Abstract
Olfactory neurons show an extreme diversity of cell types with each cell usually expressing one member from a large family of 60 Odorant receptor (Or)genes in Drosophila. Little is known about the developmental processes and transcription factors that generate this stereotyped pattern of cellular diversity. Here we investigate the molecular and cellular basis of defects in olfactory system function in an unusual dominant mutant, Scutoid. We show that the defects map to olfactory neurons innervating a specific morphological class of sensilla on the antenna, large basiconics. Molecular analysis indicates defects in neurons expressing specific classes of receptor genes that map to large basiconic sensilla. Previous studies have shown that in Scutoid mutants the coding region of the transcriptional repressor snail is translocated near the no-ocelli promoter, leading to misexpression of snail in the developing eye-antenna disc. We show that ectopic expression of snail in developing olfactory neurons leads to severe defects in neurons of the antennal large basiconics supporting the model that the dominant olfactory phenotype in Scutoid is caused by misexpression of snail.
INTRODUCTION
The generation of intricate patterns of differentiated neurons is an intriguing question in developmental biology. The olfactory organs house a complex arrangement of distinct classes of olfactory neurons. Drosophila melanogaster like most animals contain a large family of odorant receptor (Or) genes of which usually one is selected to be expressed in a single class of olfactory neuron, which along with a broadly-expressed non-canonical Or83b, forms the functional heteromeric receptor and determines the odor-response properties of the cell (Benton et al., 2006; Fuss and Ray, 2009). Different neuron classes express different Or genes and this enables the organism to detect and discriminate amongst a wide variety of odor molecules. The developmental mechanisms and transcription factors that give rise to this pattern of neuron types and the associated map of Or gene expression have remained largely elusive (Fuss and Ray, 2009).
The fruit fly Drosophila melanogaster has a numerically simple olfactory system where a complete map of Or gene expression and a variety of genetic tools are available (Dahanukar et al., 2005; Vosshall and Stocker, 2007). It has two pairs of olfactory organs, the maxillary palps and the third segments of the antennae, containing ~120 and ~1200 olfactory receptor neurons (ORNs) respectively. The ORNs are housed, usually in stereotypical pairs, in hair-like structures called sensilla. There are three morphological categories of sensilla, basiconica, coeloconica and trichoidea which are found in characteristic but overlapping zones on the antennal surface, while only sensilla basiconica are present on the surface of the palp (Shanbhag et al., 1999; Stocker, 1994). Within the antenna, expression of a particular Or gene is restricted to a subset of a morphological category of sensilla and hence to a specific zone (Fuss and Ray, 2009). Previously we have shown that organ-specific cis-regulatory mechanisms restrict expression of a specific subset of Or genes to the maxillary palps (Ray et al., 2007). It is conceivable that sensilla-specific zonal expression within the antenna serves to further restrict the possible repertoire of Or genes that can be expressed within each zone, thus progressively simplifying receptor gene regulation. Interesting similar zone-specific expression patterns are also observed in vertebrate OR genes (Fuss and Ray, 2009).
Forward genetic approaches and quantitative genetic and genomic analyses have yielded insights about the genetic architecture underlying olfactory behavior (Anholt et al., 2003; Anholt et al., 2001; Ayer and Carlson, 1991; Ayer et al., 1989; Fedorowicz et al., 1998; Rollmann et al., 2005). One of the first transcription factors that was shown to play a role in Or gene choice, Acj6, was identified using a forward genetic screen in Drosophila melanogaster for mutants that affected responses to a specific subset of odors tested (Ayer and Carlson, 1991). Acj6 was later shown to be a POU domain transcription factor that could directly affect Or gene expression (Bai and Carlson, 2010; Bai et al., 2009; Clyne et al., 1999). Subsequently a candidate gene approach was used to find a related POU domain transcription factor, Pdm3 which was also shown to play a role in Or gene expression and neural development in Drosophila (Tichy et al., 2008). Apart from these examples there also exists indirect evidence that supports a role for two other transcription factors, lozenge (Ray et al., 2007) and scalloped (Ray et al., 2008), that may play a role in Or gene expression. Lozenge was also previously shown to regulate the pattern of basiconic and trichoid sensilla on the antenna (Gupta et al., 1998). However little else is known about transcription factors that affect patterning of the antenna and its zonal distribution of the three morphological classes of sensilla.
In order to identify other transcription factors that may participate in regulation of Or gene expression, we decided to characterize extant mutants that are known to have defects in their odor response. The dominant mutant Scutoid (Sco) is unusual amongst them since it has previously been shown to have an interesting “dominant” phenotype which leads to defects in olfactory responses of the antenna to specific short chain ketones and esters (Dubin et al., 1995). Scutoid is a gain-of -function mutation of Drosophila which is associated with a chromosomal transposition that leads to a fusion of two genes no-ocelli (noc) and snail (sna)(Ashburner et al., 1982; McGill et al., 1988). This aberrant gene fusion has heterozygous dominant phenotypes that include loss of mechanosensory bristles in the thorax and eye (Fuse et al., 1999).
In this study we characterize the Sco mutant at the cellular level using electrophysiology and demonstrate that the odor defects map to the ORNs of the large basiconic sensilla on the surface of the antenna. Analysis of Or gene expression supports these observations and a strong defect was found in the large basiconic sensilla. Defects were not observed in ORNs that innervate other morphological categories of sensilla in the antenna and the maxillary palps. Moreover guidance of ORN axons is also unaffected in the mutant. Using genetic analysis we show that a mutation in no-ocelli enhances the Sco olfactory phenotype, while revertants of Sco that remove sna regions are normal. Finally we show that ectopic expression of snail in the developing olfactory system is sufficient to cause dominant olfactory defects in the large basiconic sensilla.
These results provide a molecular and developmental explanation for the olfactory phenotype of Sco in the ORNs of large basiconic sensilla and identify misexpression of snail as a powerful dominant genetic tool that can be used to disrupt the olfactory system. Such a tool could be useful in the future to genetically disrupt odor mediated host-seeking behavior in insects that destroy crops and transmit deadly diseases.
RESULTS
Scutoid flies have defects in responses to specific odorants
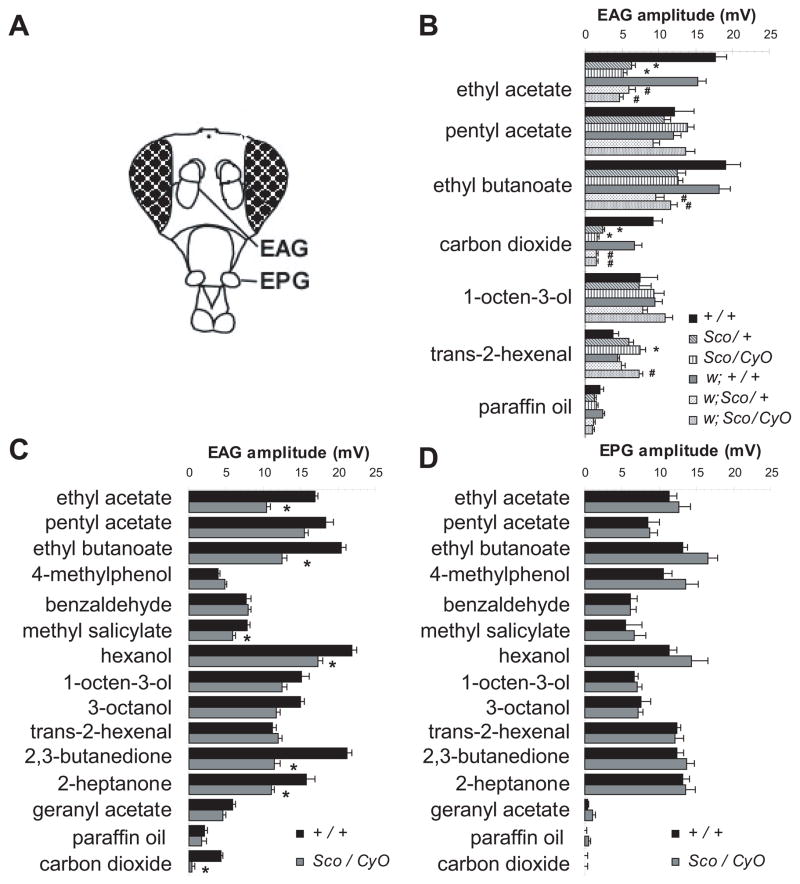
In order to attribute defects in odor responses in Sco flies to specific ORN classes we performed whole-organ recordings from antennae and maxillary palps (Figure 1A) using a diverse panel of odors chosen to stimulate different ORNs. Electroantennograms (EAG) indicate that responses to half of the odorants were significantly decreased in antenna of the mutant as compared to the wild-type controls (Figure 1B,C). By contrast, electropalpograms (EPG) from the maxillary palps using the same panel of odors demonstrated that the responses were normal (Figure 1D). These results strongly suggest that only subsets of olfactory neurons in the antenna are affected in the Sco mutant.
Figure 1. The Sco mutation affects olfactory responses from the antenna to a subset of odorants.
A, Electroantennograms (EAG) and electropalpograms (EPG) are electrophysiological recordings taken from the surface of the antenna and maxillary palps respectively. B, Mean EAG responses from Sco flies carrying the w and/or CyO chromosomes, (n=8), * significantly changed compared to +/+, # significantly changed compared to w; +/+ (P < 0.01) in a Bonferroni-corrected t test). C, Mean responses from EAGs of wild type (CS, n=15) and mutant (Sco/CyO, n=13) flies to a panel of indicated odorants (10−2 dilution). D, Mean responses from EPGs of the same (CS-5, n=6, Sco/CyO, n=6). Error bars = s.e.m.
Severe defects in Sco ORNs that innervate large basiconic sensilla
The EAG in Sco flies reveals significantly lower response to ethyl acetate, ethyl butanoate, methyl salicylate, 2,3-butanedione, 2-heptanone and carbon dioxide (CO2), which have previously been shown to strongly activate ORNs in the large basiconic sensilla of the antenna (de Bruyne et al., 2001). These are limited to a specific area on the dorso-medial and central posterior side of the antenna (Figure 2A). Scanning electron micrographs of the surface of the antenna show that all olfactory sensillum types occur on both wild type and Sco antenna (not shown) (Dubin et al., 1995). However, we find some deformed and fused sensilla in the centre of the dorso-medial region which is normally occupied by an orderly array of large basiconics (Figure 2B). Since the odor response properties of ORNs depend upon the Or genes expressed (Hallem et al., 2004), we decided to directly test whether expression of Or genes in large basiconics was affected in the Sco flies.
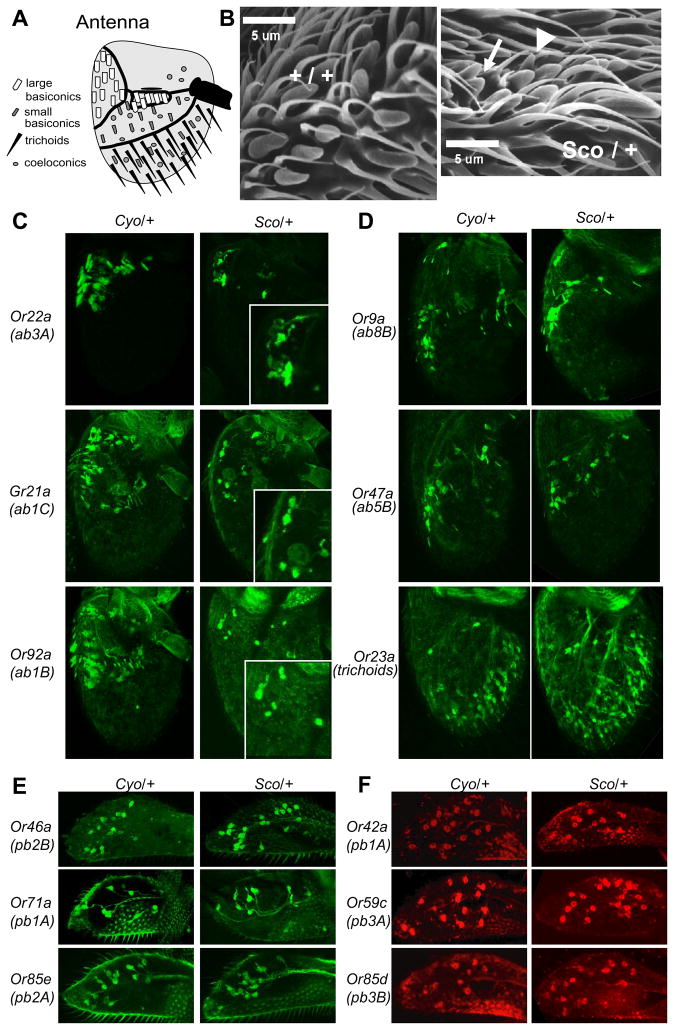
Figure 2. Sco have defects in Or-Gal4-expressing neurons of the large basiconic sensilla.
A, The distribution of different morphological categories of olfactory sensilla on the posterior surface of the 3rd segment of the antenna. B, Scanning electron micrograph of large basiconic sensilla on the dorso-medial aspect of the antenna for Sco and wild type flies respectively. Deformed (arrowhead) and fused (arrow) sensilla. C–E, Representative Z-projections of confocal micrographs from flies containing 1 copy of indicated Or-Gal4 and 2 copies of UAS-mCD8:GFP in either a w;Cyo/+ or w;Sco/+ background for C, large basiconic expressing genes in the antenna (insets show close up views of GFP signal), D, small basiconic and trichoid expressing genes in the antenna, and E, maxillary palp expressing genes. F, Representative Z-projections of confocal micrographs from fluorescent RNA in situ hybridizations for indicated Or gene probes in maxillary palps of Cyo/+ or Sco/+ flies.
Transgenic flies that contain promoter-GAL4 fusions of selected Or genes were used to drive expression of a membrane-bound form of GFP (mGFP) to label the ORNs of choice and evaluate their morphology. We tested three Or genes that drive expression specifically in ORNs that innervate the large basiconic sensilla of which there are 3 physiologically distinct types: ab1 (4 ORNs), ab2 (2 ORNs) and ab3 (2 ORNs) each of which express different Or genes. In all three cases we tested, Or22a (expressed in ab3A) that detects ethyl butanoate (Dobritsa et al., 2003), Gr21a (expressed in ab1C) that detects CO2 (Suh et al., 2004), and Or92a (expressed in ab1B) that detects 2,3-butanedione, we find a severe reduction in the number of mGFP positive neurons in the Sco antenna when compared to control Cyo antenna (Figure 2C). Furthermore we find that in the case of most cells that do express the mGFP, the dendrites present in the sensilla shafts are not clearly visible (Figure 2C, right, zoomed insets). In each antenna we can detect at most one or two mGFP positive dendrites in the large basiconic sensilla, as opposed to the controls where mGFP is detected in the sensillum shaft in almost every case (Figure 2C, left).
Sco mutant does not affect ORNs that innervate other sensillum types
In order to test whether the Sco mutant has obvious defects in Or gene expression in other sensilla we tested Or genes that are expressed in ORNs that innervate two other classes of sensilla in the antenna; the small basiconic sensilla, Or47a (ab5B) and Or9a (ab8B) (Hallem et al., 2004), and the trichoid Or23a (at2) (Couto et al., 2005). For all three genes GFP expression in neurons of the Sco antenna is unaffected (Figure 2D). Moreover, the dendritic GFP signal can also be observed in the majority of sensillum shafts.
We also performed a comprehensive analysis of Or gene expression in all 6 ORN classes of the maxillary palp in the Sco mutant. We used promoter-GAL4 driven expression of mGFP to label neurons expressing Or46a (pb2B), Or71a (pb1B), and Or85e (pb2A) (Figure 2E); as well as RNA in-situ hybridization to Or42a (pb1A), Or59c (pb3A), and Or85d (pb3B) (Figure 2F). We find that Or gene expression in none of the 6 ORN classes is affected in the Sco mutant maxillary palps, which is consistent with the functional EPG analysis.
Taken together these results support the EAG based predictions and suggest that the Sco mutation affects the neurons in large basiconic sensilla but not in small basiconic or trichoid sensilla of the antenna nor in any of the ORNs of the maxillary palp.
Normal axon-guidance of olfactory neurons in Sco
Previous studies have demonstrated that transcription factors that play a role in Or gene regulation like Acj6 and Pdm3 can also affect the coordinated process of guidance of ORN axons to the appropriate glomeruli in the antenna lobe (Komiyama et al., 2004; Tichy et al., 2008). In order to investigate whether axon growth and/or axon-guidance was affected in the Sco flies, we analyzed the projection patterns of GFP- labeled axons of a variety of ORN classes in the antennal lobes of mutant flies.
We were unable to detect obvious defects in the projection patterns for any of the ORN classes we tested including ORNs innervating maxillary palp basiconic, large basiconic, small basiconic, and trichoid sensilla (Supplementary Figure 1). The simplest interpretation is that the olfactory defects caused by the Sco mutation are restricted to the periphery.
Abnormal odor responses in individual neurons of Sco mutant large basiconic sensilla
In order to characterize the physiological defects at a cellular resolution we made single-sensillum electrophysiological recordings from the antenna. This type of extracellular recording has been used to quantitatively analyze the responses of individual neurons housed within olfactory sensilla in Drosophila, and we used a diagnostic panel of 10 diverse odorants to systematically compare ORN function from most classes of antennal sensilla between Sco and control flies (Bai et al., 2009; Clyne et al., 1999; de Bruyne et al., 2001; Tichy et al., 2008).
The large basiconic sensilla are spatially clustered in the dorso-medial area of the third segment of the antenna and there are three functional types, ab1, ab2 and ab3 (de Bruyne et al., 2001). The ab1 sensillum houses 4 ORNs, while the ab2 and ab3s house 2 ORNs each. We first measured the responses to the panel of 10 odors from each of these different sensilla in the control antenna (Table 1, Figure 3A) (de Bruyne et al., 2001). We next examined the responses of ORNs in 58 large basiconic sensilla of this area of the antenna in Sco/+ mutant flies and 66 sensilla in Sco/Cyo flies to the same odorant panel. In general it was more difficult to obtain good quality recordings from sensilla in both Sco genotypes and many had a lower signal to noise ratio than large basiconic sensilla of control flies. We observed several major defects in the large basiconic sensilla of the Sco mutant (Table 1, Figure 3). Firstly, several sensilla showed aberrant odorant responses while still being recognizable as either ab1, ab2 or ab3 types. The most severely affected sensillum type is the ab1, with a large fraction of sensillum in the Sco/+ (76%) and Sco/Cyo (70%) flies presenting abnormal responses (Table 1). Secondly, we recorded from a number of sensilla that showed odor responses that prevented a clear classification (Figure 3B). We did not attempt to classify these as any of the other sensillum types normally present on other parts of the antenna and register them as “unidentifiable”. Finally, in approximately 22–26% of sensilla we were unable to detect action potentials from the neurons although the electrode had clearly penetrated the sensillum shaft. We never observed this in the control antennae. In total a large fraction of sensilla we recorded from showed some form of defect in their odor response (Table 1).
Table 1.
Summary of odor response defects in large basiconic sensilla of Sco mutant
| Normal | Defective Sensilla | |||||||
|---|---|---|---|---|---|---|---|---|
| Odor Response Defects | Silent Sensilla | Odor Response Defects + Silent | ||||||
| Total (wt) | 18/20 | 90.00% | 2/20 | 10.00% | 0/20 | 0.00% | 2/20 | 10.00% |
| Total (Sco/Cyo) | 20/66 | 30.30% | 29/66 | 43.94% | 17/66 | 25.76% | 46/66 | 69.70% |
| Total (Sco/+) | 14/58 | 24.14% | 31/58 | 53.45% | 13/58 | 22.41% | 44/58 | 75.86% |
| ab1 (Sco/Cyo) | 5/21 | 23.81% | 16/21 | 76.19% | ||||
| ab2 (Sco/Cyo) | 6/7 | 85.71% | 1/7 | 14.29% | ||||
| ab3 (Sco/Cyo) | 9/12 | 75.00% | 3/12 | 25.00% | ||||
| ab1 (Sco/+) | 0/9 | 0.00% | 9/9 | 100.00% | ||||
| ab2 (Sco/+) | 5/5 | 100.00% | 0/5 | 0.00% | ||||
| ab3 (Sco/+) | 9/14 | 64.28% | 5/14 | 35.71% | ||||
| Unidentifiable (Sco/Cyo) | 9/66 | 13.64% | ||||||
| Unidentifiable (Sco/+) | 17/58 | 29.31% | ||||||
Numbers and proportions of neurons tested in large basiconic sensilla on antenna of flies showing functional defects as assayed using single-sensillum electrophysiology to a panel of 10 diagnostic odorants in wild-type (w (Canton S)) indicated as (wt) and Scutoid mutant (w; Sco/+) and (w; Sco/Cyo).
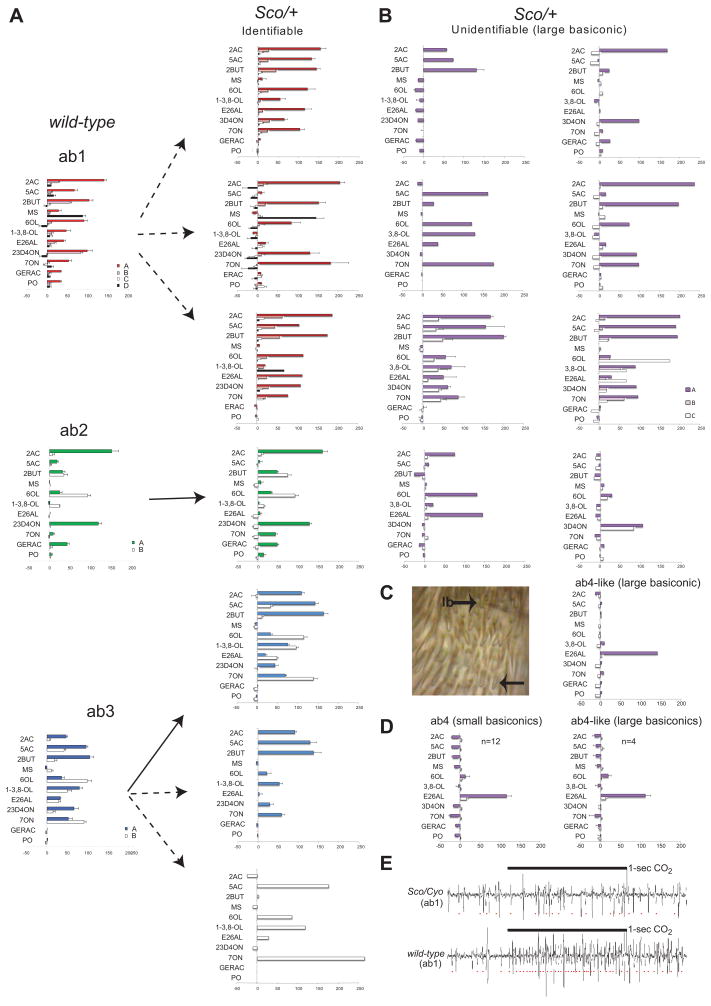
Figure 3. Sco have defects in olfactory responses of neurons of the large basiconic sensilla.
A, Comparison of odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the three types of large basiconic sensilla recognized in wild-type (wt) and Sco/+ flies. Solid arrows point to normal sensillum types while dotted arrows indicate a defective variant type. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. B, Response profiles from neurons in large basiconic sensilla that could not be classified as one of the three known sensillum types. N=20 in wt and N=58 in Sco/+, error bars= s.e.m. Abbreviations: 2AC, Ethyl Acetate; 5AC, Pentyl Acetate; 2BUT, Ethyl Butyrate; MS, Methyl Salicylate; 6OL, Hexanol; 1–3,8-OL, 1-octen-3-ol; E26AL, E2-hexenal; 23D4ON, 2,3-Butanedione; 7ON, Heptanone; GERAC, Geranyl Acetate and PO, paraffin oil (used as a diluent). C, Light micrograph of an antenna with a large basiconic sensilla indicated (l.b, top arrow) from which the recordings were taken to generate the odor-response spectra on the top right. Glass recording electrode is faintly visible to the right of indicated sensillum. A representative sample of a small basiconic sensillum is indicated by lower arrow. D, Mean odor response profile of an ab4 sensilla in small basiconic of wild-type antenna (left) and of an ab4-like type of sensillum in large basiconics of Sco/+ antenna (right). E, Example of recordings from ab1 sensilla stimulated with 1 –sec 0.3% CO2. Note the presence of action potentials from the C neuron in both traces but a lack of response in the Sco neuron.
Odor response profiles illustrate the specific defects we identified in the large basiconic sensilla of Sco/+ (Figure 3) and Sco/Cyo flies (Supplementary Figure 2). In the control flies we find that each large basiconic sensillum type has a characteristic odor response spectrum and ORNs are present in stereotypical combinations, each responding to their characteristic odorants as has been reported previously (Figure 3A, left) (de Bruyne et al., 2001). In Sco flies some ab2 and ab3 sensilla show response profiles that are similar to the control, indicating the Sco mutation does not systematically remove these sensillum types (Figure 3A, right and Supplementary Figure 2A, right). The majority of sensilla however shows a variety of defects, which vary from one fly to another (Table 1, Figure 3A, Supplementary Figure 2A, Supplementary Figure 3). Amongst the Sco/+ large basiconic sensilla whose response spectra can still be classified based on the diagnostic odor response spectrum of at least one unaffected neuron in the sensillum we find 3 variant forms of ab1, one where the D neurons do not respond to methyl salicylate (n=6), one that show lower responses in the B neuron (n=2) and one that have defects in both B and D neurons (Figure 3A, Supplementary Figure 3). In the Sco/Cyo flies we also found a variant of what is most likely an ab2 type of sensilla where the A neuron has a higher response level to geranyl acetate, but lower responses to ethyl acetate (Supplementary Figure 2A). Finally, some ab3 sensilla of Sco/+ flies lack the B neuron completely (n=4) and in 1 sensillum the A neuron is missing (Figure 3A). Figure 3B and Supplementary Figure 2B show several sensilla with response spectra which we could not classify as either of the three types.
Surprisingly, we were able to identify a number of sensilla that were embedded in the field of large basiconics of the dorsal medial region, and were clearly distinguishable as large basiconics by virtue of their size (Figure 3C,D), but had characteristic odor response profiles indistinguishable from the ab4 sensillum which is always found in small basiconics in a neighboring zone in wild type flies (Figure 3D) (de Bruyne et al., 2001). These results suggest that in Sco mutant flies a few transformed large basiconic sensilla contain ORNs and receptors that are normally present only in small basiconics.
In a separate experiment we tested the CO2 response of the ab1C neurons and we found that a large number of the ab1 sensilla did not respond to CO2 even if action potentials from ab1C neurons could be detected (Figure 3E). Taken together these results show that the Sco mutation severely disrupts the odor responses of the large basiconic sensilla in a variety of ways.
In order to test whether the defects were restricted to large morphological class of antennal basiconics we systematically surveyed a number of the small basiconic classes of sensillum for all of which diagnostic odors are available in the odor panel. By comparing the odor response spectra of the small basiconics between Sco/+ and wild type flies we are able to identify comparable classes of sensilla (ab4, ab5, ab6, ab7 and ab8) at comparable frequencies and locations (Table 2, Supplementary Figure 4). Furthermore we do not find sensilla that are either empty or missing a neuron (Table 2) as is the case for (25%) of large basiconic sensilla. In a small fraction of cases (10–13%) in both wild-type and Sco/+ we find response spectra that either cannot be classified or have defects.
Table 2.
Summary of odor response defects in small basiconic sensilla of Sco mutant
| Normal | Defective Sensilla | |||||||
|---|---|---|---|---|---|---|---|---|
| Odor Response Defects | Silent Sensilla | Odor Response Defects + Unidentifiable | ||||||
| Total (wt) | 41/46 | 89.13% | 5/46 | 10.87% | 0/46 | 0.00% | 5/46 | 10.87% |
| Total (Sco/+) | 53/61 | 86.89% | 8/61 | 13.11% | 0/61 | 0.00% | 8/61 | 13.11% |
| ab4 (Sco/+) | 5/5 | 100.00% | 0/5 | 0.00% | ||||
| ab5 (Sco/+) | 12/15 | 80.00% | 3/15 | 20.00% | ||||
| ab6 (Sco/+) | 12/12 | 100.00% | 0/12 | 0.00% | ||||
| ab7 (Sco/+) | 15/15 | 100.00% | 0/15 | 0.00% | ||||
| ab8 (Sco/+) | 9/9 | 100.00% | 0/9 | 0.00% | ||||
| Unidentifiable (Wt) | 4/46 | 8.70% | ||||||
| Unidentifiable (Sco/+) | 5/61 | 8.20% | ||||||
Numbers and proportions of neurons tested in large basiconic sensilla on antenna of flies showing functional defects as assayed using single-sensillum electrophysiology to a panel of 10 diagnostic odorants in wild-type (wCS) indicated as (wt) and Scutoid mutant (w; Sco/+).
Using a second diagnostic panel of odorants we also surveyed the coeloconic sensilla on the Sco/+ antenna using single-sensillum electrophysiology. All 4 classes of coeloconic sensilla were identified using the diagnostic panel (Supplementary Figure 5).
The results of the systematic electrophysiology survey are consistent with the expression analysis, and suggest that the Sco mutation primarily affects large basiconic sensilla on the surface of the antenna.
Genetic mapping of olfactory phenotype to the noc-Sco fusion
Sco is an example of a classical dominant mutant and it has been associated with a chromosomal transposition event which leads to a fusion of the no-ocelli (noc) and snail (sna) genes. In order to test whether there is genetic interaction between Sco and no-ocelli we generated a trans-heterozygote containing Sco and GT8, which is an allele of no-ocelli caused by a translocation (Cheah et al., 1994).
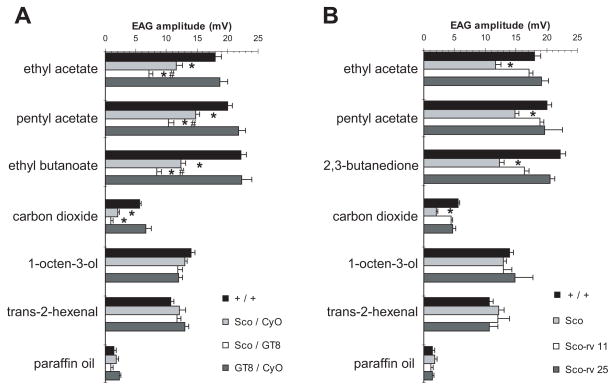
EAG analysis indicates that the decreased responses to specific odorants in Sco/Cyo are enhanced in the Sco/GT8 antenna (Figure 4A). All four odors ethyl acetate, ethyl butyrate, pentyl acetate and CO2 that are affected in the Sco/Cyo flies show significantly more reduction in EAG amplitudes. Therefore the effects of Sco on ORNs in large basiconic sensilla involve the noc gene.
Figure 4. EAG phenotypes of mutations affecting the no-ocelli and snail genes.
A, Mean EAG responses from trans-heterozygotes with GT8, an allele of no-ocelli, show enhancement of the Sco olfactory phenotype (+/+, n=9; Sco/CyO, n=6; Sco/GT8, n=7; GT8/CyO, n=5) * decreased compared to wild type, # decreased compared to Sco/CyO (P < 0.008 in a Bonferroni-corrected t test). B, Mean EAG responses show that two revertant Sco alleles that affect the juxtaposition of sna and no-ocelli do not have the Sco olfactory phenotype (data for +/+ and Sco/CyO are same as A, Sco-rv11/CyO, n=6, Sco-rv25/CyO, n=6).
To determine whether the olfactory phenotype is caused by the fusion of the noc locus with the sna locus we tested two revertant versions of the Sco chromosome that affect this juxtaposition. The Sco-rv11 revertant has a breakpoint in the region between noc and sna, while the Sco-rv25 has a small deletion in there. Both revertants show a complete rescue of the olfactory defects (Figure 4B). This shows that the olfactory phenotype in Sco maps to this fusion locus and depends on both sna and noc that are brought together by the transposition event.
Misexpression of snail in olfactory neurons causes loss of odor response
It has been demonstrated that the Sco eye and bristle phenotype is primarily caused by the ectopic expression of snail under the control of the no-ocelli enhancer caused by the chromosomal transposition (Fuse et al., 1999). Snail is a transcription factor of the C2-H2- type zinc finger family that binds to a sequence called the E-Box and has been shown to antagonize neurogenesis through its inhibitory interaction with basic Helix-Loop-Helix proteins (Ip et al., 1992). In Sco larvae, ectopic expression of Snail has been observed in the region of the eye-antennal disc that later on develops into the adult antenna (Fuse et al., 1999). In wild-type larvae snail is not expressed in the eye antennal disc. This suggests that ectopic expression of snail in the eye antennal disc could be responsible for the olfactory phenotype as well.
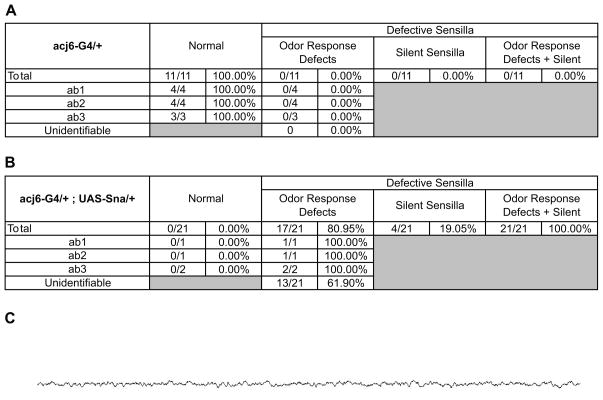
In order to test directly whether misexpression of snail in the developing olfactory neurons can cause the defects in olfactory function we used a Gal4 enhancer trap line of acj6 to drive expression of UAS-snail in the majority of olfactory neurons. Acj6 is expressed in most olfactory neurons in the antenna starting at 16 hours after pupa formation (Clyne et al., 1999). We used the single-sensillum electrophysiology assay with the panel of 10 diagnostic odors to test odor responses of the large basiconic sensilla on the dorso-medial surface of the antenna. All 21 large basiconic sensilla we recorded from were defective in the P(Gal4)/+;UAS-Sna/+ flies (Figure 5). We could not detect spikes from ~19% of the sensilla (Figure 5B). The majority of large basiconic olfactory neurons with responses could not be classified due to changes in their odor responses. The control P(Gal4)/+ flies had no detectable changes (Figure 5A). When we used another driver, Or83b-promoter-Gal4, that drives expression in a large number of ORNs (all ORNs innervating large basiconics) starting at a later developmental stage (Supplementary Figure 6) after eclosion from pupae, we did not find any defects in the ORNs of the large basiconic sensilla (data not shown).
Figure 5. Ectopic expression of snail causes defects in olfactory neurons.
Numbers and proportions of neurons present in large basiconic sensilla on antenna of flies showing functional defects as assayed using single-sensillum electrophysiology to a panel of 10 diagnostic odorants in A, acj6-Gal4/+ control enhancer trap line, and B, acj6-Gal4/+; UAS-snail/+ line.
Taken together these results demonstrate that the misexpression of snail in the eye antennal disc at an early developmental stage can cause severe defects in the ORNs similar to those observed in the Sco mutant antenna.
DISCUSSION
A mutation that specifically disrupts development of large Basiconic sensilla
The simplest interpretation of our results is that the Sco mutation has a dominant effect that specifically affects ORNs that innervate the large basiconic sensilla of the antenna. Our observations on ORN number and morphology using promoter-GAL4s indicate both a drastic reduction of neurons and a loss of dendrites. This observation is consistent with single unit electrophysiology performed on large basiconic sensilla on the antenna of Sco flies whose response to odors is affected. Some sensilla seem to have lost one or all functional neurons. In other sensilla we found neurons that fire action potentials but have lost their typical response to odors. We also found instances where novel odor responses are seen, probably indicating a change in cell identity. By contrast electrophysiological recordings and analysis of promoter-GAL4 visualization indicate that the maxillary palp sensilla, small basiconics sensilla, coeloconic sensilla and at least one class of trichoid sensilla on the antenna are unaffected.
It remains to be seen whether the effect of the Sco mutation on the large basiconics is due to developmental defects in sensilla formation or defects in Or gene expression, or both. The different morphological types of sensilla arise from different lineages of proneural clusters. The proneural gene Amos (Goulding et al., 2000; zur Lage et al., 2003) gives rise to the basiconic and trichoid sensilla of the antenna, while Atonal (Gupta and Rodrigues, 1997; Jhaveri et al., 2000) gives rise to coeloconic sensilla on the antenna and the basiconics on the palp. Mutants for these genes and others lack subsets of sensilla. For example the lozenge gene regulates amos and strong lozenge mutants lack both large and small basiconic sensilla (Stocker and Gendre, 1988). Scutoid is the first member of a class of mutants that specifically affect large basiconics. However, its phenotype is highly variable from sensillum to sensillum. In Sco antenna, large basiconic sensilla are still present, but often their ORNs are defective. The observation that ORNs in some large basiconic sensilla have modified response profiles that are similar to the ab4 small basiconic profiles suggest expansion of Or gene expression zones.
Interestingly, transcription factors that affect Or gene expression like acj6 and pdm3 also affect axon-guidance of ORNs (Bai et al., 2009; Komiyama et al., 2004; Tichy et al., 2008). In contrast Sco affects Or gene expression but does not appear to affect axon-guidance which indicates differences in the two mechanisms.
Mechanism underlying Sco olfactory phenotype
Our data supports the model that the loss of ORN function in Sco mutants is caused by the ectopic expression of a zinc finger transcriptional repressor Snail during early pupal development. Since Snail is normally not expressed in the developing olfactory tissue, the ectopic expression leads to an unusual dominant gain-of-function neomorphic phenotype. A similar effect has been previously characterized in the development of the eye in Sco mutants where Snail misexpression causes morphological disruption as well as neuronal fate change of some cells in the ommatidium (Birkholz et al., 2009). The latter study also shows that the disruption in photoreceptor cells depends on the corepressor CtBP. It is possible that Snail and CtBP can together repress transcription of target genes that are important for development of the olfactory system as well. Although the identification of genes downstream of Snail that cause the phenotype in the Sco mutant antenna are beyond the scope of this study, we note with interest that ~1/3rd of the Or genes, including two expressed in large basiconics, contain E-box Snail binding sites within regulatory regions 1 kb of upstream and downstream. The E-box site (CACCTG) is known to be a target for proneural factors and bHLH transcription factors (Orian et al., 2003), however their presence raises the question whether ectopically expressed Snail can now bind these sequences and cause repression of Or gene expression directly.
Our analysis provides a detailed characterization of the dominant Sco olfactory phenotype at a cellular and molecular level. The ability of Snail to severely affect ORN function can have important consequences in the generation of a transgenic genetically dominant strategy to disrupt olfactory systems of other insects (Hill et al., 2005). Such dominant strategies are currently lacking to develop novel transgenic strategies to genetically control disease transmitting mosquitoes that use the sense of smell to identify human hosts.
EXPERIMENTAL METHODS
Fly stocks
Wild type flies were Canton-S strain either (+/+), with one copy of the CyO balancer chromosome (+/CyO) and/or two copies of the white (w) eye-color mutation. UAS-sna flies were a kind gift of S. Hayashi. P(Gal4) enhancer-trap line of acj6 and other mutants used were obtained from the Bloomington Stock center.
Electropysiological recordings
We recorded extracellular electrical signals from whole antennae (electroantennogram, EAG), maxillary palps (electropalpogram, EPG) using AgCl-coated silver wire inserted in saline filled glass capillaries. A single fly was immobilized in a plastic pipette tip. The reference electrode was inserted in the eye. For EAG recordings the recording electrode was placed on the surface of the dorso-medial aspect of a fly’s antenna. EPGs were taken in a similar manner by contacting the medial side of the palp. Signals were amplified via an analog 10x active probe and recorded digitally (serial-IDAC, Syntech, Hilversum, the Netherlands) Amplitude maxima (mV) were determined during a 1 second stimulation period relative to baseline before stimulation. EAG and EPG signals are thought to represent the summed receptor potentials of a population of ORNs. A more detailed description is given in (Ayer and Carlson, 1991). Odor stimulation was as in (de Bruyne et al., 2001). Briefly, a glass tube supplied continuous humidified air to the preparation. Volatiles were injected into the air from 5 ml disposable syringes holding 20 μl of odorant solution on filter paper, giving a headspace dilution factor of ~10%. All odorants were at highest available purity (>98%, Aldrich, Milwaukee) and dissolved in paraffin oil at 1% v/v.
Single-sensillum electrophysiology was performed essentially as in (Dobritsa et al., 2003). Changes in spike firing rate during 500 ms stimulation relative to pre-stimulus activity were analysed offline using Axoscope software (Axon Laboratory).
All odorants were at highest available purity (>98%, Sigma) and dissolved in paraffin oil or water at 1% dilution.
In situ hybridization and immunolabeling
In situ hybridization and immunohistochemical localization were performed as in Goldman et al (2005). Whole mount brain staining was performed as in Komiyama et al (2007). Mouse anti-nc82 antibody (1:5) was obtained from the Developmental Studies Hybridoma Bank, and rabbit anti-GFP (1:250) were obtained from Invitrogen.
Electron-microscopy
Scanning electron micrographs (SEM) of structures on the surface of the antenna were taken with an ISI SS-40 Scanning Electron Microscope as in Riesgo-Escovar et al. (1997). Fly heads were mounted on standard SEM stubs, antennae pulled away from the head capsule and fixed with double-sided sticky tape before being sputter coated with gold/palladium.
Supplementary Material
Representative Z-projections of confocal micrographs of antenna lobe from flies containing 1 copy of indicated Or-Gal4 and 2 copies of UAS-mCD8:GFP in either a Cyo (control) or Sco/+ background.
A, Comparison of odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the three types of large basiconic sensilla recognized in wild-type (wt) and Sco/CyO (Sco) flies. Solid arrows point to normal sensillum types while dotted arrows indicate a defective variant type. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. B, Response profiles from neurons in large basiconic sensilla that could not be classified as one of the three known sensillum types. N=20 in wt and N=66 in Sco/Cyo, error bars= s.e.m. All odors tested at 10−2 dilution. Abbreviations: 2AC, Ethyl Acetate; 5AC, Pentyl Acetate; 2BUT, Ethyl Butyrate; MS, Methyl Salicylate; 6OL, Hexanol; 1–3,8-OL, 1-octen-3-ol; E26AL, E2-hexenal; 23D4ON, 2,3-Butanedione; 7ON, Heptanone; GERAC, Geranyl Acetate and PO, paraffin oil (used as a diluent).
Representative traces from indicated large-basiconic sensillum in wild-type (wt) flies and w;Sco/Cyo (Sco) flies to 0.5-sec stimulus of indicated odorant at 10−2 dilution. Examples show: an ab1 sensillum in Sco with no D neuron response to MS (Methyl Salicylate); ab2 sensillum in Sco with the A neuron showing an increase in response to GERAC (Geranyl Acetate); and ab3 sensillum in Sco with no B neuron as seen by lack of response to 7ON (Heptanone).
A, Comparison of odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the five types of small basiconic sensilla recognized in wild-type (wt) and Sco/+ flies. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. N=41 in wt and N=52 in Sco/+, error bars= s.e.m. All odors tested at 10−2 dilution. Abbreviations: 2AC, Ethyl Acetate; 5AC, Pentyl Acetate; 2BUT, Ethyl Butyrate; MS, Methyl Salicylate; 6OL, Hexanol; 1–3,8-OL, 1-octen-3-ol; E26AL, E2-hexenal; 23D4ON, 2,3-Butanedione; 7ON, Heptanone; GERAC, Geranyl Acetate and PO, paraffin oil (used as a diluent).
A, Mean odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the four types of coeloconic sensilla from Sco/+ flies. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. All odors tested at dilution of 10−2. NH3, ammonia; PO, paraffin oil (used as a diluent).
Representative confocal micrographs of antenna at indicated developmental stage (APF = After Pupa Formation) where UAS-mcd8:GFP is expressed using indicated GAL4 driver line acj6-Gal4 (left) and Or83b-Gal4 (right).
Acknowledgments
We acknowledge the Z. Wisotsky and S. McInally for assistance. The anti-nc82 antibody developed by E. Buchner was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA. This work was partly supported by NIH grants to J.C; and a Whitehall Foundation grant to A.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anholt RR, Dilda CL, Chang S, Fanara JJ, Kulkarni NH, Ganguly I, Rollmann SM, Kamdar KP, Mackay TF. The genetic architecture of odor-guided behavior in Drosophila: epistasis and the transcriptome. Nat Genet. 2003;35:180–184. doi: 10.1038/ng1240. [DOI] [PubMed] [Google Scholar]
- Anholt RR, Fanara JJ, Fedorowicz GM, Ganguly I, Kulkarni NH, Mackay TF, Rollmann SM. Functional genomics of odor-guided behavior in Drosophila melanogaster. Chem Senses. 2001;26:215–221. doi: 10.1093/chemse/26.2.215. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Tsubota S, Woodruff RC. The genetics of a small chromosome region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. IV: scutoid, an antimorphic mutation. Genetics. 1982;102:401–420. doi: 10.1093/genetics/102.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer RK, Carlson J. acj6: a gene affecting olfactory physiology and behavior in Drosophila. Proc Nat Acad Sci USA. 1991;88:5467–5471. doi: 10.1073/pnas.88.12.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer RK, Monte P, Carlson J. The isolation of antennal mutants and their use in Drosophila olfactory genetics. Plenum; New York: 1989. [Google Scholar]
- Bai L, Carlson JR. Distinct functions of acj6 splice forms in odor receptor gene choice. J Neurosci. 2010;30:5028–5036. doi: 10.1523/JNEUROSCI.6292-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Goldman AL, Carlson JR. Positive and negative regulation of odor receptor gene choice in Drosophila by acj6. J Neurosci. 2009;29:12940–12947. doi: 10.1523/JNEUROSCI.3525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkholz DA, Chou WH, Phistry MM, Britt SG. Disruption of photoreceptor cell patterning in the Drosophila Scutoid mutant. Fly (Austin) 2009;3:253–262. doi: 10.4161/fly.10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah PY, Meng YB, Yang X, Kimbrell D, Ashburner M, Chia W. The Drosophila l(2)35Ba/nocA gene encodes a putative Zn finger protein involved in the development of the embryonic brain and the adult ocellar structures. Mol Cell Biol. 1994;14:1487–1499. doi: 10.1128/mcb.14.2.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 1999;22:339–347. doi: 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr Opin Neurobiol. 2005;15:423–430. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson J. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Heald NL, Cleveland B, Carlson JR, Harris GL. Scutoid mutation of Drosophila melanogaster specifically decreases olfactory responses to short-chain acetate esters and ketones. J Neurobiol. 1995;28:214–233. doi: 10.1002/neu.480280208. [DOI] [PubMed] [Google Scholar]
- Fedorowicz GM, Fry JD, Anholt RR, Mackay TF. Epistatic interactions between smell-impaired loci in Drosophila melanogaster. Genetics. 1998;148:1885–1891. doi: 10.1093/genetics/148.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N, Matakatsu H, Taniguchi M, Hayashi S. Snail-type zinc finger proteins prevent neurogenesis in Scutoid and transgenic animals of Drosophila. Dev Genes Evol. 1999;209:573–580. doi: 10.1007/s004270050291. [DOI] [PubMed] [Google Scholar]
- Fuss SH, Ray A. Mechanisms of odorant receptor gene choice in Drosophila and vertebrates. Mol Cell Neurosci. 2009;41:101–112. doi: 10.1016/j.mcn.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Goulding SE, zur Lage P, Jarman AP. amos, a proneural gene for Drosophila olfactory sense organs that is regulated by lozenge. Neuron. 2000;25:69–78. doi: 10.1016/s0896-6273(00)80872-7. [DOI] [PubMed] [Google Scholar]
- Gupta B, Rodrigues V. atonal is a proneural gene for a subset of olfactory sense organs in Drosophila. Genes Cells. 1997;2:225–233. doi: 10.1046/j.1365-2443.1997.d01-312.x. [DOI] [PubMed] [Google Scholar]
- Gupta BP, Flores GV, Banerjee U, Rodrigues V. Patterning an epidermal field: Drosophila lozenge, a member of the AML-1/Runt family of transcription factors, specifies olfactory sense organ type in a dose-dependent manner. Dev Biol. 1998;203:400–411. doi: 10.1006/dbio.1998.9064. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992;6:1518–1530. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- Jhaveri D, Sen A, Reddy GV, Rodrigues V. Sense organ identity in the Drosophila antenna is specified by the expression of the proneural gene atonal. Mech Dev. 2000;99:101–111. doi: 10.1016/s0925-4773(00)00487-1. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Carlson JR, Luo LQ. Olfactory receptor neuron axon targeting: intrinsic transcriptional control and hierarchical interactions. Nature Neuroscience. 2004;7:819–825. doi: 10.1038/nn1284. [DOI] [PubMed] [Google Scholar]
- McGill S, Chia W, Karp R, Ashburner M. The molecular analyses of an antimorphic mutation of Drosophila melanogaster, Scutoid. Genetics. 1988;119:647–661. doi: 10.1093/genetics/119.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian A, van Steensel B, Delrow J, Bussemaker HJ, Li L, Sawado T, Williams E, Loo LW, Cowley SM, Yost C, Pierce S, Edgar BA, Parkhurst SM, Eisenman RN. Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev. 2003;17:1101–1114. doi: 10.1101/gad.1066903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, van der Goes van Naters W, Carlson JR. A Regulatory Code for Neuron-Specific Odor Receptor Expression. PLoS Biol. 2008;6:e125. doi: 10.1371/journal.pbio.0060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, van der Goes van Naters W, Shiraiwa T, Carlson JR. Mechanisms of odor receptor gene choice in Drosophila. Neuron. 2007;53:353–369. doi: 10.1016/j.neuron.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollmann SM, Mackay TF, Anholt RR. Pinocchio, a novel protein expressed in the antenna, contributes to olfactory behavior in Drosophila melanogaster. J Neurobiol. 2005;63:146–158. doi: 10.1002/neu.20123. [DOI] [PubMed] [Google Scholar]
- Shanbhag S, Muller B, Steinbrecht A. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int J Insect Morphology & Embryology. 1999;28:377–397. [Google Scholar]
- Stocker R. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Research. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Gendre N. Peripheral and central nervous effects of lozenge3: a Drosophila mutant lacking basiconic antennal sensilla. Dev Biol. 1988;127:12–24. doi: 10.1016/0012-1606(88)90184-4. [DOI] [PubMed] [Google Scholar]
- Suh GSB, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Tichy AL, Ray A, Carlson JR. A new Drosophila POU gene, pdm3, acts in odor receptor expression and axon targeting of olfactory neurons. J Neurosci. 2008;28:7121–7129. doi: 10.1523/JNEUROSCI.2063-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- zur Lage PI, Prentice DR, Holohan EE, Jarman AP. The Drosophila proneural gene amos promotes olfactory sensillum formation and suppresses bristle formation. Development. 2003;130:4683–4693. doi: 10.1242/dev.00680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative Z-projections of confocal micrographs of antenna lobe from flies containing 1 copy of indicated Or-Gal4 and 2 copies of UAS-mCD8:GFP in either a Cyo (control) or Sco/+ background.
A, Comparison of odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the three types of large basiconic sensilla recognized in wild-type (wt) and Sco/CyO (Sco) flies. Solid arrows point to normal sensillum types while dotted arrows indicate a defective variant type. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. B, Response profiles from neurons in large basiconic sensilla that could not be classified as one of the three known sensillum types. N=20 in wt and N=66 in Sco/Cyo, error bars= s.e.m. All odors tested at 10−2 dilution. Abbreviations: 2AC, Ethyl Acetate; 5AC, Pentyl Acetate; 2BUT, Ethyl Butyrate; MS, Methyl Salicylate; 6OL, Hexanol; 1–3,8-OL, 1-octen-3-ol; E26AL, E2-hexenal; 23D4ON, 2,3-Butanedione; 7ON, Heptanone; GERAC, Geranyl Acetate and PO, paraffin oil (used as a diluent).
Representative traces from indicated large-basiconic sensillum in wild-type (wt) flies and w;Sco/Cyo (Sco) flies to 0.5-sec stimulus of indicated odorant at 10−2 dilution. Examples show: an ab1 sensillum in Sco with no D neuron response to MS (Methyl Salicylate); ab2 sensillum in Sco with the A neuron showing an increase in response to GERAC (Geranyl Acetate); and ab3 sensillum in Sco with no B neuron as seen by lack of response to 7ON (Heptanone).
A, Comparison of odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the five types of small basiconic sensilla recognized in wild-type (wt) and Sco/+ flies. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. N=41 in wt and N=52 in Sco/+, error bars= s.e.m. All odors tested at 10−2 dilution. Abbreviations: 2AC, Ethyl Acetate; 5AC, Pentyl Acetate; 2BUT, Ethyl Butyrate; MS, Methyl Salicylate; 6OL, Hexanol; 1–3,8-OL, 1-octen-3-ol; E26AL, E2-hexenal; 23D4ON, 2,3-Butanedione; 7ON, Heptanone; GERAC, Geranyl Acetate and PO, paraffin oil (used as a diluent).
A, Mean odor responses recorded using single-sensillum electrophysiology from olfactory receptor neurons in the four types of coeloconic sensilla from Sco/+ flies. The x axis indicates the tested odors and the y axis indicates the change in the number of action potentials/s following initiation of the stimulus. All odors tested at dilution of 10−2. NH3, ammonia; PO, paraffin oil (used as a diluent).
Representative confocal micrographs of antenna at indicated developmental stage (APF = After Pupa Formation) where UAS-mcd8:GFP is expressed using indicated GAL4 driver line acj6-Gal4 (left) and Or83b-Gal4 (right).