Abstract
Multiple sclerosis (MS) is a severely debilitating neurodegenerative diseases marked by progressive demyelination and axonal degeneration in the CNS. Although inflammation is the major pathology of MS, the mechanism by which it occurs is not completely clear. The primary symptoms of MS are movement difficulties caused by conduction block resulting from the demyelination of axons. The possible mechanism of functional loss is believed to be the exposure of potassium channels and increase of outward current leading to conduction failure. 4-aminopyridine (4-AP), a well-known potassium channel blocker, has been shown to enhance conduction in injured and demyelinated axons. However, 4-AP has a narrow therapeutic range in clinical application. Recently, we developed a new fast potassium channel blocker, 4-Aminopyridine-3-Methanol (4-AP-3-MeOH). This novel 4-AP derivative is capable of restoring impulse conduction in ex vivo injured spinal cord without compromising the ability of axons to follow multiple stimuli. In the current study, we investigated whether 4-AP-3-MeOH can enhance impulse conduction in an animal model of MS. Our results showed that 4-AP-3-MeOH can significantly increase axonal conduction in ex vivo experimental autoimmune encephalomyelitis mouse spinal cord.
Keywords: Multiple sclerosis, EAE, compound action potential, conduction block, myelin damage, potassium channel
Introduction
It is well documented that myelin damage could expose potassium channels at juxtaparanodal regions which are covered by myelin in healthy axons (Chiu and Ritchie, 1980; Sherratt et al., 1980; Haghighi et al., 1995; Nashmi et al.. 2000; Karimi-Abdolrezaee et al., 2004;. The potassium efflux through fast potassium channels is believed to cause axonal conduction block by preventing sufficient depolarization and initiation of the action potential at the node of Ranvier. Consistent with this notion, 4-AP, a known potassium channel blocker, has been shown to restore action potential conduction in spinal cord injury where demylination is a major pathological phenomenon. The significance of 4-AP-mediated axonal conduction restoration was further highlighted by the recent approval of this drug by the FDA to improve walking in multiple sclerosis (MS) patients (Ampyra, Acorda Therapeutics, Inc.).
However, 4-AP has a narrow therapeutic range with side effects including dizziness, nausea, and seizures . In an effort to produce an alternative therapy that targets the fast potassium channels, we have developed a new potassium channel blocker, 4-aminopyridine-3-methanol (4-AP-3-MeOH). This compound has been shown to block fast potassium channels and restore impulse conduction in mechanically-injured spinal cord where myelin damage is evident. Interestingly, the lowest effective concentration of 4-AP-3-MeOH in stretched spinal cords is between 0.01 µM and 0.1 µM and the lowest effective concentration of 4-AP in compression injury is between 0.1 µM and 1 µM. However, 4-AP-3-MeOH has not been examined in animal models of MS where myelin damage is caused by non traumatic biochemical events . In the current study, we plan to determine whether 4-AP-3-MeOH can enhance axonal conduction in the EAE mouse, an animal model of MS with diffuse myelin damage and associated motor deficits. Our study demonstrated that 4-AP-3-MeOH can significantly enhance axonal conduction in ex vivo EAE mouse spinal cord.
Experimental Procedures
EAE mice were established using an MOG35–55/CFA emulsion kit followed by pertussis toxin injections (EK-0115, Hooke Laboratories). The emulsion was injected subcutaneously in the back of C57BL/6 mice that were 9 – 10 weeks old. An intraperitoneal injection of pertussis toxin was administered after the emulsion and a second dose of pertussis toxin followed 22 – 24 hours later. The behavior of the mice was assessed daily for 30 days and the symptoms of EAE began to emerge approximately 12 – 15 days after the emulsion injection. The average score of EAE mice used in this study was 2.2 ± 0.5 (n = 12), based on a well established 5-point behavioral scoring system. The 5-point scale begins at 0 which represents a healthy animal without any deficit. The symptoms will progress from a limp tail to weakness in the hind limbs and then finally hind limb paralysis. These experiments were approved by the Purdue Animal Care and Use Committee, West Lafayette, Indiana.
After confirmation of the behavioral deficits, the mice were anesthetized with a combination of Ketamine (90 mg/kg) and Xylazine (10 mg/kg) and then perfused with a cold, oxygenated Krebs’ solution. The spinal column was quickly removed from the body and the spinal cord then isolated as previously described. The samples were incubated in continuously oxygenated Krebs’ solution before recording. Whole mouse spinal cord (around 3.5 cm long) was placed into the double sucrose-gap recording chamber, allowing the white matter tracts to extend across the apparatus.
The amplitude of the compound action potential (CAP) was recorded from isolated EAE mouse spinal cord using a double sucrose-gap recording system which is similar to that described previously. Baseline CAP measurements of EAE spinal cord were recorded before applying 4-AP-3-MeOH. 100 µM of fresh 4-AP-3-MeOH was then applied through Krebs’ circulation in the central well to the spinal cord. Lastly, the spinal cord was then washed with Krebs’ solution. The temperature was maintained at 37°C.
For immunofluorescence staining, mice were perfusion fixed and then the whole spinal cord was extracted as previously mentioned . Three EAE mice were used as well as three control mice. Subsequently, 15 µm spinal cord cross sections were cut and labeled with Fluoromyelin (Invitrogen, CA) and Anti-Neurofilament 200 antibody produced in rabbit (dilution ratio 1:150, Sigma-Aldrich). Alexa Fluor® 488 goat anti-rabbit IgG (H+L) was used as a secondary antibody (dilution ratio 1:100, Invitrogen, CA).
Results
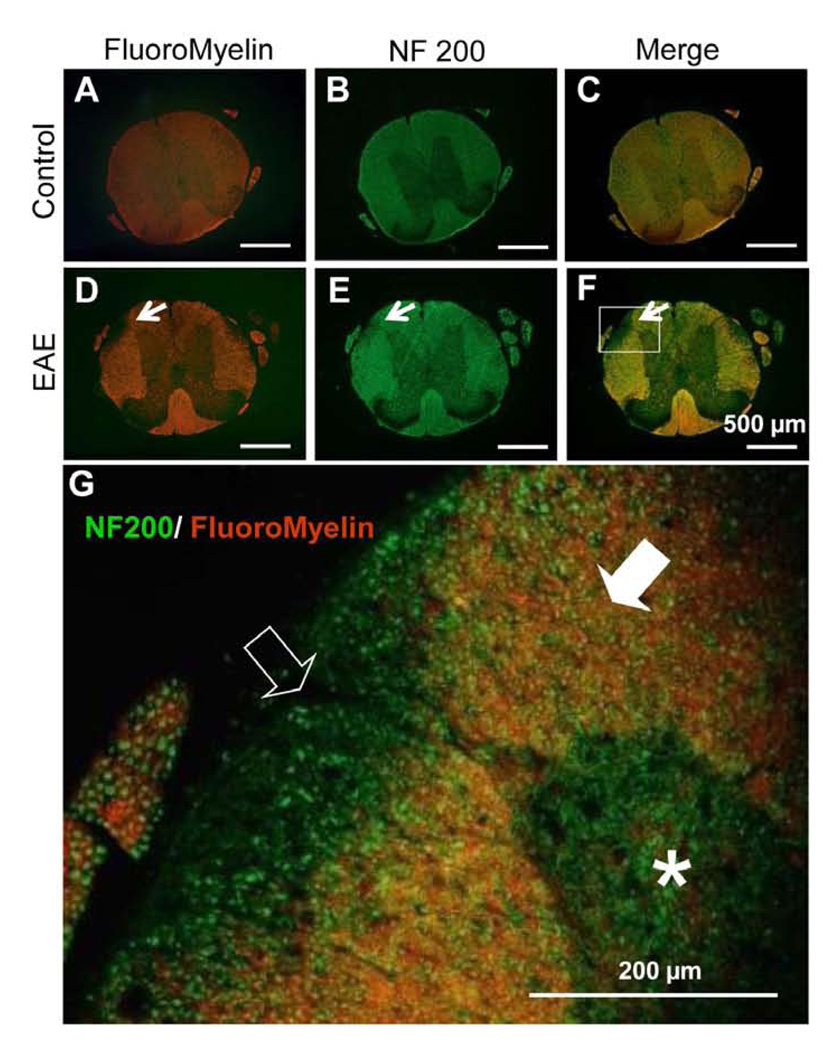
Fluoromyelin (myelin sheath) and anti-NF200 (axons) were used to define areas of demyelination in spinal cord cross sections at the thoracic level. Areas with low or no Fluoromyelin were noted in EAE mice, indicating the region of demyelination, typical of multiple sclerosis. No noticeable demyelinated areas were found throughout the thoracic region in control cords (Fig. 1).
Figure 1.
Images of thoracic cross sections of spinal cords from control (A-C) and EAE (D-F) mice labeled with fluoromyelin (red) and anti-NF200 (green). No noticeable demyelinated areas were found in the white matter of control mice spinal cord (A – C). In EAE mouse spinal cord sections, a typical demyelinated region in the white matter is shown (arrow) (D - F). G. A photomicrograph displays the higher magnification of the region circumscribed in F. Note that many axons are labeled without corresponding myelin staining. The open arrow indicates area where axons lack of myelin and filled arrow denotes area of axons with normal myelin appearance. Asterisk indicates gray matter of spinal cord. Scale bar = 500 µm (A - F); 200µm (G).
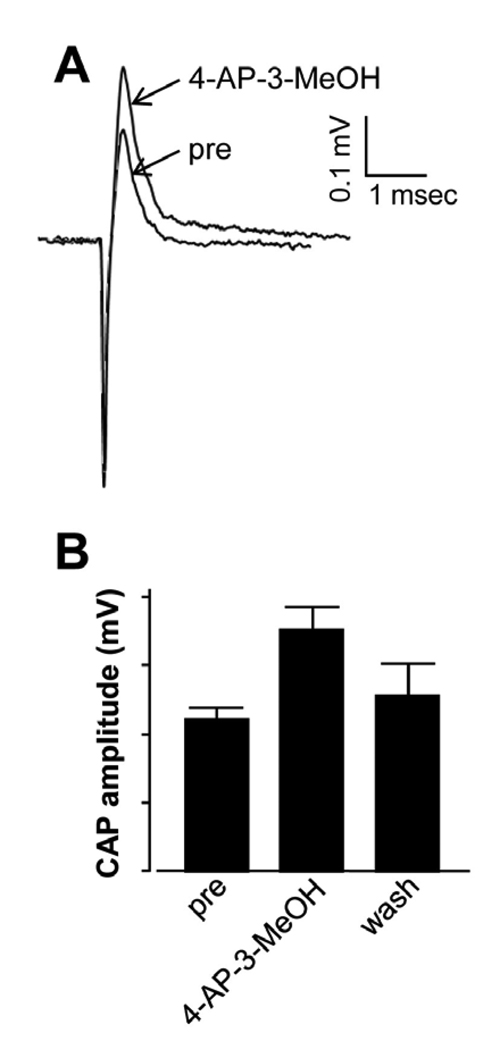
The effect of 4-AP-3-MeOH on axonal conduction in EAE mice was examined using extracted spinal cord placed in a double sucrose-gap recording chamber. The CAP amplitude before drug treatment was 0.11 ± 0.01 mV (n = 5). 4-AP-3-MeOH at 100 µM significantly improved CAP to 0.17 ± 0.02 mV following a 45 min. application, representing a 55% increase in amplitude (P < 0.05, n = 5). The CAP amplitude returned to 0.13 ± 0.02 mV following wash for 45 – 60 minutes (Fig. 2B). In contrast, 4-AP-3-MeOH did not cause significant changes in CAP amplitude in control healthy mice. Specifically, CAP amplitudes for pre-drug, drug, and wash are 0.285±0.04 mV, 0.298±0.06 mV, and 0.285±0.06 mV respectively (n=3 for all groups, P>0.5).
Figure 2.
The electrophysiological response of ex vivo EAE mouse spinal cord to the application of 100 µM 4-AP-3-MeOH showed an increased in CAP amplitude. Representative CAPs of pre and post 4-AP-3-MeOH treatment were superimposed and shown in A. Graph (B) demonstrates that 4-AP-3-MeOH treatment significantly enhanced CAP amplitude from 0.11 ± 0.01 mV to 0.17 ± 0.02 mV (n = 5, P < 0.05). The CAP amplitude returned to 0.13 ± 0.02 mV following wash for 45 – 60 minutes. Error bars represent SEM.
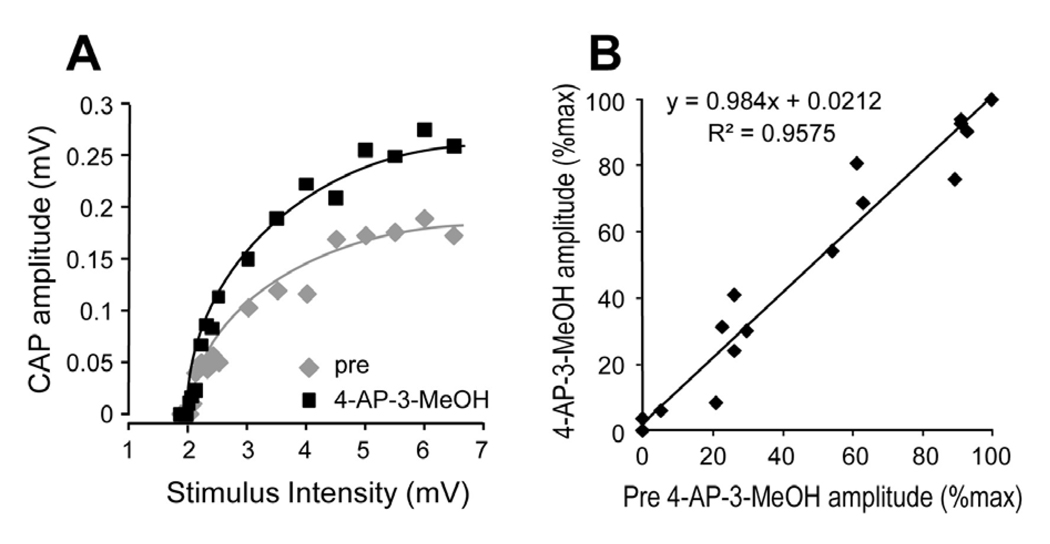
When a series of stimulus voltages with intensities ranging from 1.85 to 6.5 V were applied before and during the presence of 4-AP-3-MeOH in EAE mice, it is clear that the increase of CAP amplitude as a result of 4-AP-3-MeOH was evident in a wide range of stimulus intensities (Fig. 3A). In addition, a comparison of the response amplitude, with and without 4-AP-3-MeOH, showed no significant differences in the relative increase of amplitude (Fig. 3A). This relationship is further demonstrated in absolute terms in Fig. 3B. The near unity of the slope indicates that 4-AP-3-MeOH-mediated CAP conduction was not biased based on axonal caliber and threshold (Fig. 3B).
Figure 3.
Assessment of relation between stimulus intensity and response amplitude before and after treatment with 4-AP-3MeOH. Stimulus intensities ranging from 1.85 to 6.5V were applied to EAE spinal cord prior to drug exposure and at 100 µM 4-AP-3MeOH-treated conditions. A) Comparison of these two conditions is presented as a graphic plot, with trend lines added for the two conditions. Clearly the response amplitude increased with 4-AP-3MeOH at nearly all the stimulus intensities. B. Normalized CAP responses (% of maximum CAP in each condition) were plotted as pre-drug against 4-AP-3MeOH-treated (100 µM). Original data in B are the same as for A. Overall trends demonstrated a linear correlation between pre-drug and the 4-AP-3MeOH-treated group, indicating that there is little difference in the sensitivity of axons with different stimulus thresholds to 4-AP-3MeOH-mediated amplitude enhancement.
Discussion
Similar to our prior study in mechanically injured spinal cord, we have shown in this study that 4-AP-3-MeOH can also significantly enhance axonal conduction in the spinal cord of EAE mice (Figure 2B). Since 4-AP-3-MeOH has been shown to block fast potassium channels, it is likely that 4-AP-3-MeOH enhances CAP by blocking this type of channel unmasked due to myelin damage. To our knowledge, this is the first time that a potassium channel blocker other than 4-AP has been shown to enhance axonal conduction in an animal model of MS.
Based on available evidence, there are two major differences between 4-AP-3-MeOH and 4-AP. First of all, the lowest concentration of 4-AP-3-MeOH that can produce significant axonal conduction restoration is between 0.01 µM to 0.1 µM while 4-AP is between 0.1 µM and 1 µM. Second, unlike axons affected by 4-AP, axons affected by 4-AP-3-MeOH conduct action potentials in a manner similar to normal axons based on ex vivo examinations. Specifically, while producing a significant conduction recovery, 4-AP (100 µM) induced an increase of refractoriness. On the other hand, 4-AP-3-MeOH at the same concentration did not affect the axonal refractoriness while inducing conduction recovery. However, caution should be taken when extrapolating these data to in vivo situations where the safe effective concentration could be hundreds of times lower than used ex vivo. Similar to that in 4-AP, our data indicates that axons with various axonal diameters benefit equally from 4-AP-3-MeOH-induced axonal conduction restoration.
Based on these findings using ex vivo testing, it appears that 4-AP-3-MeOH could potentially be an alternative therapeutic option in addition to 4-AP in enhancing axonal conduction in demyelinated axons. In the current study, we chose the most beneficial concentration determined in ex vivo traumatic spinal cord injury study to determine its effectiveness in EAE mouse spinal cord. Further experiments testing a range of concentrations are necessary to establish a dose response for 4-AP-3-MeOH-mediated axonal conduction restoration in EAE mice.
Since these are the initial findings of 4-AP-3-MeOH on isolated spinal cord extracted from EAE mice, there are still more possible differences compared to 4-AP when applied in vivo. Specifically, future studies in vivo will need to assess various factors that could affect the concentration achieved in CNS which include metabolism, absorption, and excretion of 4-AP-3-MeOH. These factors are crucial to determine the eventual efficacy, potency and side effects and therefore clinical value of 4-AP-3-MeOH in the treatment of MS patients.
Research Highlights
4-AP-3-MeOH enhances axonal conduction in EAE mouse spinal cord.
Such axonal conduction restoration appears to be reversible upon wash.
Small and large axons benefit equally from-AP-3-MeOH-mediated restoration.
Acknowledgment
The authors would like to acknowledge the financial support of the State of Indiana and the Indiana Clinical and Translational Sciences Institute (PHS NCCR # TL1RR025759). We would also like to recognize Todd Rickett and Sean Connell for critical review of the manuscript.
Abbreviations
- MS
Multiple sclerosis
- 4-AP
4-aminopyridine
- 4-AP-3-MeOH
4-Aminopyridine-3-Methanol
- EAE
experimental autoimmune encephalomyelitis
- CAP
compound action potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blight AR. Effect of 4-aminopyridine on axonal conduction-block in chronic spinal cord injury. Brain Res. Bull. 1989;22:47–52. doi: 10.1016/0361-9230(89)90126-3. [DOI] [PubMed] [Google Scholar]
- Blight AR, Toombs JP, Bauer MS, Widmer WR. The effects of 4-aminopyridine on neurological deficits in chronic cases of traumatic spinal cord injury in dogs: a phase I clinical trial. J. Neurotrauma. 1991;8:103–119. doi: 10.1089/neu.1991.8.103. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature. 1980;284:170–171. doi: 10.1038/284170a0. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN, Blight AR. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- Haghighi SS, Pugh SL, Perez-Espejo MA, Oro JJ. Effect of 4-aminopyridine in acute spinal cord injury. Surg. Neurol. 1995;43:443–447. doi: 10.1016/0090-3019(95)80087-w. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Poter PJ, Wolfe DL, Hsieh JTC, Delaney GA, Blight AR. 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. J. Neurotrauma. 1994;11:433–446. doi: 10.1089/neu.1994.11.433. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Shi R. Effects of 4-aminopyridine on stretched mammalian spinal cord: the role of potassium channels in axonal conduction. J. Neurophysiol. 2003;90:2334–2340. doi: 10.1152/jn.00868.2002. [DOI] [PubMed] [Google Scholar]
- Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Fehlings MG. Temporal and spatial patterns of Kv1.1 and Kv1.2 protein and gene expression in spinal cord white matter after acute and chronic spinal cord injury in rats: implications for axonal pathophysiology after neurotrauma. Eur. J. Neurosci. 2004;19:577–589. doi: 10.1111/j.0953-816x.2004.03164.x. [DOI] [PubMed] [Google Scholar]
- Korenke AR, Rivey MP, Allington DR. Sustained-release fampridine for symptomatic treatment of multiple sclerosis. Ann. Pharmacother. 2008;42:1458–1465. doi: 10.1345/aph.1L028. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Jones OT, Fehlings MG. Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury. Eur. J. Neurosci. 2000;12:491–506. doi: 10.1046/j.1460-9568.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- Pena F, Tapia R. Relationships among seizures, extracellular amino acid changes, and neurodegeneration induced by 4-aminopyridine in rat hippocampus: a microdialysis and electroencephalographic study. J. Neurochem. 1999;72:2006–2014. doi: 10.1046/j.1471-4159.1999.0722006.x. [DOI] [PubMed] [Google Scholar]
- Pena F, Tapia R. Seizures and neurodegeneration induced by 4-aminopyridine in rat hippocampus in vivo: role of glutamate- and GABA-mediated neurotransmission and of ion channels. Neuroscience. 2000;101:547–561. doi: 10.1016/s0306-4522(00)00400-0. [DOI] [PubMed] [Google Scholar]
- Potter PJ, Hayes KC, Segal JL, Hsieh JT, Brunnemann SR, Delaney GA, Tierney DS, Mason D. Randomized double-blind crossover trial of fampridine-SR (sustained release 4-aminopyridine) in patients with incomplete spinal cord injury. J. Neurotrauma. 1998;15:837–849. doi: 10.1089/neu.1998.15.837. [DOI] [PubMed] [Google Scholar]
- Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980;283:570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- Shi R, Blight AR. Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J. Neurophysiol. 1996;76:1572–1580. doi: 10.1152/jn.1996.76.3.1572. [DOI] [PubMed] [Google Scholar]
- Shi R, Blight AR. Differential effects of low and high concentrations of 4-aminopyridine on axonal conduction in normal and injured spinal cord. Neuroscience. 1997;77:553–562. doi: 10.1016/s0306-4522(96)00477-0. [DOI] [PubMed] [Google Scholar]
- Shi R, Whitebone J. Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J. Neurophysiol. 2006;95:3384–3390. doi: 10.1152/jn.00350.2005. [DOI] [PubMed] [Google Scholar]
- Shi R, Kelly TM, Blight AR. Conduction block in acute and chronic spinal cord injury: Different dose-response characteristics for reversal by 4-Aminopyridine. Exp. Neurology. 1997;148:495–501. doi: 10.1006/exnr.1997.6706. [DOI] [PubMed] [Google Scholar]
- Stork CM, Hoffman RS. Characterization of 4-aminopyridine in overdose. ClinToxicol. 1994;32:583–587. doi: 10.3109/15563659409011063. [DOI] [PubMed] [Google Scholar]
- Sun W, Smith D, Fu Y, Cheng JX, Bryn S, Borgens R, Shi R. Novel potassium channel blocker, 4-AP-3-MeOH, inhibits fast potassium channels and restores axonal conduction in injured guinea pig spinal cord white matter. J. Neurophysiol. 2010;103:469–478. doi: 10.1152/jn.00154.2009. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Demyelination in spinal cord injury and multiple sclerosis: what can we do to enhance functional recovery? J. Neurotrauma. 1992;9 Suppl:S105–S117. [PubMed] [Google Scholar]
- Waxman SG, Utzschneider DA, Kocsis JD. Enhancement of action potential conduction following demyelination: experimental approaches to restoration of function in multiple sclerosis and spinal cord injury. Prog. Brain Res. 1994;100:233–243. doi: 10.1016/s0079-6123(08)60790-6. [DOI] [PubMed] [Google Scholar]