Abstract
In wild-type (WT) mice, the antibiotic minocycline inhibits development of cocaine-induced locomotor sensitization. Some of the actions of minocycline may involve the 5-lipoxygenase (5-LOX) pathway. We used the model of 5-LOX-deficient mice to investigate whether 5-LOX participates in minocycline’s influence on the effects of cocaine. Locomotor sensitization was induced by 4 daily cocaine injections and the phosphorylation status of GluR1 glutamate receptors was assayed in brain samples. Minocycline failed to affect cocaine sensitization in 5-LOX-deficient mice. In these mice, neither cocaine nor minocycline 4-day treatment altered GluR1 phosphorylation. In WT mice in which minocycline inhibited development of cocaine sensitization, a 4-day cocaine treatment increased GluR1 phosphorylation at both Ser831 and Ser845 sites in the frontal cortex but not the striatum; further, this effect was prevented by minocycline. Under basal conditions and in response to a single cocaine injection the levels of GluR1, GluR2, and GluR3 AMPA receptor subunits did not differ between WT and 5-LOX-deficient mice, but the response of GluR1 phosphorylation to a single cocaine injection was greater under the 5-LOX deficiency. Hence, in WT mice GluR1 phosphorylation increased only in the frontal cortex and only at the Ser831 site. In 5-LOX-deficient mice, acute cocaine injection increased both Ser831 and Ser845 phosphorylation both in the frontal cortex and in the striatum. We suggest that in studying minocycline’s action on cocaine’s effects and/or addiction in humans, it would be important to consider the characterization of the subjects’ 5-LOX system.
Keywords: 5-lipoxygenase (5-LOX), minocycline, cocaine, glutamate, AMPA, addiction
1. Introduction
Recent research has pointed to the role of the 5-lipoxygenase (5-LOX) pathway in brain pathologies (Chu and Praticò, 2009) and in modifying behavioral and cellular effects of cocaine. Briefly, behavioral sensitization to repeated administration of cocaine to rodents and the phosphorylation status of the GluR1 subunit of the glutamate/AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptors (which are involved in cocaine sensitization) are influenced by 5-LOX gene status (Kurtuncu et al., 2008) and by drugs acting on the 5-LOX system (Imbesi et al., 2007; Ménard et al., 2005).
Minocycline, a brain-penetrable antibiotic (Fagan et al., 2004) is capable of modifying brain functioning in conditions ranging from neurodegeneration (Chu et al., 2010; Elewa et al., 2006; Li et al., 2009) to psychiatric disorders (Miyaoka et al., 2008; Neigh et al., 2009; Pae et al., 2008) and addiction (Chen et al., 2009; Habibi-Asl et al., 2009; Kofman et al., 1990; Sofuoglu et al., 2009). Some of these minocycline actions have been attributed to its ability to inhibit the 5-LOX pathway (Chu et al., 2007, 2010; Song et al., 2004, 2006).
Similar to 5-LOX inhibitors, minocycline increases GluR1 phoshorylation in neuronal cultures in-vitro and administered intraperitoneally (i.p.) to mice it increases GluR1 phosphorylation in the brain (Imbesi et al., 2008). It is believed that both Ser831 and Ser845 phosphorylation of GluR1 receptors determine the AMPA channel activity and membrane insertion of this receptor and thereby modify the behavioral actions of cocaine (Boudreau and Wolf, 2005; Chen et al., 2010). It has been shown that in addition to its ability to alter GluR1 phosphorylation minocycline affects cocaine-triggered locomotor sensitization (Chen et al., 2009).
5-LOX-deficient mice (Chen et al., 1994) have been used as an experimental model to investigate physiological and pathological implications of 5-LOX metabolites such as the pro-inflammatory leukotrienes and the anti-inflammatory lipoxins. This mouse model was recently applied to research relevant for understanding of brain physiology and pathology including Alzheimer’s disease (Firuzi et al., 2008), depression (Dzitoyeva et al., 2008), and addiction (Kurtuncu et al., 2008). In this work, we used the model of 5-LOX-deficient mice to investigate whether 5-LOX participates in minocycline’s influence on the effects of cocaine.
2. Material and methods
2.1. Animals and drug treatment
Breeding pairs of wild type C57BL/6J mice (WT; 5-LOX +/+) and mice with a 5-LOX-targeted gene disruption [5-LOX (−/−)] (B6.129S2-Alox5tm1Fun/J; Stock #004155; Bar Harbor, ME) were purchased from Jackson Laboratories. Heterozygous 5-LOX +/− colonies were used to obtain WT and 5-LOX (−/−) male mice for experiments. They were genotyped prior to use. Two-month-old mice weighing 25–30 g were housed in groups of five in a temperature controlled room on a 12-h light/dark cycle (lights on at 7AM). Mice had a free access to laboratory chow and water except during behavioral experiments. The experimental protocol was approved by the Institutional Animal Care Committee. Cocaine hydrochloride (Sigma, St. Louis, MO) was dissolved in sterile saline and administered intraperitoneally (i.p.) in an injection volume of 0.05 ml/25 g body weight (10 mg/kg for repeated injections and 20 mg/kg for acute experiments). Minocycline hydrochloride (Sigma) was dissolved in sterile saline with a pH adjustment and injected in the same volume as cocaine (Chen et al., 2009). Controls received the same volume of the vehicle.
2.2. Behavioral assay
Behavioral, i.e., locomotor sensitization was assayed as previously described (Chen et al., 2009). Briefly, mice were accustomed to experimental conditions by three daily i.p. saline injections. Starting from the first day of experiment, mice were placed individually in activity cages (Cage Rack Photobeam Activity Measurement System, San Diego Instruments, San Diego, CA) equipped with computer-monitored photobeam frames for a 25-min adaptation period. Immediately after this 25-min adaptation period, animals received the i.p. injections and were returned to the activity cages for another 30-min measurement period. The movement of each animal in this 30-min period was individually recorded as the number of beam interruptions and is reported as locomotor activity.
2.3. Western blot assay
Mice were killed 30 min after the last i.p. injection and their brains were dissected into frontal cortex and striatum samples that were immediately stored at −80°C. Also included in analysis were brain samples collected from mice whose behavioral response to treatment was previously reported (Chen et al., 2009: Fig. 2). Brain samples were homogenized in ice-cold 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 320 mM sucrose, 1 mM dithiothreitol with protease inhibitors (5 µg/ml aprotinin, 5 µg/ml leupeptin, 5 µg/ml pepstatin, 1 mM phenylmethanesulfonylfluoride) and a phosphatase inhibitor cocktail (Sigma, St. Louis, MO). Samples were centrifuged at 1000 g for 10 min. The supernatant was centrifuged again at 17000 g for 30 min and the pellet was resuspended in 10 mM Tris-HCl (pH 7.4), 1 mM EDTA in the presence of the indicated inhibitors. After measuring protein concentration by BCA protein assay kit (Pierce, Rockford, IL), samples were boiled in loading buffer and run on 7.5% gels. Proteins were then transferred to nitrocellulose membranes. The non-specific binding was blocked by incubating the blots with 5% non-fat dry milk for 1 h. The membranes were then incubated overnight at 4°C with rabbit-anti-phospho-GluR1 Ser831 (1:1000; Millipore, Billerica, MA), rabbit-anti-phospho-GluR1 Ser845 (1:1000; Affinity Bioreagents, Golden, CO), rabbit-anti-GluR1 (1:1000, Anaspec, San Jose, CA), rabbit-anti-GluR2 (1:1000, Novus Biologicals, Littleton, CO), rabbit-anti-GluR3 (1:1000, Cell Signaling, Danvers, MA), and mouse-anti-β-actin (1:5000, Sigma, St. Louis, MO). Thereafter, blots were incubated for 2 h with horseradish-peroxidase-linked secondary antibody (anti-rabbit IgG or anti-mouse IgG, 1:2000, Amersham, Piscataway, NJ). Subsequent to treatment with an ECL Kit (Amersham), the membranes were exposed to the Hyperfilm ECL (Amersham). Band densities were quantified using the NIH ImageJ software. The optical density of phospho-GluR1 bands was corrected by the optical density of the corresponding total GluR1 bands; the optical density of GluR bands was corrected by the densities of corresponding β-actin bands.
Figure 2.
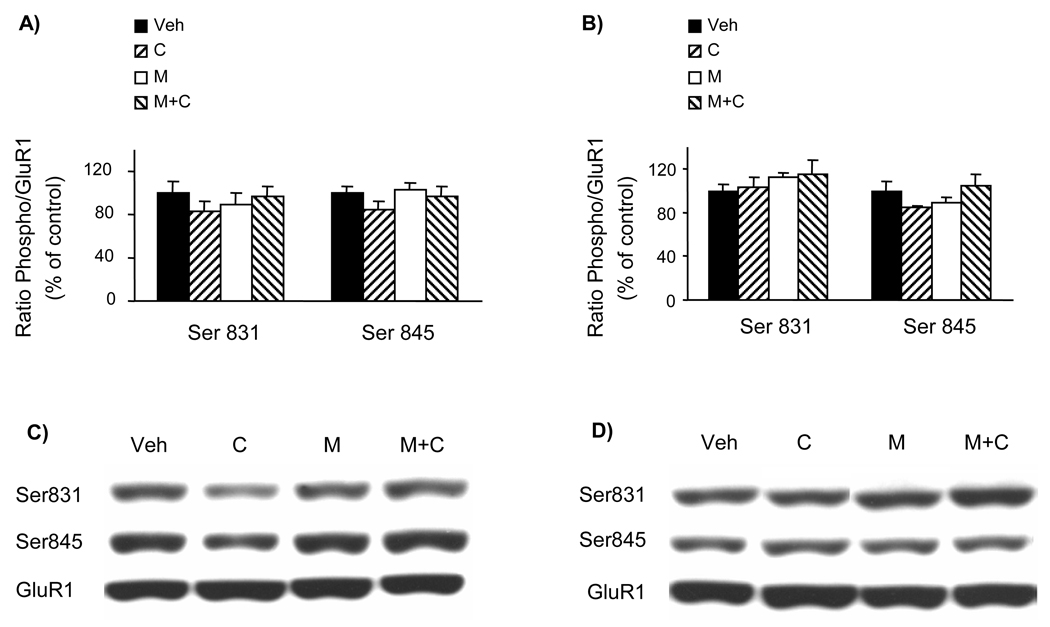
Effect of a 4-day treatment with cocaine and minocycline on GluR1 phosphorylation in 5-LOX (−/−) mice. The 4-day treatment was conducted as described in Fig. 1 (Veh = vehicle, C = cocaine, M = minocycline). Samples were collected 30 min after the last injection. The phosphorylation status of GluR1 at Ser831 and Ser845 sites was assayed by quantitative Western blot in the frontal cortex (A, C) and the striatum (B, D). The optical density of the phospho Ser831 and phospho Ser845 bands was corrected by the density of the corresponding total GluR1 bands (examples shown in C and D). The results are expressed as a percentage of the corresponding vehicle-treated controls (mean ± S.E.M.; n = 6). There were no significant differences between groups (one-way ANOVA).
2.4. Statistics
Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL). Data (shown as mean ± S.E.M.) were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, or repeated measures ANOVA followed by t-test for two-group comparisons. Significance was accepted at p<0.05.
3. Results
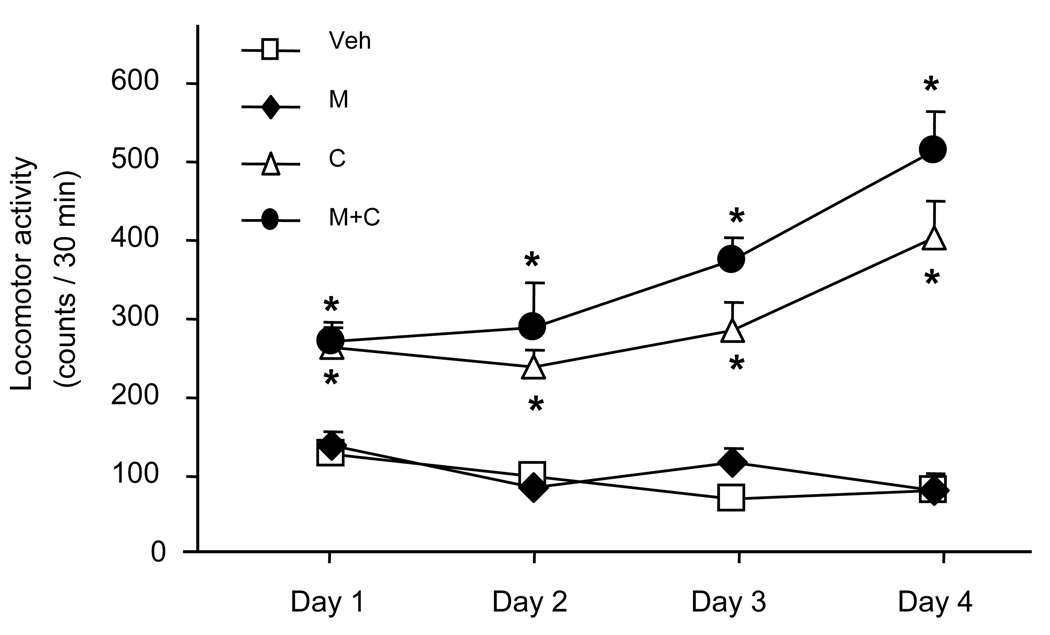
To investigate whether 5-LOX deficiency affects the ability of minocycline to attenuate the development of cocaine-induced locomotor sensitization, we replicated in 5-LOX (−/−) mice our experimental protocol used earlier in WT mice (Chen et al., 2009). Similar to WT mice, 5-LOX deficient mice also respond to daily administration of cocaine with increased locomotor activity (sensitization) but this action of cocaine was not affected by minocycline co-treatment in 5-LOX (−/−) mice (Fig. 1).
Figure 1.
Effect of a 4-day treatment with cocaine and minocycline on the locomotor activity of 5-LOX (−/−) mice. Minocycline (M; 40 mg/kg) or vehicle (Veh) were administrated daily 3 h before i.p. injection of cocaine (C; 10 mg/kg) or its vehicle (n = 6). Locomotor activity was measured for 30 min starting immediately after the last daily i.p. injection. Repeated ANOVA revealed that significant difference occurred among Veh and C or M+C groups [day, F (1, 15) = 4.61, p<0.05; group, F (3, 15) = 14.57, p<0.05; day × group, F (3, 15) = 3.68, p<0.05], but not between C and M+C groups (* p<0.05 vs the corresponding control groups, i.e., Veh and M, respectively; t-test).
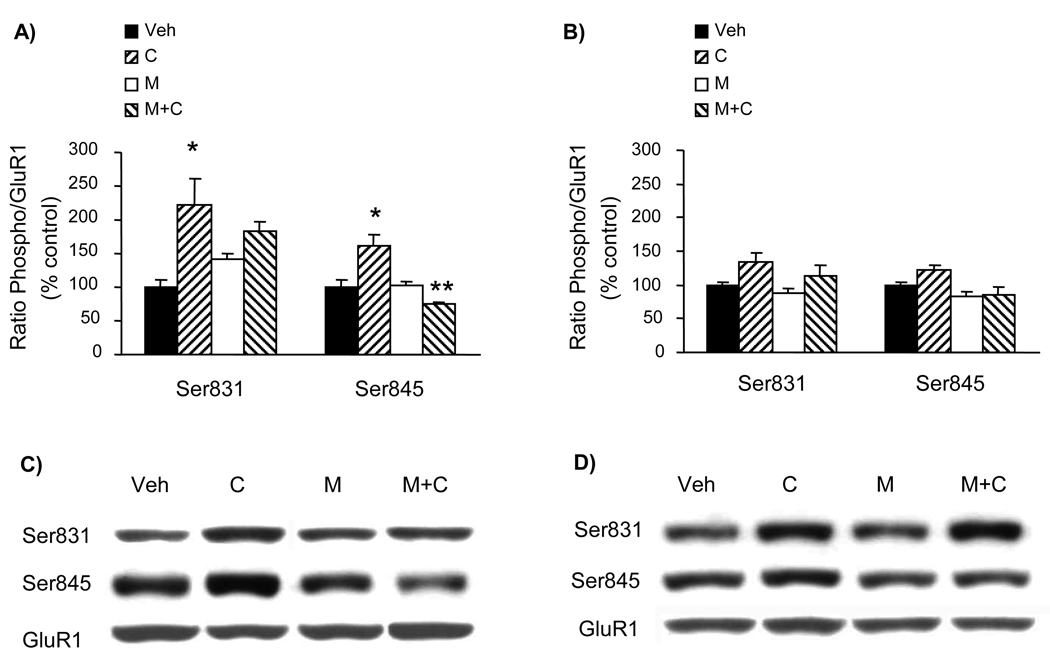
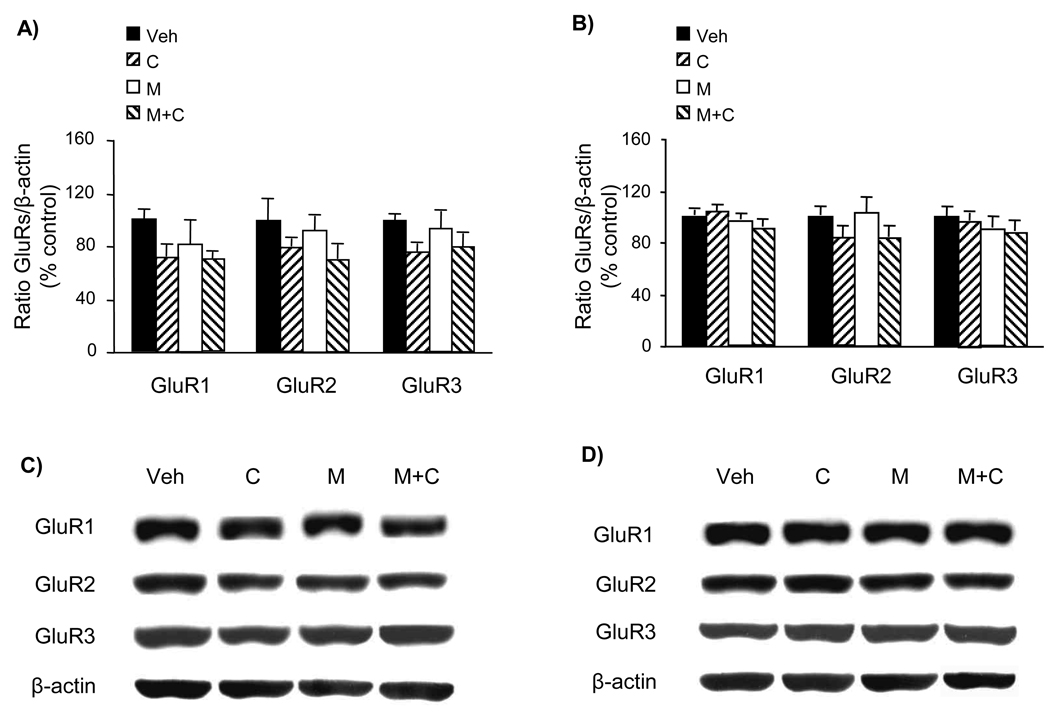
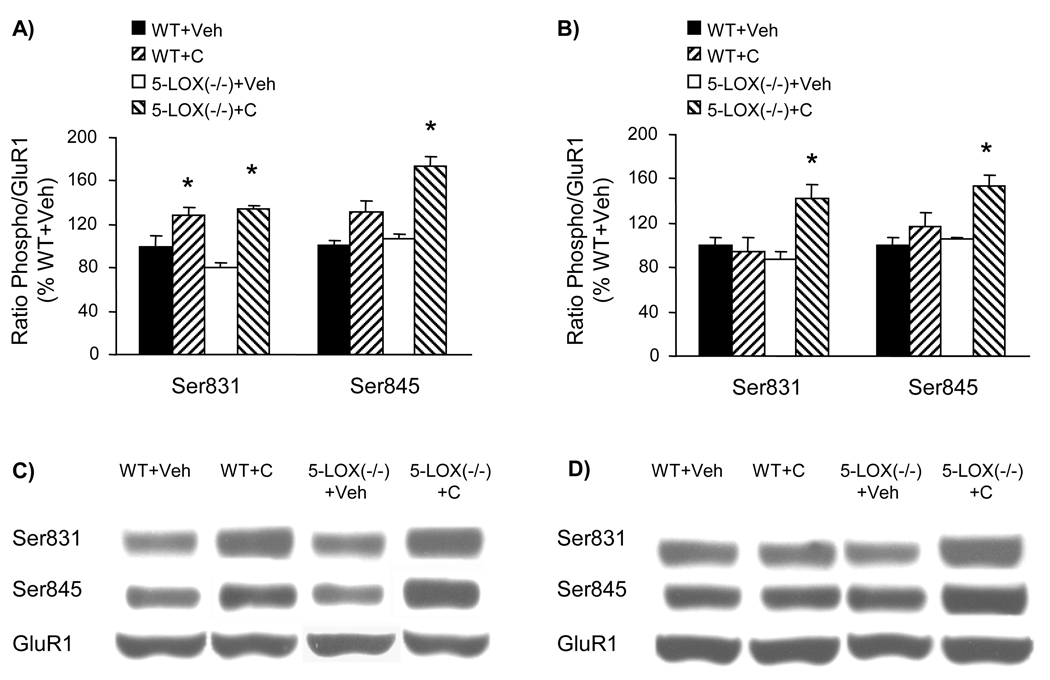
The phosphorylation status of brain (frontal cortex and striatum) GluR1 receptors at both Ser831 and Ser845 sites was not affected by the above-described 4-day drug treatment of 5-LOX (−/−) mice (Fig. 2). On the other hand, the same drug treatment schedule applied to WT mice (brain samples collected from mice whose behavioral response to treatment was previously reported; Chen et al., 2009) resulted in significant alterations of GluR1 phosphorylation in the frontal cortex but not in the striatum (Fig. 3). However, none of the treatments altered the amount of proteins of GluR1, GluR2, and GluR3 AMPA receptor subunits (Fig. 4). Hence, in the absence of minocycline GluR1 phosphorylation at both Ser831 and Ser845 sites was increased by cocaine treatment. Whereas minocycline alone did not alter GluR1 phosphorylation, in combination with cocaine it prevented cocaine-enhanced Glu1 phosphorylation in the frontal cortex (Fig. 3).
Figure 3.
Effect of a 4-day treatment with cocaine and minocycline on GluR1 phosphorylation in wild-type (WT) mice. The 4-day treatment was conducted as described in Fig. 1 (Veh = vehicle, C = cocaine, M = minocycline). Samples were collected 30 min after the last injection. The phosphorylation status of GluR1 at Ser831 and Ser845 sites was assayed by quantitative Western blot in the frontal cortex (A, C) and the striatum (B, D). The optical density of the phospho Ser831 and phospho Ser845 bands was corrected by the density of the corresponding total GluR1 bands (examples shown in C and D). The results are expressed as a percentage of the corresponding vehicle-treated control (mean ± S.E.M.; n = 6; *p<0.05 compared to Veh; **p<0.05 compared to C).
Figure 4.
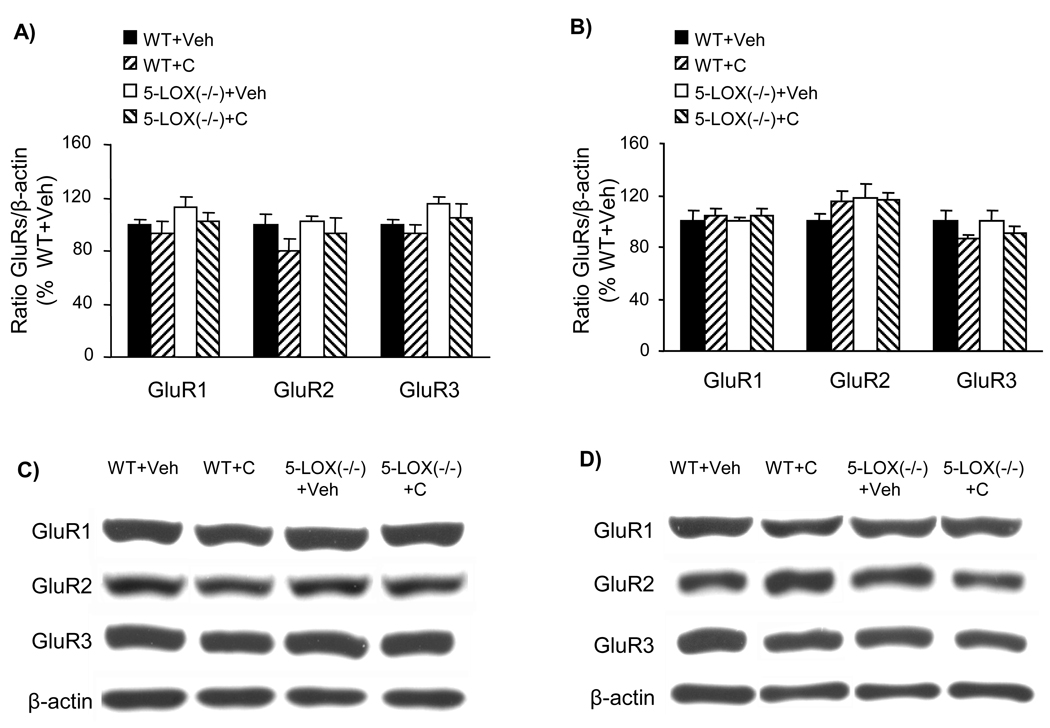
Effect of a 4-day treatment with cocaine and minocycline on the content of GluR1–3 AMPA subunits in the frontal cortex and striatum of WT mice. The 4-day treatment was conducted as described in Fig. 3 (Veh = vehicle, C = cocaine, M = minocycline). Total GluR1, GluR2, and GluR3 levels were assayed by quantitative Western blot in the frontal cortex (A, C) and the striatum (B, D). The optical density of the GluR bands was corrected by the density of the corresponding β-actin bands (examples shown in C and D). The results are expressed as a percentage of the corresponding vehicle-treated WT (mean ± S.E.M.; n = 6). There were no significant differences between groups (one-way ANOVA).
Since the above results point to a significant contribution of 5-LOX deficiency to the response of GluR1 receptor phosphorylation to repeated cocaine treatment, we investigated whether WT and 5-LOX (−/−) mice differ at the level of glutamate receptors in the absence of cocaine treatment and in their response to a single cocaine administration. The amount of proteins of GluR1, GluR2, and GluR3 AMPA receptor subunits did not differ between WT and 5-LOX (−/−) mice either in the absence of cocaine or 30 min after a single cocaine injection (Fig. 5). On the other hand, the status of GluR1 phosphorylation in response to a single cocaine injection differed between WT and 5-LOX (−/−) mice; i.e., the 5-LOX (−/−) mice responded to cocaine more readily; with an increase in GluR1 phosphorylation; than the WT mice did (Fig. 6). Hence, in WT mice acute cocaine administration increased GluR1 phosphorylation only in the frontal cortex and only at the Ser831 site. In contrast, in 5-LOX (−/−) mice, acute cocaine injection increased both Ser831 and Ser845 phosphorylation both in the frontal cortex and in the striatum (Fig. 6).
Figure 5.
Content of GluR1–3 AMPA subunits in the frontal cortex and striatum of WT and 5-LOX (−/−) mice under control conditions and after a single cocaine injection. Samples were collected 30 min after i.p. injection (Veh = vehicle; C = cocaine, 20 mg/kg). Total GluR1, GluR2, and GluR3 levels were assayed by quantitative Western blot in the frontal cortex (A, C) and the striatum (B, D). The optical density of the GluR bands was corrected by the density of the corresponding β-actin bands (examples shown in C and D). The results are expressed as a percentage of the corresponding vehicle-treated WT (mean ± S.E.M.; n = 6). There were no significant differences between groups (one-way ANOVA).
Figure 6.
GluR1 phosphorylation in the frontal cortex and striatum of WT and 5-LOX (−/−) mice under control conditions and after a single cocaine injection. Samples were collected 30 min after i.p. injection (Veh = vehicle; C = cocaine, 20 mg/kg). The phosphorylation status of GluR1 at Ser831 and Ser845 sites was assayed by quantitative Western blot in the frontal cortex (A, C) and the striatum (B, D). The optical density of the phospho Ser831 and phospho Ser845 bands was corrected by the density of the corresponding total GluR1 bands (examples shown in C and D). The results are expressed as a percentage of the corresponding vehicle-treated WT (mean ± S.E.M.; n = 6). *p<0.05 compared to corresponding vehicle-treated controls.
4. Discussion
The main finding in this study is that behavioral and cellular/biochemical effects of cocaine and minocycline differ significantly in 5-LOX-deficient mice compared to WT mice. The same experimental protocol of repeated cocaine and minocycline administration developed and used previously in our work with WT mice in which minocycline inhibited the development of locomotor cocaine sensitization (Chen et al., 2009) repeated in 5-LOX (−/−) mice revealed the failure of minocycline to interfere with behavioral effects of cocaine. This finding suggests that 5-LOX and possibly its metabolites may participate in minocyline-triggered attenuation of the development of cocaine sensitization. However, it remains to be elucidated whether this interaction of minocycline with the 5-LOX system involves an inhibitory action of minocycline on 5-LOX enzymatic activity or some alternative mechanism. To this end, a systemic administration to mice of a 5-LOX inhibitor MK-886 along with cocaine produced only a transient potentiation of locomotor activity (Kurtuncu et al., 2008), i.e., an effect different from the action of minocycline.
Analysis of brain samples collected at the end of the 4-day drug treatment showed that 5-LOX deficiency prevented both cocaine and minocycline from altering GluR1 phosphorylation. Hence, it appears that a normally functioning 5-LOX system is needed to enable repeated cocaine injections to increase the phosphorylation status of cortical GluR1 receptors we observed in the cortical samples of WT mice. Furthermore, a WT status of the 5-LOX system was required for minocycline to inhibit the stimulatory effects of repeated cocaine injections on GluR1 phosphorylation in the frontal cortex.
Although the basal levels of the three AMPA receptor subunits, GluR1, GluR2, and GluR3 (measured in the frontal cortex and striatum) did not differ among WT and 5-LOX (−/−) mice and were unaffected by a single or repeated cocaine injections into WT mice, 5-LOX-deficiency facilitated the susceptibility of the GluR1 subunit to become increasingly phosphorylated in response to a single acute cocaine injection. Hence, whereas a single cocaine administration to WT mice increased only Ser831 phosphorylation and only in the frontal cortex, in 5-LOX (−/−) mice this treatment increased phosphorylation of both Ser831 and Ser845 and both in the frontal cortex and in the striatum. These results suggest that the 5-LOX system and possibly the biologically active molecules it generates restrict the activity of biochemical pathways that cocaine mobilizes when it increases GluR1 phosphorylation. By the same token, these 5-LOX-dependent restrictive mechanisms appear to participate in increased GluR1 phosphorylation caused by repeated cocaine injections because in their absence [i.e., 5-LOX (−/−) mice] repeated cocaine injections do not alter GluR phosphorylation.
It is believed that Ser831 phosphorylation (by calcium/calmodulin-dependent protein kinase II) and Ser845 phosphorylation (by cAMP-dependent protein kinase) of GluR1 receptors increase AMPA channel activity and that Ser845 phosphorylation increases GluR1 surface insertion (Oh et al., 2006). It has been proposed that the status of GluR1 phosphorylation can be related to cocaine locomotor sensitization (Boudreau and Wolf, 2005; Chen et al., 2010). However, to establish such a correlation between behavioral and biochemical changes GluR1 phosphorylation must be studied at the cellular and subcellular level (Ghasemzadeh et al., 2009a; Lane et al., 2010) including its detailed regional distribution (Ghasemzadeh et al., 2009b). In interpreting our results, it has to be stressed that in our experimental conditions GluR1 phosphorylation was evaluated as a relatively crude biochemical marker of cocaine’s actions; i.e., it was assayed in the homogenates obtained from relatively large brain areas (i.e., frontal cortex and striatum) as opposed to smaller subregions (e.g., nucleus accumbens). It is possible that the absence of phosphorylation changes we observed in the striatum samples is due to this technical limitation. Nevertheless, our approach was capable of detecting the stimulatory effects of four daily cocaine injections in the frontal cortex of WT mice and revealing the inhibitory effect of minocycline on this action of cocaine. With respect to the correlation between behavioral and biochemical effects of four daily cocaine injections, it appears that the observed increased GluR1 phosphorylation in the frontal cortex (observed after the fourth cocaine injection) is unrelated to the establishment of locomotor sensitization (i.e., greater locomotor activity after the fourth cocaine injection compared to the activity after the first cocaine injection) since sensitization occurred both in WT and 5-LOX (−/−) mice and no changes in GluR1 phosphorylation were observed in 5-LOX (−/−) mice.
In the central nervous system, the 5-LOX pathway generates 5-LOX metabolites (Chinnici et al., 2007; Hynes et al., 1991), possibly via a transcellular synthesis of cysteinyl leukotrienes in neurons and glia (Farias et al., 2007). In addition, transcellular synthesis of 5-LOX metabolites could take place in the periphery (Sala et al., 2010) and could determine the type of metabolite (e.g., leukotrienes or lipoxins) that are produced and are ultimately involved in biological effects including in the central nervous system. These metabolites affect cell functioning via corresponding G-protein-coupled receptors (Wada et al., 2006) and play a critical role in neuroplasticity such as the hedgehog-dependent neurite projection (Bijlsma et al., 2008). Hence, it is possible that interplay between leukotriene and AMPA receptors also plays a role in the observed involvement of the 5-LOX system in cocaine actions and that leukotriene receptor-mediated events are involved in modifications of the GluR1 AMPA receptors observed in our studies. Interestingly, when the receptor activity and affinity of a natural product, Chlorella, were tested in 129 in vitro binding assay systems, it was observed that this compound acts on both leukotriene CysLT receptors and glutamate AMPA receptors (Cheng et al., 2010).
In conclusion, our results along with the previously published data show that behavioral effects of minocycline, i.e., its inhibitory action on development of cocaine-induced locomotor sensitization may involve the 5-LOX system and that minocycline is ineffective when the 5-LOX gene is disrupted. There is a significant polymorphism in the human 5-LOX gene with presumable implications for the activity of the 5-LOX system (Listì et al., 2010; Tantisira and Drazen, 2009; Vikman et al., 2009). Hence, if minocycline’s action on cocaine’s effects and/or addiction is to be investigated in human subjects, it would be important to include the characterization of the subjects’ 5-LOX system in these studies. In this study, we did not find a direct correlation between behavioral cocaine sensitization and the status of GluR1 phosphorylation but this could be due to technical limitations of our biochemical assay (large brain regions as opposed to specific neuronal pathways). Nevertheless, we found that 5-LOX gene disruption enhances the sensitivity of GluR1 receptors to be phosphorylated in response to an acute cocaine administration. Considering the significant polymorphism in the genes of the human 5-LOX system (Tantisira and Drazen, 2009) it is possible that such a 5-LOX-dependent variable GluR1 sensitivity to cocaine exists in the human central nervous system. The functional relevance of the observed 5-LOX-dependent changes in GluR1 phosphorylation needs further elucidation.
Acknowledgements
This study was supported by the National Institutes of Health grant from the National Institute on Drug Abuse (NIDA) 1R21 DA024099-01 (H.M.). We thank Marta Imbesi for help in maintaining and genotyping mouse colonies and collecting brain samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bijlsma MF, Peppelenbosch MP, Spek CA, Roelink H. Leukotriene synthesis is required for hedgehog-dependent neurite projection in neuralized embryoid bodies but not for motor neuron differentiation. Stem Cells. 2008;26:1138–1145. doi: 10.1634/stemcells.2007-0841. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uz T, Manev H. Minocycline affects cocaine sensitization in mice. Neurosci. Lett. 2009;452:258–261. doi: 10.1016/j.neulet.2009.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhu X, Zhang Y, Wetsel WC, Lee TH, Zhang X. Integrin-linked kinase is involved in cocaine sensitization by regulating PSD-95 and synapsin I expression and GluR1 Ser845 phosphorylation. J. Mol. Neurosci. 2010;40:284–294. doi: 10.1007/s12031-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- Cheng FC, Feng JJ, Chen KH, Imanishi H, Fujishima M, Takekoshi H, Naoki Y, Shimoda M. Receptor binding activities of Chlorella on cysteinyl leukotriene CysLT, glutamate AMPA, ion channels, purinergic P 2Y, tachykinin NK2 receptors and adenosine transporter. Phytother. Res. 2010;24:43–48. doi: 10.1002/ptr.2864. [DOI] [PubMed] [Google Scholar]
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol. Aging. 2007;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Chu J, Praticò D. The 5-lipoxygenase as a common pathway for pathological brain and vascular aging. Cardiovasc. Psychiatry Neurol. 2009. 2009 doi: 10.1155/2009/174657. 174657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LS, Fang SH, Zhou Y, Yu GL, Wang ML, Zhang WP, Wei EQ. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2007;28:763–772. doi: 10.1111/j.1745-7254.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Chu LS, Fang SH, Zhou Y, Yin YJ, Chen WY, Li JH, Sun J, Wang ML, Zhang WP, Wei EQ. Minocycline inhibits 5-lipoxygenase expression and accelerates functional recovery in chronic phase of focal cerebral ischemia in rats. Life Sci. 2010;86:170–177. doi: 10.1016/j.lfs.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Dzitoyeva S, Imbesi M, Uz T, Dimitrijevic N, Manev H, Manev R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J. Neural. Transm. 2008;115:823–827. doi: 10.1007/s00702-008-0034-7. [DOI] [PubMed] [Google Scholar]
- Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Edwards DJ, Borlongan CV, Xu L, Arora A, Feuerstein G, Hess DC. Optimal delivery of minocycline to the brain: implication for human studies of acute neuroprotection. Exp. Neurol. 2004;186:248–251. doi: 10.1016/j.expneurol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Farias SE, Zarini S, Precht T, Murphy RC, Heidenreich KA. Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells. J. Neurochem. 2007;103:1310–1318. doi: 10.1111/j.1471-4159.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol. Biochem. Behav. 2009a;92:383–392. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009b doi: 10.1016/j.brainres.2009.01.047. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Habibi-Asl B, Hassanzadeh K, Charkhpour M. Central administration of minocycline and riluzole prevents morphine-induced tolerance in rats. Anesth. Analg. 2009;109:936–942. doi: 10.1213/ane.0b013e3181ae5f13. [DOI] [PubMed] [Google Scholar]
- Hynes N, Bishai I, Lees J, Coceani F. Leukotrienes in brain: natural occurrence and induced changes. Brain Res. 1991;553:4–13. doi: 10.1016/0006-8993(91)90222-h. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Zavoreo I, Uz T, Sharma RP, Dimitrijevic N, Manev H, Manev R. 5-Lipoxygenase inhibitor MK-886 increases GluR1 phosphorylation in neuronal cultures in vitro and in the mouse cortex in vivo. Brain Res. 2007;1147:148–153. doi: 10.1016/j.brainres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Uz T, Manev R, Sharma RP, Manev H. Minocycline increases phosphorylation and membrane insertion of neuronal GluR1 receptors. Neurosci. Lett. 2008;447:134–137. doi: 10.1016/j.neulet.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O, Klein E, Newman M, Hamburger R, Kimchi O, Nir T, Shimon H, Belmaker RH. Inhibition by antibiotic tetracyclines of rat cortical noradrenergic adenylate cyclase and amphetamine-induced hyperactivity. Pharmacol. Biochem. Behav. 1990;37:417–424. doi: 10.1016/0091-3057(90)90006-4. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Battista N, Uz T, D'Agostino A, Dimitrijevic N, Pasquariello N, Manev R, Maccarrone M, Manev H. Effects of cocaine in 5-lipoxygenase-deficient mice. J. Neural Transm. 2008;115:389–395. doi: 10.1007/s00702-007-0848-8. [DOI] [PubMed] [Google Scholar]
- Lane DA, Jaferi A, Kreek MJ, Pickel VM. Acute and chronic cocaine differentially alter the subcellular distribution of AMPA GluR1 subunits in region-specific neurons within the mouse ventral tegmental area. Neuroscience. 2010;169:559–573. doi: 10.1016/j.neuroscience.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J. Cereb. Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listì F, Caruso C, Lio D, Colonna-Romano G, Chiappelli M, Licastro F, Candore G. Role of cyclooxygenase-2 and 5-lipoxygenase polymorphisms in Alzheimer's disease in a population from northern Italy: implication for pharmacogenomics. J. Alzheimers Dis. 2010;19:551–557. doi: 10.3233/JAD-2010-1260. [DOI] [PubMed] [Google Scholar]
- Ménard C, Valastro B, Martel MA, Chartier E, Marineau A, Baudry M, Massicotte G. AMPA receptor phosphorylation is selectively regulated by constitutive phospholipase A2 and 5-lipoxygenase activities. Hippocampus. 2005;15:370–380. doi: 10.1002/hipo.20061. [DOI] [PubMed] [Google Scholar]
- Miyaoka T. Clinical potential of minocycline for schizophrenia. CNS Neurol. Disord. Drug Targets. 2008;7:376–381. doi: 10.2174/187152708786441858. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Karelina K, Glasper ER, Bowers SL, Zhang N, Popovich PG, DeVries AC. Anxiety after cardiac arrest/cardiopulmonary resuscitation: exacerbated by stress and prevented by minocycline. Stroke. 2009;40:3601–3607. doi: 10.1161/STROKEAHA.109.564146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed. Pharmacother. 2008;62:308–311. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Sala A, Folco G, Murphy RC. Transcellular biosynthesis of eicosanoids. Pharmacol. Rep. 2010;62:503–510. doi: 10.1016/s1734-1140(10)70306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, O'Malley SS. Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol. Biochem. Behav. 2009;92:135–140. doi: 10.1016/j.pbb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wei EQ, Zhang WP, Zhang L, Liu JR, Chen Z. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport. 2004;15:2181–2184. doi: 10.1097/00001756-200410050-00007. [DOI] [PubMed] [Google Scholar]
- Song Y, Wei EQ, Zhang WP, Ge QF, Liu JR, Wang ML, Huang XJ, Hu X, Chen Z. Minocycline protects PC12 cells against NMDA-induced injury via inhibiting 5-lipoxygenase activation. Brain Res. 2006;1085:57–67. doi: 10.1016/j.brainres.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Tantisira KG, Drazen JM. Genetics and pharmacogenetics of the leukotriene pathway. J. Allergy Clin. Immunol. 2009;124:422–427. doi: 10.1016/j.jaci.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman S, Brena RM, Armstrong P, Hartiala J, Stephensen CB, Allayee H. Functional analysis of 5-lipoxygenase promoter repeat variants. Hum. Mol. Genet. 2009;18:4521–4529. doi: 10.1093/hmg/ddp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, Serhan CN. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]