Abstract
Diastolic dysfunction in the aging heart is a grave condition that challenges the life and lifestyle of a growing segment of our population. This report seeks to examine the role and interrelationship of inflammatory dysregulation in interstitial myocardial fibrosis and progressive diastolic dysfunction in aging mice. We studied a population of C57BL/6 mice that developed progressive diastolic dysfunction over 30 months of life. This progressive dysfunction was associated with increasing infiltration of CD45+ fibroblasts of myeloid origin. In addition, increased rates of collagen expression as measured by cellular procollagen were apparent in the heart as a function of age. These cellular and functional changes were associated with progressive increases in mRNA for MCP-1 and IL-13 which correlated both temporally and quantitatively with changes in fibrosis and cellular procollagen levels. MCP-1 protein was also increased and found to be primarily in the venular endothelium. Protein assays also demonstrated elevation of IL-4 and IL-13 suggesting a shift to a Th2 phenotype in the aging heart. In vitro studies demonstrated that IL-13 markedly enhanced monocyte fibroblast transformation.
Our results indicate that immunoinflammatory dysregulation in the aging heart induces progressive MCP-1 production and an increased shift to a Th2 phenotype paralleled by an associated increase in myocardial interstitial fibrosis, cellular collagen synthesis, and increased numbers of CD45+ myeloid-derived fibroblasts that contain procollagen. The temporal association and functional correlations suggests a causative relationship between age-dependent immunoinflammatory dysfunction, fibrosis and diastolic dysfunction.
Keywords: Aging myocardium, fibrosis, MCP-1, IL-13, diastolic dysfunction
INTRODUCTION
Age-related diastolic dysfunction has a significant impact on the healthy elderly. Left ventricular filling is impaired with normal aging, limiting the maximum exercise that healthy old persons can perform, and reducing their quality of life. The decrease in diastolic filling with age explains up to 60% of their decrease in exercise performance [1]. Perhaps more importantly, age-related diastolic dysfunction strongly predisposes older people to congestive heart failure (CHF), the most common reason for their hospitalization [2]. More than 50% of older men with CHF have preserved systolic function (LVEF>50%) and presumably impaired diastolic function [3]. The proportion of CHF with preserved systolic function is even higher in older women [4]. The prognosis of CHF and preserved LV systolic function is dismal [3]; there are no effective drugs for treatment of diastolic heart failure [5]. Therefore, preventing age-related diastolic dysfunction is likely to have a major additional benefit by decreasing the frequency of CHF with preserved systolic function [6].
Multiple aspects of diastolic function are significantly impaired with age. While active relaxation is impaired due to age-associated decreases in sarcoplasmic reticulum calcium uptake [7–10], passive stiffness also contributes significantly to the impaired diastolic function [11, 12]. Stiffness is clearly elevated in the old mouse, old rat, old dog and old human heart and reductions in stiffness in the mouse improve diastolic function [11–14]. For example, in the aged TGF-β1+/− mouse, decreased age-related fibrosis and preserved diastolic function are seen [15]. Specifically, in the diastolic pressure/volume relationship, the 24 month wild type heart was almost twice as stiff as the hearts from six month wild type or 24 month TGF-β1+/− mice and this paralleled the lack of age-associated increase in collagen in the aged TGF-β1+/− mouse [15]. Therefore aging is associated with increased interstitial fibrosis that increases left ventricular stiffness and impairs (passive) diastolic function.
Importantly, all of the major components of the Renin-Angiotensin System (RAS) appear to exhibit pro-fibrotic activity, although Ang II may be the hormone most responsible for cardiac fibrosis [16]. Furthermore, there is substantial evidence that the aging heart has an augmented endogenous RAS, including ACE1 and ATR-1 in old rats [17, 18] and cardiac Ang II levels in old mice [19] and rats [20]. Hydroxyproline levels, a marker for collagen content, have been known for many years to be elevated in the old rat heart [21]. Basso recently showed that six months of ACE inhibition or AT receptor blockade inhibited the tripling of fibrosis reached by 18 months in the Wistar rat [22]. Only four weeks of candesartan was needed to reduce age-related fibrosis [23]. Similar data in the mouse (increased fibrosis with age that is prevented with ACE1 or ATR blockers) were provided by Inserra [24]. The aging heart appears to be sensitive to pathologic effects of the RAS ligands, primarily Ang II, produced locally by ACE1.
In this report, we examined changes in diastolic function in C57BL/6 mice as they aged from 3 months to almost 3 years utilizing Doppler and two-dimensional echocardiography. Increases in left atrial size and altered left ventricular filling correlated with increased interstitial fibrosis; both progressed throughout the animal's life. There was also a progressive increase in induction of MCP-1, IL-4, and IL-13 that correlated both temporally and quantitatively with the fibrosis, cellular procollagen and diastolic dysfunction. In vitro studies demonstrated that IL-13 was effectively obligate for monocyte-fibroblast transformation. Thus, we quantitated the presence of CD45+ fibroblasts in the aging myocardium using flow cytometry. The presence of CD45+ fibroblasts in the heart correlated temporally and quantitatively with myocardial fibrosis and chemokine/lymphokine induction over the 13–30 month period. Importantly, the majority of the myeloid-derived fibroblasts contained procollagen and so were actively synthesizing collagen type I. These findings suggest that age-associated interstitial fibrosis and the associated diastolic dysfunction are consequences of immunoinflammatory dysregulation.
METHODS
Animals
C57BL/6 male wild-type (WT) mice of varying age were obtained from NIA (13–30 months of age) or from the barrier facility of Baylor College of Medicine Center for Comparative Medicine (3 months of age). All mice were fed standard mouse chow and water ad libitum. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All animals were treated in accordance with the guidelines of the Baylor College of Medicine Animal Care and Research Advisory Committee. Mice used for the various studies were grouped into different age ranges. For example, the aged groups included animals 13–16 months of age and animals 20–24 months of age. The specific age of the animals used for a particular study was always indicated in the text.
Protein Microarray
Protein was isolated from snap-frozen whole hearts using Cell Disruption Buffer from the Paris Kit (Ambion, Austin, TX) with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, Rockford, IL). Protein (250 μg) from each of three young (3 month) and three aged (30 month) hearts was loaded onto mouse cytokine antibody array 1 membranes (RayBiotech, Inc., Norcross, GA). Membranes were processed according to manufacturer's instructions, images on film were scanned, and densitometry was assessed by ImageJ software. Data are expressed as the mean ± SE of the signal compared with background chemiluminescence. A representative membrane is shown in Fig. S1.
Immunohistochemistry
Hearts were perfused with ZnCl/acetate-tris fixative [25] for 15 minutes and left in fixative for a total of 4 hours before dehydration and embedding in paraffin. Sections (5 μm) were deparaffinized and processed using Vectastain Elite ABC kits with DAB substrate and nickel (Vector Laboratories, Burlingame, CA). The primary antibody was an affinity-purified rabbit anti-collagen type I (Rockland Immunochemicals, Gilbertsville, PA). The negative control was a rabbit monoclonal antibody (DA1E) against an irrelevant antigen (Cell Signaling Technology, Beverly, MA). All sections were processed together and for the same length of time in substrate.
Quantitative PCR (q-PCR)
Total RNA was isolated from whole hearts with TRizol reagent (Invitrogen), purified by RNeasy kit (Qiagen) and transcribed to cDNA by iScript cDNA Synthesis kit (Bio-Rad). Q-PCR was performed on an iQ5 Multicolor Real Time PCR Detection System (Bio-Rad) using SYBR Green Super mix (Bio-Rad) and specific primers. Gene expression was measured by the comparative CT method to calculate the amount of target mRNA normalized to an endogenous reference (18S). The data were expressed as the fold of mRNA level relative to mRNA expression detected in 3 month old hearts. Each sample was tested in triplicate to assure reproducibility.
Primer sequences were designed using Beacon Designer software version 7 (Premier Biosoft, Palo Alto, CA):
ACE1: sense 5'-GCACTCTCCGTCTCTACC-3' and antisense 5'-AGCAGGTAATTGATGTCACTC;
ATR1a: sense: 5'-GAGAACACCAATATCACT-3' and antisense 5'-GATTAGGAAAGGGAACAG-3';
ATR2: sense 5'-TGTGATGGCTTGTCTATCCTC -3', and antisense 5'-ATTCACACCTAAGTATTCAATG -3';
IL-13: sense 5'-GTAGCCCACTTTATAACA-3' and antisense 5'-GATGGTCTCTCCTCATTA-3';
MCP-1: sense 5'-TCCACAACCACCTCAAGCACTTC-3'and antisense 5'-GGCATCACAGTCCGAGTCACAC-3',
18S RNA: sense 5'-ACCGCAGCTAGGAATAATGGA-3' and antisense 5'-GCCTCAGTTCCGAAAACCA-3',
Cell Isolation
Mice were anesthetized via isoflurane inhalation and sacrificed. Hearts were immediately excised, washed to remove excess blood, and finely minced with a scalpel. The minced tissue was then digested with buffered Liberase IV (Roche Applied Science, Indianapolis, IN) with regular trituration by pipet to obtain a single cell suspension. Cell suspensions were filtered to remove large debris, washed with cold HBSS supplemented with taurine and HEPES and stained for immunofluorescence [26].
Immunofluorescence and Flow Cytometry
Cells were incubated with antibodies to CD45-PE (clone 30-F11, BD Pharmingen, San Jose, CA) or the appropriate isotype controls followed by streptavidin-PE-Cy5 (Beckman Coulter). 50 nM calceinAM (which is taken up and forms a fluorescent green product only in metabolically active cells) was added to some samples to delineate living cells before they were analyzed by cytometry. Samples to be analyzed for internal antigens were fixed in 2% paraformaldehyde with 0.1% saponin then incubated with either an antibody to collagen type I (Rockland Immunochemicals, Gilbertsville, PA) or rabbit IgG followed by an anti-rabbit secondary conjugated to AlexaFluor 488 (Molecular Probes, Eugene, OR). Cells were analyzed on a Cell Lab Quanta SC flow cytometer (Beckman Coulter) using the Quanta Analysis software.
Transendothelial Migration
Human cardiac microvascular endothelial cells were obtained from Lonza (Walkersville, MD), grown as directed by the supplier and used at passages 2–8. Migration assays were performed as previously described [27]. Briefly, endothelial cells were grown on collagen type IV-coated inserts, and mononuclear leukocytes were added. MCP-1 was used as a chemoattractant and other mediators were added as indicated. After 4 days, the number of fibroblasts in the bottom of the wells was counted. Normal donor blood was obtained from human volunteers under a protocol approved by the Institutional Review Board of Baylor College of Medicine. The investigation conformed with the principles outlined in the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMC) were isolated as described previously. Cells harvested from the interface of a Ficol-Hypaque gradient were washed, counted, and aliquots frozen in liquid nitrogen until use in an assay. Each donor's cells were measured in triplicate. Due to differences in absolute numbers of fibroblasts between donors, the number of cells in the experimental well was divided by the number in the corresponding control well (diluent) to normalize the data. Human recombinant proteins (IL-12 and IL-13) and chimeric constructs (IL-13 Rα2/Fc and IL-11 Rα/Fc) were purchased from R&D Systems (Minneapolis, MN).
Doppler and Two-dimensional Echocardiography
B-mode and M-mode images were acquired using a Vevo 770 RMV-707B 30-MHz probe (VisualSonics, Toronto, Canada). To collect the mitral inflow and aortic outflow Doppler measurements for the C57BL/6 mice, a Doppler signal processing workstation (DSPW, Indus Instruments, Houston, TX) was employed using a 10-MHz (1 mm diameter) pulsed Doppler probe.
Ultrasound Acquisition Procedure
Mice were anesthetized in a closed chamber with 1.5% isoflurane in oxygen at 2 L/min. When the mice were recumbent, they were removed, weighed and body fur was completely shaved from the anterior thorax and upper abdominal area and acoustic coupling gel was applied. They were taped in the supine position to a temperature-controlled laminated plastic board with copper electrodes placed appropriately so that all four limb leads could be used for electrocardiographic monitoring and were maintained with 1% isoflurane in oxygen by nose cone.
Quantitation of LV function and structure was done with two-dimensionally guided M-mode images from parasternal short-axis views at midpapillary level. Left atrial dimensions were collected to obtain the chamber volume. The mediolateral (D2) and superoinferior dimensions (L) were assessed in parasternal short-axis and four-chamber views, respectively, using ECG-based Kilohertz Visualization (EKV). EKV reconstructs one representative heart cycle that is spatially precise and synchronized to the animal's ECG, and allows visualizing the image in slow motion and detecting the largest left atrial dimension. This is particularly useful in the mouse due to its fast heart rate. The anteroposterior (D1) dimension of the left atrium was measured with M-mode in the parasternal long-axis view. The left atrial volume was calculated using the formula for a prolate ellipse: LA Volume = (4π × D1 × D2 × L)/(3×2×2×2).
Using the above methods, we confirmed and extended our studies of diastolic dysfunction in the aging mouse. Over the period from 3 to 24 months, there were significant decreases in Peak E filling velocity and increases in iso-volumic relaxation time. After 24 months of age, both of these parameters changed in direction as the older heart gets even stiffer (Fig. 1A and B). By contrast, the left atrial volume increased monotonically as left ventricular filling pressures increased (Fig. 1C). These changes were not accompanied by any significant changes in ejection fraction (51±3, 52±2, 59±3, and 55±2 at 3, 13, 24, and 30 months of age, respectively). Thus, the population of aging mice used for the cellular and molecular studies developed a progressive reduction in diastolic function throughout their life.
Figure 1.
Noninvasive measurement of diastolic function in mice of different ages. The transmitral Peak E filling velocity decreased with age from three months to twenty four months, but the values increased in thirty month old mice (A). The isovolumic relaxation time was prolonged in the 24 month old mice, but was shorter in the 30 month old mice (B). The left atrial volume increased monotonically with age (C).
Statistics
Regression analysis was done using the linear fit function of Origin version 7.5 (OriginLab Corp., Northampton, MA). Parametric comparisons, including Student's t test were also performed with Origin software. ANOVA with Tukey's correction was performed with Primer software.
RESULTS
Interstitial Cardiac Fibrosis in the Aging Mouse
Histologic examination demonstrated a progressive increase in the density of fibrosis within the left ventricle. Fig. 2 contrasts collagen density in hearts of different ages using immunohistochemical staining for collagen type I. At 14 and 24 months there was significant interstitial fibrosis between the large bundles of cardiac myocytes without evidence of patches of fibrosis. By 30 months, the fibrosis engulfed virtually every myofibrillar bundle and the staining was denser.
Figure 2.
Immunohistochemistry of collagen type I in hearts from mice of different ages: 3 (A), 14 (B), 24 (C), and 30 month (D) at 20× magnification, and 3 (E), 14 (F), 24 (G) and 30 month (H) at 40× magnification.
Interstitial Fibrosis in the Aging Mouse is Associated with Increased Levels of CD45+ Fibroblasts
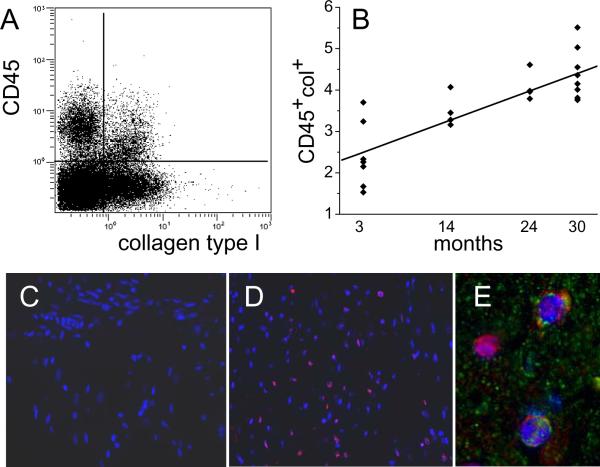
Our previous studies with repeated brief daily ischemic episodes leading to a fibrotic cardiomyopathy [26–30] and Ang II infusion [31] demonstrate a clear relationship between interstitial fibrosis and the appearance of CD45+ fibroblasts of myeloid origin. Since Ang II is causally associated with fibrosis [31], including aging models [32], we postulated that CD45+ fibroblasts may play an important part in the cardiac dysfunction in the aging mouse model. Freshly-isolated cardiac cells were examined by flow cytometry for the presence of CD45+ fibroblasts. In Fig. 3A is shown a representative flow cytometry histogram of CD45+ cells that also had internal stores of collagen type I, indicating their fibroblast phenotype. In Fig. 3B we show that the percent of viable non-cardiomyocytes that expressed these two markers (double positives) increased with age. In contrast to the lack of CD45+ cells in the 3 month old heart assessed by immunofluorescence (Fig. 3C), there is a uniform distribution of those cells in the old heart (Fig. 3D). CD45+ cells were also positive for procollagen type I (Fig. 3E).
Figure 3.
Expression of collagen type I in CD45+ cells. Nonmyocytes were isolated from hearts of different ages. (A) Flow cytometry of those cells demonstrated that a proportion of the nonmyocytes expressed both CD45 (hematopoietic marker) and collagen type I (fibroblast marker) as seen in the upper right quadrant (cells isolated from a 14 month old animal). (B) The proportion of the nonmyocytes that were double positives (CD45 and collagen type I) increased with age. Linear regression R2= 0.66 and p=0.0001 (n=7, 4, 4, and 9 for 3, 14, 24, and 30 months). Immunofluorescence in paraffin sections of hearts with antibodies to CD45 (red) was negative in 3 month old hearts (C) and positive with a random distribution in 14 month old hearts (D). (E) Some CD45+ cells were also positive for procollagen type I (green). Cell nuclei are DAPI stained (blue).
Aging mice Demonstrate Increasing Rates of Production of Collagen Type I Indicated by Procollagen Type I
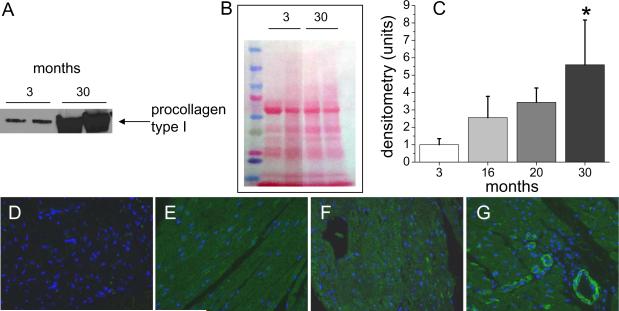
Collagen type I is synthesized as a precursor molecule (procollagen) that undergoes posttranslational modification prior to secretion. Secreted mature collagen is deposited into the tissue matrix (as evaluated by immunohistochemical staining, see Fig. 2), but procollagen is found inside cells that are actively producing this molecule. To assess the rates of synthesis of collagen type I in the aging heart, we isolated whole cell lysates from young (3 month old) and aged (30 month old) mice and analyzed them for procollagen type I expression. We found that 30 month old mice had a 5 fold increase in cardiac expression of procollagen type I compared with 3 month old animals (Fig. 4A and C). These changes progressed with age; middle aged mice (16 month old) had a >2 fold elevated expression compared to young animals (Fig. 4C and Fig. S2). Since some protein expression (i.e. lamin A, GAPDH, actin; all used as loading indicators) is affected by aging (data not shown), we included photographs of membranes stained with Ponceau S with our Western blot results to assure equal protein loading (Fig. 4B, Fig. S2).
Figure 4.
Expression of procollagen type I in the aging heart. Protein extracts from whole hearts of different ages were analyzed for the presence of procollagen to indicate active collagen synthesis. (A) is an immunoblot of 3 month-old versus 30 month old hearts, (B) is the Ponceau stain of the membrane to ensure equal protein loading of the gel, and (C) is the densitometric analysis of multiple blots from hearts of the indicated ages (n=4). Immunofluorescence of paraffin sections of hearts of different ages indicates that there is no visible procollagen type I (green) in young hearts (D). Procollagen type I is visible at 14 months (E) and 24 months (F) in fibroblasts, and in blood vessel walls at 24 months and 30 months (G). Cell nuclei are DAPI stained (blue).
These data were confirmed by immunofluorescence staining for procollagen type I in heart sections (Fig. 4 D–G). We observed augmented levels of procollagen type I throughout the heart section starting at the age of 14 months, and this developed further in older animals.
Chemokines and Cytokines Mediate Fibrosis
In considering mediators that might be required for the increase in CD45+ fibroblasts with aging, we investigated two possibilities. In our previous studies, we established that the appearance of myeloid derived fibroblasts was associated with an increase in MCP-1 [29, 31]. In C57BL/6 mice, genetic deletion of MCP-1 virtually eliminates the presence of these myeloid fibroblasts and markedly reduces interstitial fibrosis [29, 31] associated with either the repeated ischemia/reperfusion model [29] or Ang II infusion [31]. Thus, we examined the presence of cardiac MCP-1 mRNA in mice spanning ages from 3–30 months. In Fig. 5A, an increase in MCP-1 followed a time course very similar to those of interstitial fibrosis and the appearance of CD45+ fibroblasts. The mean mRNA level in 30 month old hearts (2.19) was more than double that in young mice. To show that the increased mRNA levels were reflected in increased protein levels, we performed an array study that indicated a 2.6-fold increase in the MCP-1 protein from 3 month old hearts to 30 month old hearts (Fig. 5B). In heart tissue, there was an absence of MCP-1 immunofluorescence staining in the 3 month old heart (Fig. 5C) while there was striking positivity in venules as early as 14 months (Fig. 5D).
Figure 5.
Measurement of MCP-1 mRNA and protein in hearts from mice of different ages. (A) mRNA expression of MCP-1 by quantitative PCR, with a linear regression R2=0.56 and p=0.0004; n=5, 3, 5, and 5 for 3, 14, 24, and 30 month old animals. MCP-1 protein levels in 3 month old hearts were compared to that in 30 month old hearts by protein array (B)(n=3). Immunofluorescence of an antibody against MCP-1 (green) in hearts from different ages showed no visible staining in 3 month old hearts (C), whereas there was staining in the blood vessels of all other ages (D, 14 month old heart). Cell nuclei are DAPI stained (blue).
Also dependent on MCP-1 expression is the cytokine IL-13 [33]. It is one of the lymphokines that defines the Th2 state of T-lymphocytes, the products of which are associated with pathological fibrosis in various organs [16, 34, 35]. We therefore measured the protein levels of two Th2 cytokines, IL-4 and IL-13, and a Th1 cytokine, IFN-γ, in the aging heart. The levels of IL-4 were increased by 64% in 30 month old hearts compared to 3 month old hearts and the levels of IL-13 were increased by 28% (Fig. 6A). The levels of IFN-γ were unchanged. Because levels of IL-13 protein can be reduced by receptor internalization [36] and thereby give a falsely low value, we also investigated the levels of IL-13 mRNA. We found an age-dependent increase in IL-13 mRNA in the mouse heart (Fig. 6B), with the mean value (3.1) at 30 months more than three times that in young control mice. From these data we hypothesized a potential modulatory role for IL-13 in age-dependent cardiac interstitial fibrosis.
Figure 6.
Monocyte-to-fibroblast maturation after TEM in response to MCP-1. Human mononuclear leukocytes from four donors were allowed to migrate through human cardiac microvascular endothelial cells in response to 650 ng of MCP-1. The total number of fibroblasts in each well of triplicates was counted after two days of migration and two further days of maturation. (A) Data from wells treated with 10 ng/ml of either IL-13 or IL-12 were normalized to control wells with no further treatment. (B) All wells were treated with 10 ng/ml IL-13; some of those were also treated with 1 μg/ml of either an IL-13 Rα2/Fc chimera (IL13RFc) or the control construct IL-11 Rα/Fc chimera (IL11RFc) and the data normalized to the counts from the IL-13 only treated wells. An asterisk indicates which groups are statistically different from the control (p=0.01, Student's t test).
IL-13 is a Critical Modulator of Monocyte to Fibroblast Transformation In Vitro
To further examine the hypothesis that MCP-1 and IL-13 work together in establishing a fibrotic state, we used a model developed in our laboratory in which human monocytes migrate across human cardiac microvascular endothelium and mature to fibroblasts [26, 27, 30]. In our previous studies, we demonstrated that monocyte-fibroblast transformation in this model requires transendothelial migration in response to MCP-1 [26]. This in vitro model has also been shown to predict correctly the effects of FcR γ chain activation [27] and Rho-kinase-1 deletion [30] on interstitial fibrosis in an experimental ischemic cardiomyopathy model.
We investigated the influence of cytokines on the ability of human monocytes migrating through human cardiac endothelium in response to MCP-1 to mature into fibroblasts. IL-13 more than quadrupled the number of fibroblasts, while a Th1 cytokine, IL-12, reduced the number to 25% of the control (Fig. 7A). Further, the enhancement of fibroblast formation by IL-13 could be inhibited by a soluble IL-13 receptor construct and not an IL-11 receptor control construct (Fig. 7B), indicating that it was the cytokine and not a contaminant in the recombinant protein preparation that produced the effect.
Figure 7.
Measurement of cytokine mRNA and protein in hearts from mice of different ages. (A) Th2 (IL-4 and IL-13) and Th1 (IFN-γ) cytokines were measured by protein array in 3 month old versus 30 month old hearts (n=3). (B) mRNA expression of IL-13 by quantitative PCR, with a linear regression R2=0.43 and p=0.006; n=4, 3, 5, and 4 for 3, 14, 24, and 30 month old animals.
DISCUSSION
The occurrence of diastolic dysfunction in the aging heart is a significant clinical problem and has been linked to defective active relaxation as well as passive stiffening associated with increased interstitial fibrosis [7, 10–12]. The current study is the first to assess the potential role of this fibrosis as a factor in diastolic function by correlating diastolic measurements over the lifetime of the mouse with pathways that induce fibrosis. The results suggest that the fibrosis is present at the end of the first year of life and progresses throughout a mouse's lifetime up to 30 months. The finding that these changes were apparent at the end of the first year is in agreement with our previous studies and in other laboratories demonstrating a marked increase in severity of adverse remodeling subsequent to myocardial infarction in mouse models [6, 37, 38]. In the current study, the progressive fibrosis and diastolic dysfunction observed throughout this period both correlate (temporally and quantitatively) with the progressive appearance of cardiac fibroblasts that are CD45+. In our previous studies [26, 31] we demonstrated that these CD45+ cells are of myeloid origin and are common to other mouse models of interstitial fibrosis induced by repeated brief ischemic episodes [26] and by Ang II infusion [31]. In the latter two models, there is a coincident induction of MCP-1 which is essentially obligate for the occurrence of fibrosis (see below).
Immune Dysregulation in Cardiac Interstitial Fibrosis
MCP-1 is a primary chemoattractant for monocytes [39]. We postulated that MCP-1 was responsible for recruiting the myeloid cells that ultimately convert to fibroblasts in the old heart. In our in vitro model in which human monocytes transmigrate across a human cardiac microvascular endothelial barrier in response to MCP-1, a population of CD45+ monocytes became spindle-shaped fibroblasts similar to the fibroblasts found associated with interstitial fibrosis in the mouse heart [26, 31]. It is important to emphasize that CD45 is not found on resident cardiac fibroblasts; CD45 is considered a canonical marker for leukocytes and their hematopoietic progenitors [40]. Furthermore, it is only after transendothelial migration that the monocyte-fibroblast transformation occurs in this model [26]. Subsequent experiments have demonstrated that this model correctly predicts and correlates with interventions that alter the interstitial fibrosis after repeated ischemic episodes in the mouse [26, 30].
Our studies suggest that interstitial fibrosis in the aging heart arises from a progressive dysregulation of immune and inflammatory responses over the mouse lifetime. In the repeated ischemia model, daily (non-infarctive) occlusions of 15 minutes resulted in an intense interstitial fibrosis associated with induction of MCP-1 but no other inflammatory or chemokinetic cytokine [28]. This could be blocked by overexpression of Extracellular Superoxide Dismutase (EC-SOD), attenuating oxygen free radical generation and likewise preventing elevation of MCP-1 [28]. Fibrosis was also markedly inhibited in mice with genetic deletion of MCP-1 (MCP-1−/−) [29]. We then studied the effects of continuous infusion of Ang II leading to hypertension, left ventricular hypertrophy and dilatation, and interstitial fibrosis. Ang II was not only associated with elevations of MCP-1, but also with an augmented inflammatory response [31]. Critically, Ang II infusion was accompanied by an identical amount of hypertrophy and hypertension in the MCP-1−/− mice; by contrast, interstitial fibrosis was virtually eliminated [31]. Thus, while Ang II and downstream immunoregulatory responses [41–44] have many more potential effects that might stimulate fibrosis, the initial interstitial fibrosis response requires MCP-1 induction as well as transmigration to the interstitium of the heart of CD45+ cells that mature to fibroblasts [31].
MCP-1 Response in the Aging Heart
The data demonstrate a time-dependent increase in MCP-1 associated with the aging heart after the first year. The coincident correlation of interstitial fibrosis, MCP-1 induction and myeloid-derived fibroblasts certainly suggests that dysregulation of MCP-1 plays a critical role in the initiation of interstitial fibrosis in aging much as it does in experimental ischemic cardiomyopathy [29] and Ang II infusion [31]. It is important to recognize that this study only demonstrates the essential nature of this initial step and does not delineate all of the important factors that may result in a chronic progressive fibrosis. Moreover, in the Ang II and repetitive ischemia models, interstitial fibrosis arose relatively acutely and peaked after several weeks without further worsening despite continuing stimulation [28, 31]. MCP-1 induction and fibrosis disappeared after four weeks despite continued ischemia [28] and MCP-1 and CD45+ fibroblasts disappeared after two weeks of Ang II infusion [31]. By contrast, interstitial fibrosis in aging was progressive in our current studies and was associated both quantitatively and temporally with continued progressive increases in MCP-1, Th2 cytokines and myeloid-derived fibroblasts (Figs. 3 – 5). Also, unlike the acute models, other cells such as structural fibroblasts contributed to the interstitial fibrosis in aging. We speculate that part of the dysregulation in aging results from the failure to suppress this system once activated, thus allowing it to advance on a chronic basis. Future studies contrasting the acute models in aging will attempt to delineate the mechanisms of this inflammatory dysregulation.
IL-13 and the Aging Heart
One of the intriguing findings in our studies was the time-dependent increase of the Th2 cytokine IL-13 (Fig. 6B). IL-13 is profibrotic in vivo in other organs [34, 35]. It has also been reported to enhance human monocyte-to-fibrocyte maturation [45]. In the studies reported here, we confirm that human monocytes migrating through human cardiac microvascular endothelial cells in response to MCP-1 could still be influenced by Th1 (IL-12) versus Th2 (IL-13) cytokines in their ability to mature into fibroblasts. IL-13 markedly increased, and IL-12 decreased that transition (Fig. 7).
Age-associated changes in immune function have been associated with a shift from Th1 (IL-12, IFN-γ) to Th2 (IL-13, IL-4) cytokines in some animal studies [46], and not in others [47, 48]. A review of 60 studies in 2002 [49] concluded that the data were too incomplete to draw similar conclusions about a Th1 to Th2 shift in humans. Since that time, however, more studies have continued to point to impaired Th1 and enhanced Th2 responses in human aging [50, 51]. Our current finding of an age-related increase in cardiac IL-13 and IL-4 indicates that an increase in the local Th2 immune response may contribute to the increasing fibrosis seen in the aging mouse heart (Fig. 6).
It is important to note that subsets of T lymphocytes respond to MCP-1 as well [52]. In fact, genetic deletion of MCP-1 prevents mounting a Th2 response [33]. In our study, we propose the converse, wherein local increases in MCP-1 enable the increases in the profibrotic cytokine, IL-13, likely by attracting an MCP-1 responsive T lymphocyte that secretes the lymphokine.
Age-dependent Diastolic Dysfunction
We have examined the role of immunoinflammatory dysregulation in interstitial fibrosis and diastolic dysfunction in the aging mouse heart. The data suggest that this condition advances over a period of almost two years in the life of a C57BL/6 mouse and is marked by progressive increases in the left atrial volume (Fig. 1). The temporal and quantitative correlation between interstitial fibrosis, immune-inflammatory dysregulation, and anatomical changes suggests these are important factors in producing diastolic dysfunction. The etiology of age-associated changes in the heart has been studied in multiple models [6, 7, 9, 13–15]. Studies have suggested roles for reactive oxygen [19] and the RAS [24, 53] paralleling the effectors studied in our acute models of reactive interstitial fibrosis [28, 31]. Importantly, several studies, done primarily in the rat, suggest that the local or intrinsic RAS is activated in the aging heart [17, 18, 22, 23, 53]. A recent study in mice utilized mass spectroscopy to demonstrate a significant augmentation of local Ang II in the aging mouse heart [19]. We also found a progressive increase in the endogenous cardiac RAS as measured by ACE1 and AT1 mRNA levels in the mouse (Fig. S3A and B). By contrast, there was no induction of mRNA for the AT2 receptor, which is associated with cardiac protection (Fig. S3C) [54]. This concurs with the literature above but is also pertinent to the role of MCP-1 in the generation of fibrosis with Ang II infusion described by us [31].
CONCLUSION
In summary, we report a temporal and quantitative relationship of diastolic dysfunction in the aging mouse to immunoinflammatory dysregulation and cardiac interstitial fibrosis. To do this effectively we examined each parameter as a function of aging. While the immunoinflammatory mediators studied were suggested by acute models of fibrosis, the quantitative and qualitative changes are remarkably similar to those seen in aging, postulating a causative role for MCP-1 dependent attraction of CD45+ fibroblast precursors of myeloid origin in the old heart. In addition, we extend our characterization of this response to the Th2 lymphokines; Th2 responses in aging have not previously been demonstrated in the heart; MCP-1 has been shown to be an important mediator of Th2 phenotypes [33].
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Sandra Haudek for materials, Dorellyn Lee and Thuy Pham for excellent technical assistance, and Sharon Malinowski for manuscript preparation.
SOURCES OF FUNDING This work was supported by the NIH (RO1HL076661 and RO1HL089792).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES None
REFERENCES
- [1].Vanoverschelde JJ, Essamri B, Vanbutsele R, d'Hondt A, Cosyns JR, Detry JR, et al. Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J Appl Physiol. 1993;74:2225–33. doi: 10.1152/jappl.1993.74.5.2225. [DOI] [PubMed] [Google Scholar]
- [2].Luchi RJ, Taffet GE, Teasdale TA. Congestive heart failure in the elderly. J Am Geriatr Soc. 1991;39:810–25. doi: 10.1111/j.1532-5415.1991.tb02705.x. [DOI] [PubMed] [Google Scholar]
- [3].Taffet GE, Teasdale TA, Bleyer AJ, Kutka NJ, Luchi RJ. Survival of elderly men with congestive heart failure. Age Ageing. 1992;21:49–55. doi: 10.1093/ageing/21.1.49. [DOI] [PubMed] [Google Scholar]
- [4].Lindenfeld J, Krause-Steinrauf H, Salerno J. Where are all the women with heart failure? J Am Coll Cardiol. 1997;30:1417–9. doi: 10.1016/s0735-1097(97)00343-4. [DOI] [PubMed] [Google Scholar]
- [5].Ezekowitz JA, Lee DS, Tu JV, Newman AM, McAlister FA. Comparison of one-year outcome (death and rehospitalization) in hospitalized heart failure patients with left ventricular ejection fraction >50% versus those with ejection fraction <50% Am J Cardiol. 2008;102:79–83. doi: 10.1016/j.amjcard.2008.02.102. [DOI] [PubMed] [Google Scholar]
- [6].Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–61. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- [7].Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol. 1990;258:H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- [8].Lakatta E. Aging: Handbook of Physiology. American Physiological Society; New York: 1995. Cardiovascular System. In: Masoro E, editor. [Google Scholar]
- [9].Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci. 1997;52:B285–B290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- [10].Lim CC, Liao R, Varma N, Apstein CS. Impaired lusitropy-frequency in the aging mouse: role of Ca(2+)-handling proteins and effects of isoproterenol. Am J Physiol. 1999;277:H2083–H2090. doi: 10.1152/ajpheart.1999.277.5.H2083. [DOI] [PubMed] [Google Scholar]
- [11].Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–8. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- [12].Rozenberg S, Tavernier B, Riou B, Swynghedauw B, Page CL, Boucher F, et al. Severe impairment of ventricular compliance accounts for advanced age-associated hemodynamic dysfunction in rats. Exp Gerontol. 2006;41:289–95. doi: 10.1016/j.exger.2005.11.009. [DOI] [PubMed] [Google Scholar]
- [13].Alwardt CM, Yu Q, Brooks HL, McReynolds MR, Vazquez R, Watson RR, et al. Comparative effects of dehydroepiandrosterone sulfate on ventricular diastolic function with young and aged female mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R251–R256. doi: 10.1152/ajpregu.00272.2005. [DOI] [PubMed] [Google Scholar]
- [14].Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, et al. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000;97:2809–13. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–95. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- [16].Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao XJ, Li YF. Alteration of messenger RNA and protein levels of cardiac alpha(1)-adrenergic receptor and angiotensin II receptor subtypes during aging in rats. Can J Cardiol. 2009;25:415–20. doi: 10.1016/s0828-282x(09)70509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heymes C, Swynghedauw B, Chevalier B. Activation of angiotensinogen and angiotensin-converting enzyme gene expression in the left ventricle of senescent rats. Circulation. 1994;90:1328–33. doi: 10.1161/01.cir.90.3.1328. [DOI] [PubMed] [Google Scholar]
- [19].Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–97. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- [21].Tomanek RJ, Taunton CA, Liskop KS. Relationship between age, chronic exercise, and connective tissue of the heart. J Gerontol. 1972;27:33–8. doi: 10.1093/geronj/27.1.33. [DOI] [PubMed] [Google Scholar]
- [22].Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- [23].Jones ES, Black MJ, Widdop RE. Angiotensin AT2 receptor contributes to cardiovascular remodelling of aged rats during chronic AT1 receptor blockade. J Mol Cell Cardiol. 2004;37:1023–30. doi: 10.1016/j.yjmcc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- [24].Inserra F, Romano L, Ercole L, de Cavanagh EM, Ferder L. Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension. 1995;25:437–42. doi: 10.1161/01.hyp.25.3.437. [DOI] [PubMed] [Google Scholar]
- [25].Beckstead JH. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem. 1994;42:1127–34. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- [26].Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci U S A. 2008;105:10179–84. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proceedings of the National Academy of Sciences, USA. 2003;100:2700–5. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–92. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- [30].Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, et al. Rho Kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovascular Research. 2009;83:511–8. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stein M, Boulaksil M, Jansen JA, Herold E, Noorman M, Joles JA, et al. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol. 2010;299:H310–H321. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- [33].Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- [34].Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–91. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- [35].Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- [36].Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–9. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- [37].Gould KE, Taffet GE, Michael LH, Christie RM, Konkol DL, Pocius JS, et al. Heart failure and greater infarct expansion in middle-aged mice: a relevant model for postinfarction failure. Am J Physiol Heart Circ Physiol. 2002;282:H615–H621. doi: 10.1152/ajpheart.00206.2001. [DOI] [PubMed] [Google Scholar]
- [38].Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–92. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Leonard EJ, Skeel A, Yoshimura T. Biological aspects of monocyte chemoattractant protein-1 (MCP-1) Adv Exp Med Biol. 1991;305:57–64. doi: 10.1007/978-1-4684-6009-4_7. [DOI] [PubMed] [Google Scholar]
- [40].Saunders AE, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 2010;22:339–48. doi: 10.1016/j.cellsig.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [41].Westermann D, Riad A, Richter U, Jager S, Savvatis K, Schuchardt M, et al. Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol. 2009;104:499–509. doi: 10.1007/s00395-009-0014-6. [DOI] [PubMed] [Google Scholar]
- [42].Van LS, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103:319–27. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- [43].Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol. 2008;103:60–8. doi: 10.1007/s00395-007-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Heusch G. Diastolic heart failure: a misNOmer. Basic Res Cardiol. 2009;104:465–7. doi: 10.1007/s00395-009-0025-3. [DOI] [PubMed] [Google Scholar]
- [45].Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–33. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94:1–5. doi: 10.1016/s0047-6374(96)01849-0. [DOI] [PubMed] [Google Scholar]
- [47].Li SP, Miller RA. Age-associated decline in IL-4 production by murine T lymphocytes in extended culture. Cell Immunol. 1993;151:187–95. doi: 10.1006/cimm.1993.1230. [DOI] [PubMed] [Google Scholar]
- [48].Kovacs EJ, Duffner LA, Plackett TP. Immunosuppression after injury in aged mice is associated with a TH1-TH2 shift, which can be restored by estrogen treatment. Mech Ageing Dev. 2004;125:121–3. doi: 10.1016/j.mad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [49].Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–90. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- [50].Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–46. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- [51].Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719–32. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- [52].Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–6. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- [53].Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, Rosario Lores AM, et al. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128:247–52. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- [54].Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.