p < 0.0001), where a post-hoc Fisher’s LSD test revealed significant differences between each area of investigation (see Figure 4A). Collapsing across FAD and SSV hemispheres, the highest level of cytogenesis was seen at the injury site (INJ, 77.09 ± 11.2 BrdU+ cells/image), followed by the DSVZ (16.5 ± 1.42 BrdU+ cells/image), with the VSVZ exhibiting the lowest level of cytogenesis in these birds (12.24 ± 1.3 BrdU+ cells/image). There was no interaction between treatment and brain region (F(2, 28) = 1.545, p = 0.231).
Figure 4.
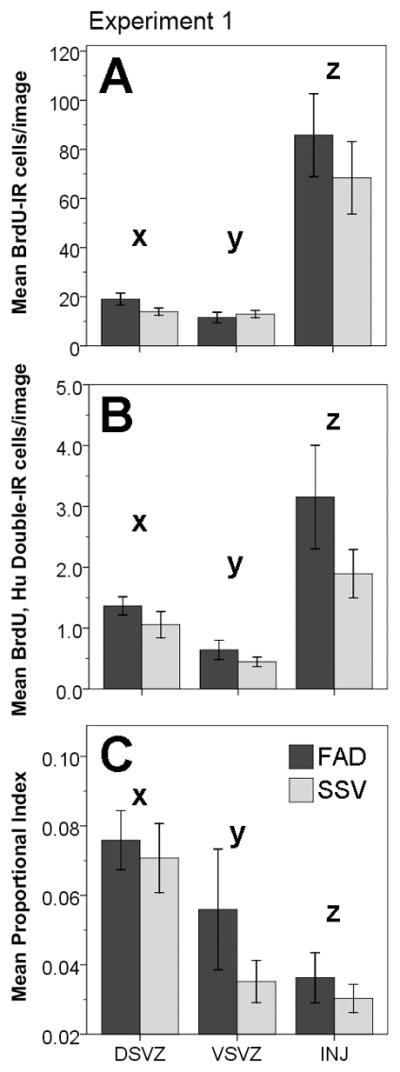
Experiment 1, cytogenesis, neurogenesis, and proportional neurogenesis in response to fadrozole and vehicle injections. For all figures, dark gray bars represent the Fadrozole (FAD) treated condition, and light gray bars represent the vehicle (SSV) treated condition.(A) Means (±1 S.E) of BrdU immunoreactive (IR) cells per (0.038 mm2) image across brain regions and treatments. FAD treatment did not have a significant main effect on cytogenesis when compared to SSV treated hemispheres (p = 0.518, repeated measures ANOVA of log transformed data). There was a significant effect of brain region (p < 0.0001), but no interaction between treatment and region (p = 0.231). Fisher’s LSD revealed significant differences for each of the pairwise comparisons of brain regions: INJ > DSVZ (z ≠ x, p < 0.0001), INJ > VSVZ (z ≠ y, p < 0.0001), DSVZ > VSVZ (x ≠ y, p = 0.041). (B) Means (±1 S.E) of the numbers of BrdU and Hu double labeled (IR) cells counted per image represented across brain region and treatment. FAD had no effect on neurogenesis when compared to SSV treated hemispheres (p = 0.21, repeated measures ANOVA on log transformed data). There was a significant effect of brain region on neurogenesis (p < 0.0001), but no interaction between treatment and brain region (p = 0.96). Fisher’s LSD revealed significant differences for each of the pairwise comparisons between brain regions: INJ > DSVZ (z ≠ x, p = 0.018), INJ > VSVZ (z ≠ y, p < 0.0001), DSVZ > VSVZ (x ≠ y, p = 0.003). (C) Means (±1 S.E) of the proportional neuronal index (double labeled cells/Brdu positive cells) across brain regions and treatments. FAD treatment did not have a significant main effect on the neuronal index when compared to SSV treated hemispheres (p = 0.306, repeated measures ANOVA). There was a significant effect of brain region (p < 0.0001), but no interaction between treatment and region (p = 0.253). Fisher’s LSD revealed significant differences for each of the pairwise comparisons of brain regions: DSVZ > VSVZ (x ≠ y, p = 0.005), DSVZ > INJ (x ≠ z, p < 0.0001), VSVZ > INJ (y ≠ z, p = 0.038).
Neurogenesis
Neurogenesis was measured as the mean number of BrdU and Hu double positive cells per (0.038mm2) image. Analysis of the log transformed data again revealed no main effect of FAD treatment as compared to SSV injected hemispheres (F(1, 14)= 1.724, p = 0.21). Collapsing across brain regions, the FAD treated hemispheres exhibited a mean value of 1.72 ± 0.262 double-labeled cells per image, and the SSV injected hemispheres exhibited a mean value of 1.13 ± 0.262 double-labeled cells per image. Collapsing across treatment, there was a significant main effect of brain region on neurogenesis (F(2, 28)= 22.787, p <0.0001), where a post-hoc Fisher’s LSD revealed significant differences between each of the brain areas (see Figure 4B). The injury site had the highest level of neurogenesis (2.52 ± 0.47 double-labeled cells/image), followed by the DSVZ (1.21 ± 0.13 double-labeled cells/image), with the VSVZ having the lowest mean number of BrdU/Hu double-labeled cells per image (0.55 ± 0.086). Again, there was no significant interaction between treatment and brain region (F(2, 28)= 0.041, p =0.96).
Neuronal Index
As a measure for proportional neurogenesis, the number of Brdu/Hu double labeled cells/image was divided into the number of BrdU positive cells/image and averaged across images for each hemisphere in each subject. Comparison of this measure between FAD and SSV treated hemispheres revealed no significant main effect of treatment (F(1, 14)= 1.128, p = 0.306). FAD treated hemispheres had a mean neuronal index of 5.7 ± 0.8%, and SSV treated hemispheres had a mean neuronal index of 4.5 ± 0.8%. Collapsing across treatment, there was a significant main effect of brain region on neuronal index (F(2, 28)= 20.26, p < 0.0001). A post-hoc Fisher’s LSD test revealed significant differences between all of the brain regions,(see Figure 4C) with the DSVZ having the highest proportional neurogenesis (7.3 ± 0.7%), followed by the VSVZ (4.7 ± 0.9%), with the injury site demonstrating the lowest level of proportional neurogenesis (3.3 ± 0.4%). There was no significant interaction between treatment and brain region (F(2, 28)= 1.444, p = 0.253).
Experiment 2: Effect of local E2 replacement on cytogenesis, neurogenesis, and neuronal morphogenesis
Cytogenesis
To test the effect of local E2 provision on cytogenesis, the mean numbers of BrdU positive nuclei were sampled from images in the DSVZ, VSVZ, and injury site, and the log transformed means were compared across these regions and in response to treatment with either FAD or FAD+E2. A repeated measures ANOVA revealed a significant effect of treatment on cytogenesis (F(1, 14)= 10.464, p = 0.006), with the FAD+E2 treated hemispheres exhibiting higher levels of cytogenesis (51.94 ± 6.028 BrdU+ cells/image) than the hemispheres treated with FAD only (39.01 ± 6.028 BrdU+ cells/image). Also, when collapsed across treatment, there was a significant main effect of brain region on cytogenesis (F(2, 28)= 80.211, p < 0.0001), and a post-hoc Fisher’s LSD comparison revealed significant differences between each of the areas with the injury site having the highest levels of cytogenesis (106.01 ± 13.24 BrdU+ cells/image), followed by the DSVZ (18.67 ± 2.33 BrdU+ cells/image), and then the VSVZ (11.74 ± 2.26 BrdU+ cells/image) which had the lowest levels of cytogenesis (see Figure 5A). There was no significant interaction between treatment and brain region (F(2, 28)= 1.598, p = 0.22).
Figure 5.
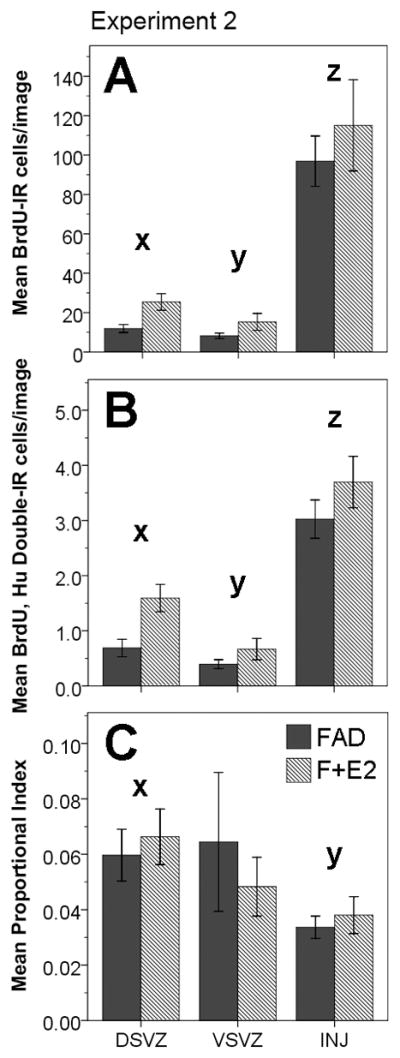
Experiment 2: cytogenesis, neurogenesis, and proportional neurogenesis in response to contralateral injections of Fadrozole (FAD) and fadrozole plus estradiol (F+E2). For all figures, dark gray bars represent the FAD treated condition, and striped bars represent the F+E2 treated condition. (A) Means (±1 S.E) of BrdU immunoreactive (IR) cells per image across brain regions and treatments. F+E2 treatment significantly increased overall cytogenesis as compared to hemispheres treated with FAD only (p = 0.006, repeated measures ANOVA of log transformed data). There was also a significant effect of brain region on cytogenesis (p < 0.0001), but no interaction between treatment and region (p = 0.22). Fisher’s LSD revealed significant differences for each of the pairwise comparisons between brain regions: INJ > DSVZ (z ≠ x, p < 0.0001), INJ > VSVZ (z ≠ y, p < 0.0001), DSVZ > VSVZ (x ≠ y, p = 0.008). (B) Means (±1 S.E) of the numbers of BrdU and Hu double labeled (IR) cells counted per image, represented across brain region and treatment. F+E2 significantly increased overall neurogenesis as compared to hemispheres treated with FAD only (p = 0.007, repeated measures ANOVA on log transformed data). Also, there was a significant effect of brain region on neurogenesis (p < 0.0001), but no interaction between treatment and brain region (p = 0.16). Fisher’s LSD revealed significant differences for each of the pairwise comparisons between brain regions: INJ > DSVZ (z ≠ x, p < 0.0001), INJ > VSVZ (z ≠ y, p < 0.0001), DSVZ > VSVZ (x ≠ y, p = 0.001). (C) Means (±1 S.E) for the proportional neuronal index (double labeled cells/Brdu positive cells) across brain regions and treatments. F+E2 treatment did not have a significant main effect on the neuronal index when compared to FAD treated hemispheres (p = 0.907, repeated measures ANOVA). There was a significant effect of brain region (p =0.04), but no interaction between treatment and region (p = 0.393). Fisher’s LSD revealed a significant difference between the proportional index for the DSVZ and that for the INJ site, but no other significant effects: DSVZ > INJ (x ≠ y, p = 0.0001).
Neurogenesis
Neurogenesis was measured by counting the number of BrdU and Hu double labeled cells per image in the 3 cytogenic regions of the injured songbird brain (DSVZ, VSVZ, and INJ). A repeated measures ANOVA of the log transformed means from these measures revealed a significant effect of treatment (F(1, 14) =10.16, p = 0.007), suggesting that the FAD+E2 treated hemispheres had higher levels of neurogenesis (1.99 ± 0.157 BrdU, Hu double labeled cells/image) than the hemispheres treated with FAD only (1.37 ± 0.157 BrdU, Hu double labeled cells/image). Collapsing across treatment, the ANOVA also revealed a significant main effect of brain region (F(2, 28)= 61.301, p < 0.0001, see Figure 5B), with the injury site exhibiting the highest level of neurogenesis (3.36 ± 0.291 double-labeled cells/image), followed by the DSVZ (1.14 ± 0.148 double labeled cells/image), and the VSVZ (0.531 ± 0.105 double labeled cells/image). A post-hoc Fisher’s LSD test revealed that the values in these regions were significantly different from one another (p ≤ 0.001). There was no significant interaction between treatment and brain region on neurogenesis (F(2, 28)= 1.962, p = 0.16).
Neuronal index
To determine whether or not local E2 provision affects aspects of neuro-or glio-genesis that influence cell fate (i.e. differentiation or discriminatory survival), we also measured the number of new neurons as a proportion of total newborn cells in each brain area and treatment group. A repeated measures ANOVA of this neuronal index (number of double labeled cells/number of BrdU positive cells) revealed no main effect of treatment (F(1, 14)= 0.014, p = 0.907), with similar mean values between FAD treated (5.3 ± 1%) and FAD+E2 treated (5.1 ± 1%) hemispheres. There was, however, a significant difference in the proportion of new cells that became neurons across the various cytogenic brain regions (F(1.32, 18.5) = 4.427, p =0.04), with the highest proportional index seen in the DSVZ (6.3 ± 0.7%), followed by the VSVZ (5.6 ± 1.4%), with the injury site demonstrating the lowest proportional index for neurogenesis (3.6 ± 0.4%). A post-hoc Fisher’s LSD test revealed that only the values in the DSVZ differed from those in INJ (p = 0.0001), while comparisons between VSVZ and INJ, and VSVZ and DSVZ revealed no significant differences (p > 0.05, see Figure 5C). There was no significant interaction between treatment and brain area for the proportional neuronal index (F(1.32, 18.5) = 0.869, p = 0.393). For this analysis of the neuronal index, a Greenhouse-Geisser correction was applied to the degrees of freedom for the within-subjects comparisons to compensate for a violation of the sphericity assumption as revealed by Mauchly’s W (W = 0.486, p = 0.009).
DISCUSSION
Songbirds are powerful models for neural plasticity. They provided the first conclusive evidence for adult neurogenesis in the homeotherm brain (Goldman and Nottebohm, 1983; Paton and Nottebohm, 1984), and the striking neuroanatomical plasticity they exhibit prompted some of the first investigations into the roles of gonadal steroids in synaptic plasticity (Bleisch et al., 1984; Devoogd et al., 1985), neurite arborization (DeVoogd and Nottebohm, 1981), neurogenesis (Rasika et al., 1994; Hidalgo et al., 1995), and neuronal survival (Arnold, 1980; Bottjer and Johnson, 1992). As an extension of this natural plasticity, the songbird brain appears to be markedly resilient in response to injury. Indeed, subsequent to lesion, the zebra finch brain demonstrates increased levels of neural cell proliferation (Lee et al., 2007) and shows a dramatic upregulation of aromatase expression that mitigates secondary degeneration (Wynne et al., 2008). Additionally, selective lesion of HVC to RA projecting neurons in zebra finches further reveals the regenerative capacity of the song circuit, where newly derived cells can repopulate HVC, renew projections to RA, and lead to the recovery of behavioral function (Scharff et al., 2000). While the songbird brain is remarkably plastic, and therefore well suited to the study of neural injury and neural repair, little else is known about subventricular neurogenesis in response to insult, or, in particular, to injury-induced aromatization.
The current study sought to extend our knowledge of this system by investigating whether or not injury-induced glial aromatization, and/or local provision of E2, affect cytogenesis or neurogenesis in the adult zebra finch brain. Subjects in the first experiment received bi-lateral penetrating injuries to the cerebrum that were treated with the aromatase inhibitor fadrozole in one hemisphere, and suspension vehicle in the contralateral hemisphere. At a dose previously shown to affect local processes of neuroprotection and gliosis (Wynne et al., 2008), FAD treatment did not elicit any significant effects on cytogenesis or neurogenesis when compared to the vehicle injected controls. In the second experiment, 17β-estradiol was added to FAD and injected into one hemisphere, while the contralateral hemispheres were treated with FAD only. The addition of E2 to FAD had the effect of increasing cytogenesis and neurogenesis as compared to hemispheres treated with FAD only. In both experiments, and regardless of treatment, the injury sites demonstrated the highest levels of cytogenesis and neurogenesis, while the DSVZ had the highest proportion of new cells that differentiated into neurons. Thus, sites of endogenous neurogenesis in the songbird SVZ (Goldman and Nottebohm, 1983; Alvarez-Buylla and Kirn, 1997; Dewulf and Bottjer, 2005) remain proliferative in response to injury, and injuries distal to the SVZ may foster the recruitment of new cells to, and/or the proliferation of cells around the site of damage. While injury induced aromatization has been shown to promote neural cell survival (Wynne and Saldanha, 2004; Saldanha et al., 2005; Wynne et al., 2008), this effect may not be sufficient to enhance measures of neurogenesis at 2 weeks post-injury. However, an acute injection of E2 to the injury site, even in the presence of an aromatase inhibitor, increases both cytogenesis and neurogenesis in the adult zebra finch brain.
Previous studies demonstrate that reducing the levels of circulating estrogens via OVX or by systemic aromatase inhibition, can decrease neural cell proliferation (Lee et al., 2007; Peterson et al., 2007; Suzuki et al., 2007). Based on this precedent, we hypothesized that inhibition of injury-induced, glial aromatization in the zebra finch brain would result in less neuro-and cyto-genesis when compared to vehicle treated controls. Interestingly, the inhibition of injury-induced aromatization by Fadrozole did not result in a detectable change in either cytogenesis or neurogenesis. As the dose of Fadrozole that was used has been previously shown to elicit dramatic, local effects on degeneration, apoptosis, and gene expression in the zebra finch brain (Saldanha et al., 2005; Walters and Saldanha, 2008; Wynne et al., 2008), it would seem that this lack of effect is not driven by an inability of Fadrozole to inhibit aromatase activity. Rather, given that local, and not peripheral, aromatization was targeted for disruption, it is likely that the unaffected peripheral sources of estrogens were sufficient to support high levels of injury-induced cyto-and neuro-genesis. Other possible causes for a lack of effect of FAD treatment on cyto-or neuro-genesis include possible variability in injury size at the time of BrdU injections (Wynne et al., 2008), the timing and extent of BrdU injections, and the survival time of the animals. Indeed, previous work in zebra finches has demonstrated effects of systemic aromatase inhibition on neural cytogenesis within days of hippocampal injury (Lee et al., 2007), suggesting that either global inhibition of estrogen synthesis, injuries more proximal to the neurogenic niche, or earlier timepoints after injury, may be necessary to reveal an effect of aromatase inhibition on neural cytogenesis. To address these issues, our lab is currently investigating whether or not neurogenic properties of local, glial aromatization may be revealed in animals that have been deprived of normal levels of circulating steroids, or at timepoints other than two weeks after injury.
While FAD administration failed to reveal an effect on cytogenesis or neurogenesis, the data do suggest that the acute administration of E2 with FAD into the injured zebra finch brain can increase both cytogenesis and neurogenesis, when compared to treatment with FAD alone. This is consistent with previous reports demonstrating the neuro-and cyto-genic effects of E2 treatment in the songbird brain (Hidalgo et al., 1995; Lee et al., 2007), however, it is interesting that aromatase inhibition had no effect on these measures, but that acute E2 injection should increase both significantly. The authors propose that the discrepancy is likely a result of the dose of E2 used in the current study which may represent a supraphysiological amount. While the local concentration of E2 that is present in the neuropil resultant to injury and aromatase upregulation is currently unknown, measures of 17β-estradiol in the circulation range from approximately 20–50 pg/mL plasma (Adkins-Regan et al., 1990; Charlier et al., 2010) and measures from individual brain areas in uninjured zebra finches are as high as 250 pg/g of tissue (Charlier et al., 2010), suggesting that an injection of 200ng/μL (for a total of 1μg) of E2 may be represent a much higher concentration than the endogenous levels of this steroid. Thus, the results presented here indicate that a likely supraphysiological dose of E2, administered acutely and intracerebrally, can enhance cyto-and neuro-genesis in gonadally intact finches. Future studies in the lab will aim to determine the quantity of endogenous E2 in the injured neuropil, and whether or not E2 dependent effects on injury-induced neurogenesis are dose dependent or can be elicited at physiologically relevant concentrations.
To determine whether or not FAD or FAD+E2 treatments affect neuronal differentiation or morphogenesis in a preferential manner, the ratio of BrdU positive neurons over total BrdU positive cells was computed as a measure of proportional neurogenesis. Comparison of this neuronal index across FAD and vehicle treated hemispheres revealed no significant differences, suggesting that the inhibition of local, injury-induced aromatization does not affect the proportion of newly derived cells that adopt a neuronal fate. Similarly, treatment of injured hemispheres with FAD+E2 did not have an effect on the neuronal index when compared to hemispheres treated with FAD only, suggesting that the acute provision of E2 to the site of injury does not affect the differentiation or survival of newly divided neurons in a preferential fashion. These data are consistent with research in mammals where E2 treatment affects glial/neuronal differentiation of neural precursor cells during development, but not in adulthood (Brannvall et al., 2002). However, due to the limited scope of the current research, further study will be needed to see if estrogens affect neuronal/glial differentiation in adult vertebrates, or in response to injury.
Reports in both birds and mammals suggest that the dorsal portion of the SVZ is highly prolific and neurogenic, both constitutively and in response to brain injury (Lee et al., 2007; Peterson et al., 2007; Suzuki et al., 2007). Additionally, newly derived cells are found around sites of brain damage, even when these sites are distal to the neurogenic SVZ (Zuo, 1998; Magavi et al., 2000). Correspondingly, in the injured adult zebra finch brain, newly proliferated cells, including neurons, were observed in the subventricular zone and at the cerebral injury sites located several millimeters away from the ventricular zone. Additionally, the injury sites demonstrated higher levels of cytogenesis and neurogenesis than the SVZ, though the DSVZ generated the greatest proportion of new neurons out of the three regions examined. Treatments with either the aromatase inhibitor FAD, or with FAD+E2 did not have any significant effects on the patterns of cytogenesis and neurogenesis in these brain areas, as they did not reveal any significant interactions between treatment and brain area for any of the three measures (cytogenesis, neurogenesis, or proportional neuronal index). Thus, neurogenesis after injury in the adult zebra finch brain conforms to patterns previously established in vertebrates, and neither aromatase inhibition, nor E2 provision, appear to alter proliferation, migration, or survival of newly derived cells in a manner that would result in different site-specific patterns of cyto-or neuro-genesis in the first 2 weeks following injury. Additionally, the fact that the INJ sites had significantly lower neuronal indices than the DSVZ in both of the experimental groups, despite that fact that they displayed higher levels of cyto-and neuro-genesis, suggests that the DSVZ remains the neurogenic niche in the injured zebra finch brain; a finding that is consistent with the established role for the DSVZ as the prominent neurogenic niche in the vertebrate brain (Taupin, 2006) and the relative inability of brain areas distal to the SVZ to foster neuronal morphogenesis (Lim et al., 2000). Alternatively, the pattern of greater numbers of BrdU positive cells around INJ, but only a low proportion of them being neuronal, may be an artifact of a particularly robust glial proliferation that is occurring at the injury site as part of the process of gliosis. While studies in rodents, suggest that brain lesions do result in increased migration of SVZ derived neuroblasts to the site of injury (Goings et al., 2004; Sundholm-Peters et al., 2005), the majority of proliferating cells at the injury site tend to be micro-and astro-glial (Norton, 1999; Ladeby et al., 2005). Additionally, there is evidence to suggest that disruption of the blood brain barrier by traumatic injury allows mitotic, hematopoeitic cells to enter the neuropil (Ladeby et al., 2005). Identification of either astroglial or macrophage cell phenotypes for BrdU positive cells surrounding injury would better inform hypotheses as to the nature of injury-induced cytogenesis in the songbird brain, and help to determine whether or not this process is similar to that seen in mammals. Our lab is currently investigating the co-immunolocalization of BrdU and proteins specific to reactive astrocytes and cells of hematopoietic origin.
Taken together, the results presented here suggest that the acute, intracerebral administration of estradiol, but not local inhibition of aromatase, can significantly affect cytogenesis and neurogenesis in the injured zebra finch brain. Increased cyto-and neuro-genesis in response to exogenous E2 is consistent with numerous previous findings in homeotherms that suggest E2 can enhance the number of newly derived cells, including neurons, in injured and uninjured brains. Open questions remain as to the role that the location and extent of injury play in neurogenesis, as well as the mechanisms by which the cyto-and neuro-genic effects of E2 are mediated in the songbird system. Future studies will seek to determine the effects of acute E2 provision on gliosis and gliogenesis as well as whether or not the effects of E2 on cyto-and neuro-genesis are dose dependent or replicable in the absence of peripheral sources of estrogens.
Figure 3.
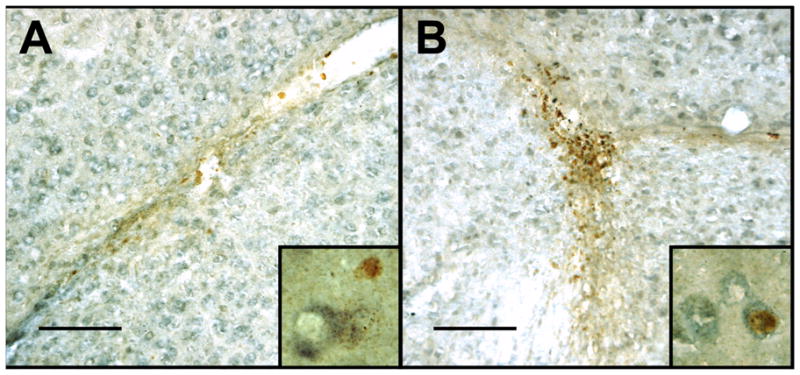
Newly Proliferated Cells in the SVZ and INJ. Photomicrographs of BrdU labeled nuclei (reddish brown) and Hu labeled cells (blue/grey) in the dorsal subventricular zone (A) and at the injury site (B). The scale bars represent 100 microns. High power photomicrographs of Hu and BrdU labeled cells are shown in the inset images. The width of each inset represents 30 microns.
Acknowledgments
This work was supported by grants from the Great Rivers Affiliate of the American Heart Association (Predoctoral Fellowship 09PRE2010168) and the National Institutes of Health (NINDS: NIH 042767).
References
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A. Neurogenesis and plasticity in the CNS of adult birds. Exp Neurol. 1992;115:110–114. doi: 10.1016/0014-4886(92)90232-f. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Arnold AP. Effects of androgens on volumes of sexually dimorphic brain regions in the zebra finch. Brain Res. 1980;185:441–444. doi: 10.1016/0006-8993(80)91083-5. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- Bleisch W, Luine VN, Nottebohm F. Modification of synapses in androgen-sensitive muscle. I. Hormonal regulation of acetylcholine receptor number in the songbird syrinx. J Neurosci. 1984;4:786–792. doi: 10.1523/JNEUROSCI.04-03-00786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Matters of life and death in the songbird forebrain. J Neurobiol. 1992;23:1172–1191. doi: 10.1002/neu.480230909. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brown CM, Suzuki S, Jelks KA, Wise PM. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med. 2009;27:240–249. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, Soma KK. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010;167:18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Devoogd TJ, Nixdorf B, Nottebohm F. Synaptogenesis and changes in synaptic morphology related to acquisition of a new behavior. Brain Res. 1985;329:304–308. doi: 10.1016/0006-8993(85)90539-6. [DOI] [PubMed] [Google Scholar]
- Dewulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone: a map of proliferative activity. J Comp Neurol. 2005;481:70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- Gahr M. Hormone-dependent neural plasticity in the juvenile and adult song system: what makes a successful male? Ann N Y Acad Sci. 2004;1016:684–703. doi: 10.1196/annals.1298.025. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Barami K, Iversen K, Goldman SA. Estrogens and non-estrogenic ovarian influences combine to promote the recruitment and decrease the turnover of new neurons in the adult female canary brain. J Neurobiol. 1995;27:470–487. doi: 10.1002/neu.480270404. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lee DW, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev Neurobiol. 2007;67:1107–1117. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morale MC, L’Episcopo F, Tirolo C, Giaquinta G, Caniglia S, Testa N, Arcieri P, Serra PA, Lupo G, Alberghina M, Harada N, Honda S, Panzica GC, Marchetti B. Loss of aromatase cytochrome P450 function as a risk factor for Parkinson’s disease? Brain Res Rev. 2008;57:431–443. doi: 10.1016/j.brainresrev.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Norton WT. Cell reactions following acute brain injury: a review. Neurochem Res. 1999;24:213–218. doi: 10.1023/a:1022505903312. [DOI] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Fernando G, Day L, Allen TA, Chapleau JD, Menjivar J, Schlinger BA, Lee DW. Aromatase expression and cell proliferation following injury of the adult zebra finch hippocampus. Dev Neurobiol. 2007;67:1867–1878. doi: 10.1002/dneu.20548. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J Neuroendocrinol. 2001;13:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc Natl Acad Sci U S A. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–118. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J Neurobiol. 2005;64:192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev. 2006;2:213–219. doi: 10.1007/s12015-006-0049-0. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J Neurobiol. 2003;56:398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- Wade J, Schlinger BA, Hodges L, Arnold AP. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. Gen Comp Endocrinol. 1994;94:53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Saldanha CJ. Glial aromatization increases the expression of bone morphogenetic protein-2 in the injured zebra finch brain. J Neurochem. 2008;106:216–223. doi: 10.1111/j.1471-4159.2008.05352.x. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Maas S, Saldanha CJ. Molecular characterization of the injury-induced aromatase transcript in the adult zebra finch brain. J Neurochem. 2008;105:1613–1624. doi: 10.1111/j.1471-4159.2008.05256.x. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16:676–683. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56:97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- Zuo MX. The studies on neurogenesis induced by brain injury in adult ring dove. Cell Res. 1998;8:151–158. doi: 10.1038/cr.1998.15. [DOI] [PubMed] [Google Scholar]


