Abstract
Background
In Gaucher disease (GD), acid-β-glucosidase (GBA1) gene mutations result in defective glucocerebrosidase and variable combinations of hematological, visceral, and diverse bone disease. Osteopenia is highly prevalent, but its age of onset during the natural course of GD is not known. It is also unclear if the degree of improvement in osteopenia, secondary to imiglucerase enzyme therapy, differs by the age of the patient.
Objective
We hypothesized that osteopenia develops early in life, during the natural course of type 1 Gaucher disease (GD1), and that its response to treatment is maximal during this period.
Methods
We examined data from the International Collaborative Gaucher Group (ICGG) Gaucher Registry of patients treated with imiglucerase between the ages of 5 and 50 years. Lumbar spine bone mineral density (BMD) (determined by dual-energy X-ray absorptiometry (DXA) and expressed as Z-scores) at baseline and for up to 10 years on imiglucerase were analyzed in children (ages≥5 to <12 years), adolescents (≥12 to <20 years), young adults (≥20 to <30 years), and older adults (≥30 to <50 years). BMD was correlated with other disease characteristics. Pre-treatment, descriptive statistics were applied to 5-year age categories. Non-linear mixed effects regression models were used to analyze DXA Z-scores over time after treatment with imiglucerase.
Results
Pre-treatment, low BMD was prevalent in all age groups, most strikingly in adolescents. DXA Z-scores were at or below −1 in 44% of children (n=43), 76% of adolescents (n=41), 54% of young adults (n=56) and 52% of older adults (n=171). The most common GBA1 genotype was N370S heteroallelic. Baseline hematological and visceral manifestations in the 4 age groups were similar. In children with DXA Z-scores ≤−1 at baseline, imiglucerase therapy for 6 years resulted in improvement of mean DXA Z-scores from −1.38 (95% CI −1.73 to −1.03) to −0.73 (95% CI −1.25 to −0.21); in young adults DXA Z-scores improved from −1.95 (95% CI −2.26 to −1.64) to −0.67 (95% CI −1.09 to −0.26). BMD also improved in older adults, but the magnitude of the improvement was lower compared to younger patients.
Conclusions
Low bone density is common in GD1 with the highest prevalence rate in adolescence, a developmental period critical to attainment of peak bone mass. Imiglucerase results in amelioration of osteopenia in all age groups, with the greatest improvements in younger patients.
Keywords: Gaucher disease, Osteopenia, Imiglucerase, Children, Adolescents, Adults
Introduction
In Gaucher disease (GD), the most common lysosomal storage disease, mutations in acid β-glucosidase (GBA1), the gene encoding lysosomal gluococerebrosidase, result in defective lysosomal glucocerebrosidase and widespread accumulation of glycolipid-laden macrophages. The resulting phenotype is diverse involving hepatomegaly, splenomegaly, thrombocytopenia, anemia, complex skeletal disease and decreased health-related quality of life. GD is a progressive disease and may lead to shortened life-expectancy [1] and debilitating complications. Three broad categories of GD are recognized: type 1 (non-neuronopathic), traditionally characterized by the absence of neurodegenerative disease typically found in types 2 and 3 of the disease, accounts for 94% of all cases.
In the skeleton, bone marrow infiltration with glucocerebroside-laden macrophages, known as Gaucher cells, occurs in the majority of the patients. The presence of Gaucher cells leads directly or indirectly to local, focal or generalized skeletal pathology. Clinically important bone manifestations of GD include severe acute “bone crises” (acute avascular osteonecrosis), medullary infarction, chronic pain, cortical and trabecular osteopenia, osteoporosis, osteolytic lesions, pathologic fractures, and growth failure in children. Nearly 100% of patients exhibit symptomatic or imaging evidence of 1 or more of these skeletal manifestations at diagnosis [2]. Many patients exhibit an Erlenmeyer flask deformity suggesting that there is defective bone remodeling in GD [2,3]. Osteopenia affects nearly 65% of type 1 Gaucher disease (GD1) patients in the International Collaborative Gaucher Group (ICGG) Gaucher Registry [2]. Osteopenia is reportedly worse in splenectomized patients and in those with GBA1 genotypes that are associated with clinically more severe phenotypes [4].
Enzyme replacement therapy with macrophage-targeted recombinant glucocerebrosidase, imiglucerase (Cerezyme®, Genzyme Corporation), has been administered to more than 4500 patients worldwide [5]. Imiglucerase therapy reverses hematological and visceral disease, reverses marrow infiltration, reduces incidence of avascular osteonecrosis, and improves health-related quality of life indicators [6]. Improvement in the osseous skeleton in response to imiglucerase occurs more slowly and incompletely compared to visceral and hematological responses [7]. Nevertheless, adult GD1 patients treated with imiglucerase showed a dose-dependent amelioration of osteopenia [7]. The extent of osteopenia at different ages in GD1 patients, or the magnitude of its response to imiglucerase enzyme therapy, has not been published or studied.
This study investigated the hypothesis that osteopenia develops early in life during the natural course of GD1 and that the response to treatment is maximal during this period. We tested our hypothesis in the largest global disease registry for a lysosomal storage disease, the ICGG Gaucher Registry, using bone mineral density (BMD) measurements over 10 years of follow-up.
Materials and methods
International Collaborative Gaucher Group Gaucher Registry
The ICGG Gaucher Registry was launched in 1991 to track the clinical, demographic, genetic, biochemical and therapeutic characteristics of patients with GD throughout the world, irrespective of disease severity, treatment status or treatment choice [3]. Governance and scientific direction is provided by an independent international group of physician experts in GD, with operational support from Genzyme Corporation (Cambridge, MA, USA). Since 1991, with Institutional Review Board/Ethics Committee approvals, physicians from 62 countries have voluntarily submitted de-identified data on over 5828 patients, capturing 51,628 patient years of follow-up. The major investigative goals of the Registry are to define the clinical spectrum of GD, assess its natural history through longitudinal follow-up, and assess the effect of treatment.
Study population
The study population included all Registry patients with GD1 from age 5–50 years who were treated with alglucerase and/or imiglucerase, and for whom lumbar spine BMD data (assessed by dual-energy X-ray absorptiometry (DXA)) were submitted by 1 April 2010. Alglucerase (Ceredase®, Genzyme Corporation placenta-derived macrophage-targeted glucocerebrosidase) and imiglucerase (Cerezyme®, Genzyme Corporation recombinant macrophage-targeted glucocerebrosidase) have been shown to be therapeutically equivalent in a randomized, 2-arm clinical trial [8]. Patients treated with alglucerase were eventually transitioned to imiglucerase once it was commercially available. For simplicity, treatment with these 2 pharmacologic glucocerebrosidases will henceforth be denoted as imiglucerase enzyme therapy. All patients included in this analysis had 1 or more DXA assessment within the range of 1 year before and through 10 years following initiation of imiglucerase. Patients who were treated with bisphosphonates were excluded from this analysis.
Patients were assigned to 1 of 4 groups according to age at initiation of imiglucerase treatment. There was no overlap among groups. The following categories were used: children ages ≥5 to <12 years; adolescents: ≥12 to <20 years; young adults: ≥20 to <30 years; and older adults: ≥30 to ≤50 years. The adults were divided into 2 groups, because one can still accrue bone mass during the third decade of life [9,10]. Approximately 70% of the patients in each group only received imiglucerase and 30% of the patients initiated treatment with alglucerase.
Baseline demographics and clinical characteristics
Baseline data is that closest to the date of the first enzyme infusion within a range of 1 year before or after the start of enzyme therapy. Baseline demographics include gender, age, treatment information, GBA1 genotype, ethnicity and country of origin. Baseline clinical characteristics include hemoglobin concentration, platelet count, liver volume, spleen volume, bone pain in the last 30 days, any previous bone crisis, and other skeletal disease manifestations.
Bone densitometry
Bone mineral density was measured using DXA of the lumbar spine with Hologic (Hologic™, Bedford, MA, USA), GE Lunar (GE Healthcare, Madison, WI, USA), and Norland (Cooper Surgical, Trumbull, CT, USA) scanners per election of the treating physician. Of the data presented, approximately 55% of the DXA measurements were from Hologic devices, 23% from GE Lunar, and less than 10% from Norland scanners (Supplemental Table 1). The DXA data were expressed in units of Z-scores, which state BMD as the number of standard deviations above or below the average BMD of a healthy subject of the same age and gender, such that a patient with a Z-score of 0 is at the mean for that age and gender. The majority (73% to 77%) of lumbar spine DXA assessments in all patient groups were of the L1–L4 vertebrae. We chose lumbar spine DXA because normative data to calculate Z-scores in children are only available for lumbar spine and not other sites.
Statistical analysis
Descriptive statistics were used for analysis of demographics and clinical characteristics. For DXA Z-score values, a plot was constructed showing mean and corresponding 95% confidence intervals at baseline according to age at initiation of therapy. To calculate changes in DXA Z-score after initiation of treatment, a non-linear mixed effects model using the Emax parameterization [11] was constructed for each age category. This parameterization allows the slope to vary with time and permits estimation of the maximal effect on bone mineral density following initiation of treatment. Results from the models are depicted in both graphical and tabular formats. Models were constructed for a subset of patients with low baseline BMD (defined as a Z-score of −1.0 or lower) and a total of 1 or more subsequent DXA measurements.
Results
As of 1 April 2010, from a total Registry population of 5643 patients, 3785 (67%) patients met the study inclusion criteria: GD1, date of diagnosis, and imiglucerase therapy, with a known starting date. Of these, 1254 patients had DXA data with 930 patients between 5 and 50 years of age at the start of imiglucerase therapy. After excluding patients who had been treated with bisphosphonates, 889 patients were eligible for stratification and analysis.
Patient demographics at first infusion of imiglucerase are shown in Table 1. In all age groups, the ratio of females to males was approximately 3:2. More than 85% had at least 1 N370S allele (Supplemental Table 2). Ninety-two to 98% of patients reported race/ethnicity as non-African–American/Caribbean. The percentage of patients with a history of splenectomy progressively declined by age group: approximately 33% in older adults, 26% in young adults, 16% in adolescents, and 7% in children. The mean dose of imiglucerase was similar among all groups, ranging from 35.5 to 39.9 U/kg/2 wks.
Table 1.
Patient demographics and characteristics.
| Age at first infusion of imiglucerase |
||||
|---|---|---|---|---|
| ≥ 5 to <12 years | ≥ 12 to <20 years | ≥ 20 to <30 years | ≥ 30 to ≤50 years | |
| Patients enrolled | 156 | 125 | 185 | 423 |
| Gender | n=156 | n=125 | n=185 | n=423 |
| Male | 71 (45.5) | 52 (41.6) | 68 (36.8) | 173 (40.9) |
| Female | 85 (54.5) | 73 (58.4) | 117 (63.2) | 250 (59.1) |
| Age at Gaucher diagnosis, y | n=156 | n=125 | n=185 | n=423 |
| Median (25th, 75th) | 6.0 (5.0, 8.0) | 11.0 (7.0, 15.0) | 20.0 (6.0, 24.0) | 29.0 (16.0, 37.0) |
| Mean (SD) | 6.3 (2.47) | 10.3 (4.99) | 15.9 (9.18) | 26.5 (13.32) |
| Year of diagnosis, n | n=156 | n=125 | n=185 | n=423 |
| <1991 | 15 (9.6) | 45 (36.0) | 86 (46.5) | 226 (53.4) |
| 1991–1999 | 63 (40.4) | 36 (28.8) | 49 (26.5) | 100 (23.6) |
| ≥2000 | 78 (50.0) | 44 (35.2) | 50 (27.0) | 97 (22.9) |
| Year of first infusion, n | n=156 | n=125 | n=185 | n=423 |
| <1995 | 22 (14.1) | 27 (21.6) | 43 (23.2) | 92 (21.7) |
| 1995–1999 | 42 (26.9) | 40 (32.0) | 64 (34.6) | 129 (30.5) |
| ≥2000 | 92 (59.0) | 58 (46.4) | 78 (42.2) | 202 (47.8) |
| Ethnicity, n | n=143 | n=112 | n=168 | n=371 |
| African–American/Caribbean | 12 (8.4) | 6 (5.4) | 7 (4.2) | 6 (1.6) |
| All othersa | 131 (91.6) | 106 (94.6) | 161 (95.8) | 365 (98.4) |
| Genotype | n=121 | n=99 | n=165 | n=387 |
| N370S/N370S | 8 (6.6) | 27 (27.3) | 49 (29.7) | 158 (40.8) |
| N370S/Other | 94 (77.7) | 65 (65.7) | 98 (59.4) | 217 (56.1) |
| Other/Other | 19 (15.7) | 7 (7.1) | 18 (10.9) | 12 (3.1) |
| Splenectomy status | n=156 | n=125 | n=185 | n=423 |
| Splenectomized | 11 (7.1) | 20 (16.0) | 47 (25.5) | 139 (32.9) |
| Intact spleen | 145 (92.9) | 105 (84.0) | 137 (74.5) | 284 (67.1) |
| Average dose of imiglucerase, U/kg/2 wks | n=152 | n=124 | n=181 | n=408 |
| Median (25th, 75th) | 37.5 (29.9, 54.9) | 36.9 (23.6, 56.6) | 30.0 (20.0, 56.9) | 30.0 (21.2, 56.2) |
| Mean (SD) | 39.9 (14.83) | 37.6 (17.05) | 35.5 (17.74) | 36.5 (17.35) |
Defined as Caucasian; Hispanic; American Indian; Asian; Jewish; Arab; or multi-ethnic.
Baseline hematological and visceral organ measurements at first infusion of imiglucerase are shown in Table 2. Here, the patients in each age stratum were basically similar. Anemia was present in 25% to 42% of patients and platelet counts were below 150×103/mm3 in 78% to 97% of patients, depending on the age group. Although the extent of splenomegaly was no different in children than in the other age groups, thrombocytopenia tended to be milder in children.
Table 2.
Hematological and visceral manifestations at first infusion of imiglucerasea.
| Age at first infusion of imiglucerase |
||||
|---|---|---|---|---|
| ≥5 to <12 years | ≥12 to <20 years | ≥20 to <30 years | ≥30 to ≤50 years | |
| Patients enrolled | 156 | 125 | 185 | 423 |
| Hemoglobinb | 91 | 65 | 77 | 148 |
| Median (25th, 75th) | 11.1 (10.0,12.0) | 11.8 (10.8,12.7) | 11.6 (10.4,13.2) | 12.4 (11.1,13.5) |
| Mean (SD) | 11.0 (1.33) | 11.7 (1.54) | 11.8 (1.86) | 12.3 (1.73) |
| Anemiab, n (%) | 91 | 65 | 77 | 148 |
| Yes | 27 (29.7) | 21 (32.3) | 32 (41.6) | 37 (25.0) |
| No | 64 (70.3) | 44 (67.7) | 45 (58.4) | 111 (75.0) |
| Platelet count (×103/mm3) c | 90 | 65 | 77 | 148 |
| Median (25th, 75th) | 115.0 (86.0,143.0) | 88.0 (68.0,118.0) | 76.0 (60.0,98.0) | 76.5 (60.0,110.0) |
| Mean (SD) | 115.8 (42.87) | 95.9 (40.32) | 77.8 (31.01) | 87.4 (47.96) |
| Platelet count below 150 (×103/mm3)c, n (%) | 90 | 65 | 77 | 148 |
| Yes | 70 (77.8) | 60 (92.3) | 75 (97.4) | 140 (94.6) |
| No | 20 (22.2) | 5 (7.7) | 2 (2.6) | 8 (5.4) |
| Spleen volume | 62 | 58 | 58 | 148 |
| Median (25th, 75th) | 13.4 (9.8,21.1) | 12.1 (7.8,20.5) | 10.6 (6.6,17.2) | 9.0 (5.5,15.0) |
| Mean (SD) | 16.2 (9.60) | 14.2 (8.29) | 13.0 (9.13) | 12.0 (10.19) |
| Splenomegaly (spleen volumed in multiples of normal), n (%) | 62 | 58 | 58 | 148 |
| Yes | 60 (96.8) | 54 (93.1) | 52 (89.7) | 116 (78.4) |
| No | 2 (3.2) | 4 (6.9) | 6 (10.3) | 32 (21.6) |
| Liver volume | 64 | 62 | 71 | 196 |
| Median (25th, 75th) | 1.9 (1.6,2.1) | 1.5 (1.3,1.9) | 1.5 (1.1,2.1) | 1.3 (1.1,1.8) |
| Mean (SD) | 1.9 (0.49) | 1.7 (0.80) | 1.7 (0.86) | 1.5 (0.61) |
| Hepatomegaly (liver volumed in multiples of normal), n (%) | 64 | 62 | 71 | 196 |
| Yes | 63 (98.4) | 49 (79.0) | 45 (63.4) | 112 (57.1) |
| No | 1 (1.6) | 13 (21.0) | 26 (36.6) | 84 (42.9) |
“At the time of first infusion” is defined as the data point closest to the first infusion date, no more than −8/+2 weeks (inclusive) from first infusion for hemoglobin/platelet, and −6 months/+6 weeks (inclusive) from first infusion for liver/spleen. Patients with no infusion date were excluded from the analysis for each hematological and visceral assessment.
Non-splenectomized patients only. Anemia is defined according to age and gender norms for hemoglobin concentrations as follows: <12 g/dL for males older than 12 years; <11 g/dL for females older than 12 years; <10.5 g/dL for children ages >2 to 12 years; <9.5 g/dL for children ages 6 months to 2 years; and <10.1 g/dL for children younger than 6 months of age.
Non-splenectomized patients only.
Spleen and liver volumes measured by ultrasound were calculated using the formulas reported by Elstein [25].
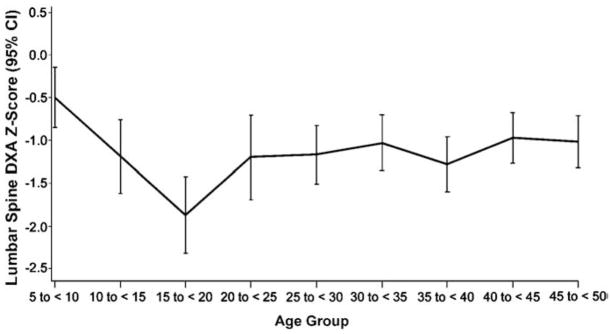
Children also had fewer reports of skeletal pain, bone infarctions and avascular necrosis (Table 3). Forty children (33% of 122) and 22 adolescents (33% of 69) were below the fifth percentile for height. DXA Z-scores were ≤−1 in 44% of children (19/43), 76% of adolescents (31/41), 54% of young adults (30/56) and 52% of older adults (89/171). Mean baseline DXA Z-scores according to age category are shown in Fig. 1 and Table 4. This graph illustrates progressive decline in DXA Z-scores within the pediatric age group with largest decline in the adolescents. The extent of osteopenia in young and older adults was similar.
Table 3.
Bone manifestations at first infusion of imiglucerasea.
| Age at first infusion of imiglucerase |
||||
|---|---|---|---|---|
| ≥5 to <12 years | ≥12 to <20 years | ≥20 to <30 years | ≥30 to ≤50 years | |
| Patients enrolled | n=156 | n=125 | n=185 | n=423 |
| Bone pain, n (%) | n=118 | n=78 | n=104 | n=258 |
| Absent | 71 (60) | 36 (46) | 43 (41) | 100 (39) |
| Present | 47 (40) | 42 (54) | 61 (59) | 158 (61) |
| Bone crisis, n (%) | n=115 | n=75 | n=97 | n=238 |
| Absent | 101 (88) | 59 (79) | 83 (86) | 213 (89) |
| Present | 14 (12) | 16 (21) | 14 (14) | 25 (11) |
| Radiologic bone disease, n (%) | n=104 | n=76 | n=112 | n=279 |
| Evidence of any bone disease | ||||
| Absent | 19 (18.3) | 8 (10.5) | 14 (12.5) | 34 (12.2) |
| Present | 85 (81.7) | 68 (89.5) | 98 (87.5) | 245 (87.8) |
| Type of bone disease reported | Any data available |
Abnormality present |
Any data available |
Abnormality present |
Any data available |
Abnormality present |
Any data available |
Abnormality present |
|---|---|---|---|---|---|---|---|---|
| n | n (%) | n | n (%) | N | n (%) | n | n (%) | |
| Avascular necrosis | 60 | 4 (6.7) | 44 | 12 (27.3) | 65 | 24 (36.9) | 175 | 47 (26.9) |
| Erlenmeyer flask deformity | 83 | 59 (71.1) | 46 | 37 (80.4) | 71 | 55 (77.5) | 171 | 120 (70.2) |
| Fractures | 43 | 0 (0) | 32 | 3 (9.4) | 37 | 3 (8.1) | 119 | 14 (11.8) |
| Infarction | 54 | 13 (24.1) | 44 | 20 (45.5) | 49 | 20 (40.8) | 164 | 73 (44.5) |
| Lytic lesions | 41 | 9 (22.0) | 30 | 6 (20.0) | 35 | 16 (45.7) | 117 | 41 (35.0) |
| Marrow infiltration | 60 | 49 (81.7) | 54 | 47 (87.0) | 75 | 70 (93.3) | 200 | 188 (94.0) |
| Age at first infusion of imiglucerase |
||||
|---|---|---|---|---|
| ≥5 to <12 years | ≥12 to <20 years | ≥20 to <30 years | ≥30 to ≤50 years | |
| Decreased bone mineral density (lumbar spine DXA Z-scoreb), n (%) | n=43 | n=41 | n=56 | n=171 |
| >−1 | 24 (55.8) | 10 (24.4) | 26 (46.4) | 82 (48.0) |
| ≤−1 to≥−2 | 14 (32.6) | 14 (34.1) | 18 (32.1) | 53 (31.0) |
| <−2 | 5 (11.6) | 17 (41.5) | 12 (21.4) | 36 (21.1) |
| Pediatric growth retardation, n (%) | n=122 | n=69 | n=0 | n=0 |
| Observed | 40 (33) | 22 (33) | 0 (0) | 0 (0) |
| Expectedc | 6 (5) | 3 (5) | 0 (5.0) | 0 (5.0) |
“At the time of first infusion” is defined as the data point closest to the first infusion date, no more than −2 years/+6 weeks (inclusive) from first infusion. Patients with no infusion date were excluded from the analysis for each bone assessment.
Standard deviations of age and gender-adjusted norms.
Pediatric growth retardation is defined as the number of patients who are below the 5th percentile for height based on age and gender of the normal healthy population and is calculated as 0.05× total number of patients [26].
Fig. 1.
DXA Z-scores in patients with type 1 Gaucher disease at the time of first imiglucerase infusion.
Table 4.
DXA Z-scores in patients with type 1 Gaucher disease at first infusion of imiglucerase.
| Age group (years) | N | Mean | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| 5 to<10 | 45 | −0.495 | −0.848 | −0.142 |
| 10 to<15 | 38 | −1.187 | −1.618 | −0.757 |
| 15 to<20 | 28 | −1.872 | −2.319 | −1.425 |
| 20 to<25 | 27 | −1.200 | −1.697 | −0.704 |
| 25 to<30 | 43 | −1.167 | −1.510 | −0.823 |
| 30 to<35 | 44 | −1.030 | −1.360 | −0.701 |
| 35 to<40 | 38 | −1.279 | −1.600 | −0.959 |
| 40 to<45 | 57 | −0.971 | −1.267 | −0.675 |
| 45 to 50 | 59 | −1.013 | −1.316 | −0.710 |
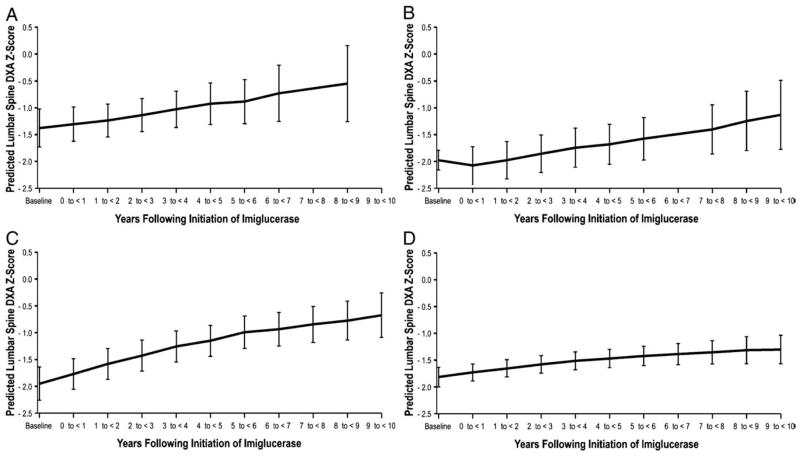
Regression models for DXA Z-scores following initiation of therapy with imiglucerase are shown in Fig. 2 for patients with baseline Z-scores less than −1. Among children (n=19), the mean DXA Z-scores improved from baseline −1.38 (95% CI −1.73 to −1.03) over 8 to 9 years of imiglucerase therapy to −0.73 (95% CI −1.25 to −0.21) (Table 5). Similar responses were observed in adolescents and both adult groups; in adolescents (n=23), mean baseline Z-scores of −2.16 (95% CI −2.53 to −1.79) increased to −1.13 (95% CI −1.78 to −0.49) after 10 years of imiglucerase (Table 6). Among young adults (n=30) after 10 years of imiglucerase therapy, mean baseline Z-scores of −1.95 (95% CI −2.26 to −1.64) increased to −0.67 (95% CI −1.09 to −0.26) (Table 7). The Z-scores for older adults (n=68), although improved, changed the least from −1.82 (95% CI −2.00 to −1.63) at baseline to −1.30 (95% CI −1.57 to −1.04) after 10 years of imiglucerase therapy (Table 8). For comparison, the distributions of the actual DXA Z-scores for each group are shown in Supplemental Table 3A–D.
Fig. 2.
DXA Z-scores of GD1 patients with 2 or more measurements during imiglucerase treatment in A. children (ages ≥5 years to <12 years), B. adolescents (ages ≥12 years to <20 years), C. young adults (ages ≥20 years to <30 years) and D. adults (ages ≥30 years to ≤50 years).
Table 5.
DXA Z-scores of children
| Years following initiation of imiglucerase | Baseline DXA ≤−1 (n=22 patients, 80 assessments) |
||
|---|---|---|---|
| Predicted DXA Z-score | Lower 95% confidence interval | Upper 95% confidence interval | |
| Baseline (−1 to<0) | −1.38 | −1.73 | −1.03 |
| 0 to<1 | −1.31 | −1.63 | −0.98 |
| 1 to <2 | −1.24 | −1.54 | −0.93 |
| 2 to <3 | −1.14 | −1.45 | −0.83 |
| 3 to <4 | −1.03 | −1.37 | −0.69 |
| 4 to <5 | −0.92 | −1.31 | −0.54 |
| 5 to <6 | −0.88 | −1.29 | −0.47 |
| 6 to <7 | −0.73 | −1.25 | −0.21 |
| 7 to <8 | . | . | . |
| 8 to <9 | −0.55 | −1.26 | 0.16 |
| 9 to <10 | . | . | . |
DXA Z-scores of patients with 2 or more measurements from a non-linear mixed model in children (ages≥5 years to <12 years).
Table 6.
DXA Z-scores of adolescents.
| Years following initiation of imiglucerase | Baseline DXA ≤−1 (n=23 patients, 89 assessments) |
||
|---|---|---|---|
| Predicted DXA Z-score | Lower 95% confidence interval | Upper 95% confidence interval | |
| Baseline (−1 to<0) | −2.16 | −2.53 | −1.79 |
| 0 to<1 | −2.08 | −2.43 | −1.73 |
| 1 to <2 | −1.98 | −2.32 | −1.63 |
| 2 to <3 | −1.86 | −2.21 | −1.51 |
| 3 to <4 | −1.75 | −2.11 | −1.38 |
| 4 to <5 | −1.68 | −2.06 | −1.31 |
| 5 to <6 | −1.58 | −1.98 | −1.18 |
| 6 to <7 | . | . | . |
| 7 to <8 | −1.41 | −1.87 | −0.95 |
| 8 to <9 | −1.25 | −1.80 | −0.69 |
| 9 to <10 | −1.13 | −1.78 | −0.49 |
DXA Z-scores of patients with 2 or more measurements from a non-linear mixed model in adolescent (ages≥12 years to <20 years).
Table 7.
DXA Z-scores of young adults
| Years Following Initiation of Imiglucerase | Baseline DXA ≤−1 (n=20 patients, 69 assessments) |
||
|---|---|---|---|
| Predicted DXA Z-Score | Lower 95% Confidence Interval | Upper 95% Confidence Interval | |
| Baseline (−1 to<0) | −1.95 | −2.26 | −1.64 |
| 0 to<1 | −1.77 | −2.05 | −1.48 |
| 1 to <2 | −1.58 | −1.87 | −1.29 |
| 2 to <3 | −1.43 | −1.71 | −1.14 |
| 3 to <4 | −1.25 | −1.54 | −0.96 |
| 4 to <5 | −1.15 | −1.44 | −0.86 |
| 5 to <6 | −0.99 | −1.29 | −0.69 |
| 6 to <7 | −0.93 | −1.25 | −0.62 |
| 7 to <8 | −0.84 | −1.18 | −0.51 |
| 8 to <9 | −0.77 | −1.14 | −0.41 |
| 9 to <10 | −0.67 | −1.09 | −0.26 |
DXA Z-scores of patients with 2 or more measurements from a non-linear mixed model in young adults (ages≥20 years to <30 years).
Table 8.
DXA Z-scores of adults.
| Years following initiation of imiglucerase | Baseline DXA ≤−1 (n=68 patients, 253 assessments) |
||
|---|---|---|---|
| Predicted DXA Z-score | Lower 95% confidence interval | Upper 95% confidence interval | |
| Baseline (−1 to<0) | −1.82 | −2.00 | −1.63 |
| 0 to<1 | −1.73 | −1.89 | −1.57 |
| 1 to <2 | −1.65 | −1.81 | −1.49 |
| 2 to <3 | −1.58 | −1.74 | −1.42 |
| 3 to <4 | −1.51 | −1.68 | −1.35 |
| 4 to <5 | −1.47 | −1.64 | −1.30 |
| 5 to <6 | −1.42 | −1.60 | −1.24 |
| 6 to <7 | −1.39 | −1.58 | −1.19 |
| 7 to <8 | −1.35 | −1.57 | −1.13 |
| 8 to <9 | −1.32 | −1.57 | −1.06 |
| 9 to <10 | −1.30 | −1.57 | −1.04 |
DXA Z-scores of patients with 2 or more measurements from a non-linear mixed model in adults (ages≥30 years to ≤50 years).
Discussion
This is the first study in patients with GD1 to examine BMD across an age spectrum ranging from childhood to adulthood, and to report BMD response to imiglucerase therapy in an age group-specific manner. We found that low BMD occurs commonly in the natural course of GD1 and that it begins to manifest as early as 5 years of age. Of all the age groups that we followed, the BMD deficit is most pronounced in the adolescent period (age ≥12 to <20 years), a period of maximal bone mass accrual. Normally, maximal bone mineral is accrued in the first 2 decades of life [12,13], and peaks in the third decade [14]. The subsequent course is of progressive decline of bone density [10]. Failure to achieve peak bone mass is believed to contribute to eventual osteoporosis in several disease states [15].
A major treatment goal in GD1 is to prevent osteoporosis and to reduce the risk of pathological and fragility fractures. Due to the rarity of GD1 and insufficient patient numbers for statistically valid analysis, fracture risk as a function of BMD has never been calculated. Nevertheless, in a recent study from the ICGG Gaucher Registry, fracture rates of 14% were reported in patients with GD1 [16]. In the general population it is estimated that the reduction of 1 standard deviation (SD) from baseline of total body BMD, as measured by DXA, doubles the risk for fractures [17]. Our findings suggest that low BMD begins early in the life of patients with GD1 and that this group is most responsive to enzyme replacement therapy with imiglucerase. Taken together, our findings underscore the importance of early diagnosis and intervention to achieve optimal peak bone mass in children and young adults as a therapeutic goal.
It should be noted that low BMD, although prevalent in all age groups, was most marked in adolescents. However, there was a disassociation from other phenotypic features of GD1 such as severity of hematological and visceral disease, which generally reflects the body burden of lipid-laden macrophages; severity of these extra-skeletal manifestations in the younger patients was similar to older patients. Compared to older adults, it is notable that in children, adolescents and younger adults, in whom osteopenia was most striking, there was the lowest prevalence of splenectomy, a procedure previously associated with a higher risk of osteopenia [4]. Taken together, these observations are consistent with recent findings that GBA1 deficiency results in impairment of osteoblast differentiation and proliferation leading to reduced bone formation [18]. The apparent failure of hematological and visceral severity to be predictive of low BMD, which is infrequently measured in children, adolescents and young asymptomatic adults with GD1 at the time of diagnosis or during follow-up visits, is clinically important because these parameters are probably the most common determinants influencing a decision to initiate treatment.
We found that the GBA1 genotype was not a critical determinant of risk for low BMD. Although heteroallelism for the N370S mutation was more common in children and adolescents, and N370S homoallelism was more prevalent in older patients, low BMD was encountered in all genotype categories. Many studies have demonstrated that patients heteroallelic for N370S genotypes tend to have more severe hematological and visceral manifestations of GD1, and are more likely to be diagnosed at a younger age than patients who are homoallelic for N370S [19]. It is not known if the apparent lack of association of GBA1 genotype with osteopenia is due to under-reporting of osteopenia in older, asymptomatic N370S homoallelic patients.
DXA is the most common method for assessing BMD and is used in routine clinical practice for this purpose. DXA provides an established, non-invasive, low radiation-exposure technique for monitoring BMD over time. The lumbar spine is one of the common measurement sites, and there is a large body of age-, gender- and race-standardized reference data. Additionally, the lumbar spine has been shown to be more sensitive to changes in systemic bone mass than other measurement sites in adults [4]. A limitation of DXA is the dependence of BMD on body size. Therefore in pediatric patients, delayed growth could contribute to apparent low BMD values in GD1. To reduce the effect of this confounder, we used Z-scores that take into account the normal growth pattern, as well as the expected normal decline of bone density in adulthood. Therefore, the Z-score is the preferred parameter to depict the bone-density phenotype of GD1 and assess the impact of enzyme replacement therapy with imiglucerase. Another possible confounder in our results is the dependence of bone density on ethnicity [20]. For this reason, we classified ethnicity as African–American/Caribbean or Other, and we utilized appropriate reference populations to calculate the Z-scores for these 2 groups. It should be noted that, although DXA is a reliable biomarker for BMD, other factors such as bone quality (i.e., bone strength or bone brittleness) are important additional determinants of fracture risk and were not measured in our study.
Due to the rarity of GD1, our study was only feasible due to the existence of the ICGG Gaucher Registry, which provided longitudinal data from a large worldwide patient population that can be analyzed by age group. Unlike clinical trials, Registry data are often not submitted at well-defined time points, and the severity at baseline and treatment effects are allowed to vary by patient. Mixed models are ideally suited for analyzing this type of longitudinal data. Nevertheless, there are limitations associated with our study that are common to most observational (non-randomized) research studies. All Registry data are retrospective and unaudited. Patients followed in the Registry are not randomized to treatment with imiglucerase. Scanner type (e.g., GE Lunar, Hologic, or Norland), as well as the specific scanner used to obtain DXA measurements, has varied among patients and may have varied for repeat visits of individual patients. Other potential confounders, such as genetic polymorphisms other than GBA1 genotype, epigenetic factors, and environmental factors such as concurrent illnesses, alcohol use and smoking, could conceivably contribute to osteopenia of GD1; we did not adjust for these potential confounders in this analysis.
Do our data contribute to an understanding of causative mechanisms for abnormal bone homeostasis in patients with GD1? Osteopenia in GD1 has generally been attributed to increased bone resorption [21]. However, biomarkers of osteoclast function are only inconsistently elevated in GD1 sera [22], and the accumulation of glucocerebroside has not been reported in GD osteoclasts. Moreover, when the bone responds to imiglucerase, there is concomitant improvement of serum biomarkers of osteoblast function, but no significant change in biomarkers of osteoclast function [21]. Additionally, inhibition of bone resorption with bisphosphonates at supra-pharmacological doses, given in conjunction with imiglucerase therapy, resulted in minimal improvement of osteopenia in adult GD1 patients [23]. Together, these findings point to a more complicated picture of determinants of osteopenia and the skeletal response to enzyme therapy. Our finding that osteopenia begins early in life, at a point when bone formation normally outpaces bone resorption, raises the possibility that osteoblast dysfunction may be more important than hitherto appreciated [24]. Indeed, the conditional deletion of the GBA1 gene in mice results in profound osteopenia due to osteoblast dysfunction, mediated by accumulating glycolipid through inhibition of protein kinase C [18]. Our results emphasize the importance of a clearer understanding of the effects of GD1 pathology and enzyme or other therapies for GD1, not only on tissue macrophages, but also on cells of other lineages.
Conclusions
Bone disease and its resulting disability are significant sources of long-term morbidity for patients with GD. Low bone density is prevalent in all age groups with GD1; it is most prevalent during adolescence, a developmental period critical to attainment of peak bone mass. Failure to achieve optimal bone mass at this age is likely to be a major determinant of peak bone mass generally attained by age 30 years. Imiglucerase results in amelioration of osteopenia in all age groups, and the effect is greatest in younger patients.
Supplementary Material
Acknowledgments
Funding sources
Logistical support for this study was provided by Genzyme Corporation. The database for the International Collaborative Gaucher Group (ICGG) Gaucher Registry is supported by Genzyme Corporation.
We thank the patients with non-neuronopathic GD and their physicians and health care personnel who submit data to the ICGG; the ICGG Publication Committee for their review of the manuscript; the ICGG Gaucher Registry support team at Genzyme Corporation; Sarah Kulke, MD; Radhika Tripuraneni, MD, MPH; John Taylor, MS for critical review of the manuscript; and Robert Brown for the graphic design of the figures.
Abbreviations
- BMD
bone mineral density
- DXA
dual energy X-ray absorptiometry
- GBA1
acid-β-glucosidase
- GD
Gaucher disease
- GD1
type 1 Gaucher disease
- ICGG
International Collaborative Gaucher Group
- SD
standard deviation
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.bcmd.2010.10.011.
Footnotes
Conflict of interests
Pramod Mistry, Neal Weinreb and Paige Kaplan receive honoraria and expense reimbursement for serving on a Board of Advisors of the ICGG Gaucher Registry. Pramod Mistry and Neal Weinreb receive travel reimbursements and/or honoraria and/or research support from Genzyme Corporation, Shire Pharmaceuticals, Amicus Therapeutics and Actelion. J. Alexander Cole is an employee of Genzyme Corporation. Andrea Gwosdow is a medical writer contracted by Genzyme Corporation.
Contributor Information
Pramod K. Mistry, Email: Pramod.Mistry@yale.edu.
Neal J. Weinreb, Email: boneal@winning.com.
Paige Kaplan, Email: Kaplan@email.chop.edu.
J. Alexander Cole, Email: alexander.cole@genzyme.com.
Andrea R. Gwosdow, Email: andrea.gwosdow@genzyme.com.
Thomas Hangartner, Email: thomas.hangartner@wright.edu.
References
- 1.Weinreb NJ, Deegan P, Kacena KA, et al. Life expectancy in Gaucher disease type 1. Am J Hematol. 2008;83:896–900. doi: 10.1002/ajh.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabowski GA, Kolodny EH, Weinreb NJ, et al. In: The Online Metabolic and Molecular Basis of Inherited Metabolic Disease. Scriver C, Beaudet A, Valle D, Slye W, editors. Chapter 146.1 McGraw-Hill Publishers; 2006. [Google Scholar]
- 3.Charrow J, Andersson HC, Kaplan P, et al. The Gaucher Registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Intern Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- 4.Pastores GM, Wallenstein S, Desnick RJ, Luckey MM. Bone density in type 1 Gaucher disease. J Bone Miner Res. 1996;11:1801–1807. doi: 10.1002/jbmr.5650111125. [DOI] [PubMed] [Google Scholar]
- 5.Weinreb NJ. Imiglucerase and its use for the treatment of Gaucher’s disease. Expert Opin Pharmacother. 2008;9:1987–2000. doi: 10.1517/14656566.9.11.1987. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb N, Barranger J, Packman S, et al. Imiglucerase (Cerezyme) improves quality of life in patients with skeletal manifestations of Gaucher disease. Clin Genet. 2007;71:576–588. doi: 10.1111/j.1399-0004.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 7.Wenstrup RJ, Kacena KA, Kaplan P, et al. Effect of enzyme replacement therapy with imiglucerase on BMD in type 1 Gaucher disease. J Bone Miner Res. 2007;22:119–126. doi: 10.1359/jbmr.061004. [DOI] [PubMed] [Google Scholar]
- 8.Grabowski GA, Barton NW, Pastores G, et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Recker RR, Davies KM, Hinders SM, et al. Bone gain in young adult women. JAMA. 1992;268:2403–2408. [PubMed] [Google Scholar]
- 10.Matkovic V, Jelic T, Wardlaw GM, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93:799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holford NH, Sheiner LB. Understanding the dose–effect relationship: clinical application of pharmacokinetic–pharmacodynamic models. Clin Pharmacokinet. 1981;6:429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab. 1991;73:555–563. doi: 10.1210/jcem-73-3-555. [DOI] [PubMed] [Google Scholar]
- 13.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 14.Teegarden D, Proulx WR, Martin BR, et al. Peak bone mass in young women. J Bone Miner Res. 1995;10:711–715. doi: 10.1002/jbmr.5650100507. [DOI] [PubMed] [Google Scholar]
- 15.Winsloe C, Earl S, Dennison EM, Cooper C, Harvey NC. Early life factors in the pathogenesis of osteoporosis. Curr Osteoporos Rep. 2009;7:140–144. doi: 10.1007/s11914-009-0024-1. [DOI] [PubMed] [Google Scholar]
- 16.Kahn A, Hangartner TN, Weinreb NJ, et al. Characterizing extra-skeletal phenotype and biomarkers of type 1 Gaucher disease patients with avascular necrosis (AVN) or fractures: a study from the ICGG Gaucher Registry—preliminary analysis. 11th International Conference of Inborn Errors of Metabolism; San Diego, California. 2009. [Google Scholar]
- 17.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 18.Mistry PK, Liu J, Yang M, et al. GBA1 deficient mice recapitulates Gaucher disease displaying system-wide cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci, USA. 2010 Oct 20; doi: 10.1073/pnas.1003308107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibille A, Eng CM, Kim SJ, Pastores G, Grabowski GA. Phenotype/genotype correlations in Gaucher disease type I: clinical and therapeutic implications. Am J Hum Genet. 1993;52:1094–1101. [PMC free article] [PubMed] [Google Scholar]
- 20.Short DF, Zemel BS, Gilsanz V, et al. Fitting of bone mineral density with consideration of anthropometric parameters. Osteoporos Int. 2010 May 21; doi: 10.1007/s00198-010-1284-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims KB, Pastores GM, Weinreb NJ, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clin Genet. 2008;73:430–440. doi: 10.1111/j.1399-0004.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciana G, Addobbati R, Tamaro G, et al. Gaucher disease and bone: laboratory and skeletal mineral density variations during a long period of enzyme replacement therapy. J Inherit Metab Dis. 2005;28:723–732. doi: 10.1007/s10545-005-0032-y. [DOI] [PubMed] [Google Scholar]
- 23.Wenstrup RJ, Bailey L, Grabowski GA, et al. Gaucher disease: alendronate disodium improves bone mineral density in adults receiving enzyme therapy. Blood. 2004;104:1253–1257. doi: 10.1182/blood-2003-11-3854. [DOI] [PubMed] [Google Scholar]
- 24.Van Dussen L, Lips P, Everts V, et al. Markers of bone metabolism: should current concept of bone disease in Gaucher patients be remodeled?. 9th European Working Group on Gaucher Disease; Cologne, Germany. 2010. [Google Scholar]
- 25.Elstein D, Hadas-Halpern I, Azuri Y, et al. Accuracy of ultrasonography in assessing spleen and liver size in patients with Gaucher disease: comparison to computed tomographic measurements. J Ultrasound Med. 1997;16:209–211. doi: 10.7863/jum.1997.16.3.209. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.