Abstract
Aims
We previously found that in mice with experimental myocardial infarction (MI), 17β-estradiol (E2) increased mortality and worsened cardiac remodeling and these deleterious effects were associated with renal enlargement and hydronephrosis in a dose-dependent manner. In the present study we questioned whether E2-induced renal damage predisposes to rather than results from its adverse effectson the heart.
Main methods
Ovariectomized (ovx) mice received either placebo (P) or E2 at 0.02 (E2-L, low dose), 0.42 (E2-M, moderatedose ) or 4.2 μg/d (E2-H, highdose ) for 8 weeks.
Key findings
E2-L partially restored uterine weight and plasma estrogen levels without affecting heart, lung and liver weight, hemodynamic parameters, or heart and kidney morphology and function. E2-M restored normal uterine weight, but this was accompanied by a significant increase in kidney weight, albuminuria, glomerular matrix formation and markers for oxidative stress. E2-H increased uterine weight 4.5-fold and resulted in higher plasma creatinine levels, severe albuminuria, renal tubular dilatation, tubulointerstitial injury, hydronephrosis, glomerulosclerosis and oxidative stress. E2-H also caused ascites, hepatomegaly and fluid retention in the uterinehorns but had no significant effect on blood pressureor heart function.
Significance
Our data demonstrated that an excessive dose of E2 that raises uterine weight beyond physiological levels adversely affects the kidney even before it damages the heart. We believe estrogen dosage should be taken into account when considering hormonal replacement therapy, sincei nappropriate doses of E2may damage not only the heart but also the kidney.
Keywords: estrogen dosage, heart, kidney, hydronephrosis andalbuminuria
INTRODUCTION
Women have a lower incidence and prevalence of cardiovascular and renal disease and slower progression from chronic renal disease to end-stage renal failure compared to age-matched men (Adams, Jr. et al. 1999; Isles et al. 1992; Brett and Madans 1995; Silbiger and Neugarten 2008; Neugarten et al. 2000; Eriksen and Ingebretsen 2006). This gender advantage for females becomes far less or disappears with increased age and reduced estrogen levels after menopause, suggesting that ovarian hormones, most likely estrogen, protect women against cardiovascular and renal disease (Lerner and Kannel 1986; Eaker et al. 1993). Observational studies have demonstrated that postmenopausal women who receive hormone replacement therapy (HRT) have a lower rate of cardiovascular disease and cardiac death than those who do not (Stampfer et al. 1991; Grodstein et al. 2000; Blum et al. 2000). However, two recent randomized prospective primary or secondary prevention trials, the Women’s Health Initiative (WHI)(Rossouw et al. 2002) and the Heart and Estrogen/Progestin Replacement Study (HERS II) (Hulley et al. 2002), showed that HRT can have an unfavorable outcome on the risk and events of CVD. Although these data are convincing, it is not clear whether such unfavorable outcomes are at least partly related to the age of the patients and the time when HRT is begun (Hodis 2008; Grodstein et al. 2003; Schnatz 2006), nor whether HRT also exerts an adverse effect on the kidney.
Estrogen dosage may contribute to the variable outcome of HRT. In most observational and clinical trials, 0.625 mg/day oral conjugated equine estrogen (CEE) plus 2.5mg/day medroxyprogesterone acetate (MPA) was used. Few studies have addressed the possible impact of a single-dose regimen on the outcome of HRT. Grodstein et al reportedthat a low dose of CEE (0.3 mg/day) lessened major coronary events, whereas at 0.625 mg or higher and combined with progestin it significantly increased the risk of stroke (Grodstein et al. 2000). Sanada et al (Sanada et al. 2003) confirmed that low-dose CEE (0.3 mg/day for 3 months) improved endothelial function and decreased LDL cholesterol nearly as well as the standard dose of 0.625 mg/day. More recently, Villa et al (Villa et al. 2010) reported that low-dose estrogen (1 mg) in combination with drospirenone (a new progestin derived from 17α-spronolactone) had a favorable effect on lipid profile and endothelial function. In animal studies, wide range of estrogen dosages (from 0.17 to 83.3 μg/day) has been used. However, nearly all studies employed only a single dose, and its effects on the heart were reported to be beneficial, ineffective or detrimental (Beer et al. 2007; Hügel et al. 1999; van Eickels et al. 2003). Thus it is difficult to be sure whether these divergent findings were dose-related or due to differences in species or strain. We previously tested the dose-effect of 17β-estradiol (E2) in mice myocardial infarction (MI) and found that at 0.42 μg/day it tended to be cardioprotective; however, at increased doses (4.2 and 18.8 μg/day) it not only increased mortality but also worsened cardiac hypertrophy, LV chamber dilatation and heart dysfunction. Moreover, when we treated mice with high doses of E2 we found dose-dependent enlargement of the liver and kidney (Zhan et al. 2008). These findings were provocative; however, there are questions remained to be answered, namely, 1) whether the low dose was low enough to provide cardioprotection; and 2) whether the renal damage was directly due to the cardiac damage or an entirely separate effect of the hormonal treatment.
In the present experiment, we added a much lower dose of E2 (0.02μg/day) to two doses from our previous study (0.42 and 4.2 μg/day), focusing on the effect of E2 on renal morphology and function in ovariectomized mice without MI in an attempt to clarify 1) whether E2 causes structural and functional injuries in the kidney in a dose-dependent manner; and 2) whether E2- induced renal damage predisposes torather than results from its adverse effects on the heart.
MATERIALS AND METHODS
Animals
Female C57BL/6J mice 4 weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). They were housed in an air-conditioned room with a 12-hour dark/light cycle and given standard chow with free access to tap water. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System in accord with the National Institutes of Health Guidelinesfor the Care and Use of Laboratory Animals.
Ovariectomy and estrogen replacement
Mice were anesthetized with pentobarbital (50 mg/kg, i.p.) and bilateral ovariectomy (ovx) was performed via a pair of flank incisions (Cavasin et al. 2004; Cavasin et al. 2003). 60-day-release pellets containing placebo or 17β-estradiol (E2 0.02, 0.42, or 4.2 μg/day; Innovative Research of America, Sarasota, FL) were implanted subcutaneously at the back of the neck immediately after ovx(Zhan et al. 2008). Sham ovx groups hadthe same procedure except that the ovaries were left intact.
Experimental protocol
Mice were divided into five groups: 1) sham-ovx; 2) ovx + placebo; 3) ovx + low dose (E2-L, 0.02 μg/d); 4) ovx + moderate dose (E2-M, 0.42 μg/d); and 5) ovx + high dose (E2-H, 4.2 μg/d). Treatment was continued for 8 weeks.
Systolic blood pressures (SBP), cardiac function and organ harvest
At the end of the experiment, SBP was determined by tail cuff (Visitech BP-2000, Apex, NC) following a 7-day training period (Xu et al. 2007). Transthoracic echocardiography was performed in conscious mice using a Doppler echocardiographic system equipped with a 15-MHz linear transducer (Acuson C512, Mountain View, CA) as described previously (Yang et al. 1999; Xu et al. 2007). Mice were then anesthetized with pentobarbital (50 mg/kg, i.p.) and a Millar micromanometer pressure catheter (Millar Instruments, Houston, TX) was inserted into the left ventricle (LV) via the right carotid artery to measure LV pressure and dP/dtmax/iP (maximum rate of the rise in LV pressure divided by pressure at maximum dP/dt), an indicator of isovolumic contraction (Cingolani et al. 2003; Sun et al. 2005). After blood was collected, the heart was stopped at diastole by injecting 15% KCl. The heart, lung, kidney, liver and uterus were removed and weighed. The LV was sectioned transversely into 3 slices, and the middle section was rapidly frozen in isopentane and stored at −70°C for determination of interstitial collagen fraction (ICF) and cardiomyocyte cross-sectional area (MCSA) as described previously (Xu et al. 2007). Kidneys were fixed in 10% formalin, processed and embedded in paraffin for histological analysis.
Blood collection and plasma estrogen and creatinine
After measuring cardiac hemodynamics and function, 500 μl of blood was withdrawn from the vena cava and plasma was kept at –70°C until assay. Plasma estrogen and creatinine were determined by enzyme immunoassay using a commercially available kit (Cayman Chemicals, Ann Arbor, MI).
Urine volume, albumin, creatinine clearance and 8-isoprostane
During the last week of the experiment, mice were placed in metabolic cages. After 5 days of acclimatization, 24-hour urine was collected, centrifuged, filtered and stored at −20°C until assay. Urinary albumin was measured with a commercially available ELISA kit (Alpha Diagnostics, San Antonio, TX) and expressed as μg/24 hr. Creatinine clearance (CrCl) was calculated using the formula: (urinary creatinine x urine volume)/(plasma creatinine x 24 hr) and expressed as μl/min/10 mg kidney weight. 8-isoprostane was measured with an enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI) (Schnackenberg and Wilcox 1999)and expressed as ng/24 hr.
Western blot for NADPH oxidase subunit gp91phox (Nox2) protein expression
Kidney tissue was homogenized in lysis buffer, the supernatant collected and protein content detected with Coomassie protein assay reagent (Thermo Scientific, Waltham, MA). Protein extracts (60 μg/lane) were separated out in 10% SDS-PAGE under reducing conditions and electrotransferred to a nitrocellulose membrane. Membranes were blocked in buffer (TBS/0.1% Tween 20 containing 5% non-fat milk) and incubated overnight at 4°C with a monoclonal antibody against Nox2 (1:500, BD Transduction, Lexington KY). They were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000, Amersham Pharmacia Biotech, Piscataway, NJ) for 1 hr at room temperature. Immunoreactive bands were detected with a chemiluminescent reaction kit (Amersham) and semi-quantified by densitometry. Nox2 protein was normalized by GAPDH.
Histology of the kidney
i) Tubulointerstitial injury
Renal sections were deparaffinized in xylene, dehydrated by ethanol gradient and stained with hematoxylin and eosin (H&E). Renal interstitial collagen was assessed by picrosirius red staining and calcium deposition assessed by von Kassa staining; dark-colored calcium crystals were visualized microscopically using a dark filed with polarized light illumination. Tubulointerstitial injury was evaluated using a modified semi-quantitative scoring system (Hayakawa and Raij 1998). Severity of injury was scored from 0 to 3 according to four criteria: 1) distance between two neighboring tubules: 0 = no change, 1 = slightly increased, 2 = moderately increased, 3 = greatly increased associated with interstitial fibrosis; 2) number of dilated tubules: 0 = none, 1 = a few, 2 = a moderate number, 3 = most of them; 3) pathology of tubules: 0 = no change, 1 = a few flattened epithelial cells combined with loss of the brush border, 2 = dead cells or cell fragments in the lumen, 3 = casts in the lumen, luminal obstruction and tubularnecrosis; 4) Calcium phosphate deposition: 0 = No calciumcrystal sidentified; 1 = a few dark-colored crystals observed in the cortex at 40x magnification; 2 = calcium crystals appeared in the cortex or medulla at 10x or 20x magnification and easily identified under 40x magnification; 3 = calcium crystals easily seen in many areas of the renal tissue at 10x and 20x magnification and extensively distributed in the tubules and interstitial space of the cortex and medulla. Each criterion was evaluated in at least 10 microscope fields and averaged. Scores from all four criteria were added together. Injury was scored independently by three investigators in a blind fashion.
ii) Glomerular matrix
Sections were deparaffinized, dehydrated and fixed in 4% formaldehyde for 20 min, then stained with Schiff’s reagent (PAS; Sigma, St. Louis, MO) for 15 min as described previously (Liao et al. 2008). Dark purple areas of the glomerulus were considered to present the extracellular matrix. Twenty-five to 30 glomeruli in each section were imaged at 40× magnification. Data were analyzed by computerized imaging software (Microsuite Biological, Olympus, Center Valley, PA). The glomerular matrix was expressed as a percentage of the glomerular area.
Data analysis
All data were summarized by mean ± SE. Student’s two-sample t-test was used to compare differences and calculate p-value between two groups. Since multiple comparisons were involved, Hochberg’s step-up procedure was used to adjust p-values so that the family-wise type I error rate (at the 0.05 level) would be controlled. Differences were considered significant if the adjusted p-value wasless than 0.05.
RESULTS
Effect of E2 replacement on organ weight
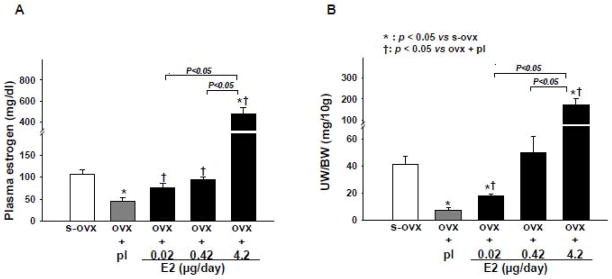
Efficacy of 8 weeks of E2 replacement was determined based on uterine weight and plasma estrogen levels (Figure 1). Ovariectomy (ovx) significantly reduced uterine weight. E2 at low doses (E2-L) partially but significantly restored uterine weight. E2-M restored uterine weight to the same level as sham-ovx but E2-H caused an excessive increase (about 5-fold higher than sham-ovx). All mice in the E2-H group exhibited fluid retention in the uterine horns and stiffening ofthe uterusas we ll asmoderate to severe urine retention in the bladder, hydronephrosis and ascites, although the ureter was not obstructed. Ovx with or without E2 replacement had no effect on heart and lung weight (Table 1).
Figure 1.
Dose-effect of 17β-estradiol (E2) replacement on plasma estrogen levels (A) and uterine weight corrected by body weight (UW/BW) (B) in ovariectomized (ovx) mice. s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose(all doses given for 8 weeks).
Table 1.
Dose-Effect of 17β Estradiol (E2) on Organ Weight.
| Groups |
Sham-ovx n = 7 |
ovx + placebo n= 8 |
ovx + E2 |
||
|---|---|---|---|---|---|
| Parameters | ovx + L n = 9 |
Ovx + M n= 8 |
ovx + H n = 8 |
||
| BW (g) | 19.3±0.6 | 20.5±0.3 | 21.5±0.5 | 19.9±0.4 | 20.0±0.4 |
| Uterine weight/BW (mg/10 g) | 41±6 | 7±1* | 14±2*† | 50±12†§ | 170±30*†§# |
| Kidney weight/BW (mg/10 g) | 116±4 | 105±4 | 109±5 | 127±3*†§ | 125±2*†§ |
| Heart weight/BW (mg/10g) | 39.6±0.8 | 39.9±1.7 | 36.7±2.5 | 41.3±1.1 | 39.6±1.4 |
| Lung weight/BW (mg/10 g) | 54±2.6 | 53±1.1 | 51±1.6 | 51±1.3 | 54±2.8 |
| Liver weight/BW (mg/10 g) | 468±22 | 430±36 | 443±42 | 481±17 | 516±16*† |
ovx: ovariectomy; E2: 17β-estradiol; L: low dose (0.02 μg/day); M: moderate dose (0.42 μg/d); H: high dose (4.2 μg/d). BW: body weight.
p < 0.05 vs sham-ovx;
p < 0.05 vs ovx + placebo;
p < 0.05 vsovx +L;
p < 0.05 vsovx + M.
Effect of E2 on systolic blood pressure, heart rate, cardiac remodeling and function
Systolic blood pressure (SBP) and heart rate (HR) were similar among groups, indicating that neither one was affected by ovx or E2 replacement (Table 2). Ovx and ovx + E2 at all doses had no significant effect on LV pressure, dP/dtmax/iP (an indicator of isovolumic contraction), dP/dtmin or ejection fraction. However, at moderate and high doses E2 increased LV chamber dimension, area and mass (Table 2). Histological evaluation showed that ovx increased both interstitial collagen fraction (ICF) and myocyte cross-sectional area (MCSA) significantly compared to sham-ovx, which wasreversed by E2-Lbut not E2-M orE2 -H (Table 2).
Table 2.
Dose-Effect of Estrogen on Left Ventricular Function, Morphologyand Histolog y.
| Groups |
Sham-ovx | ovx + placebo | ovx +E2 |
||
|---|---|---|---|---|---|
| Parameters | ovx + L | ovx + M | ovx + H | ||
| SBP (mmHg) | 105±5 | 104±2 | 106±4 | 106±3 | 101±3 |
| HR (beats/min) | 659±19 | 649±14 | 659±10 | 658±13 | 629±15 |
| dP/dtmax/iP (sec−1) | 98±3 | 91±7 | 107±13 | 122±7 | 94±1 |
| dP/dtmin (mmHg/sec) | −3022±107 | −2837±270 | −3293±431 | −3539±347 | −2957±245 |
| LVDs (mm) | 1.06±0.06 | 1.11±0.03 | 1.08±0.02 | 1.16±0.04 | 1.18±0.04* |
| LVDd (mm) | 2.07±0.09 | 2.23±0.06 | 2.19±0.04 | 2.35±0.04* | 2.27±0.06* |
| PWTd (mm) | 0.77±0.02 | 0.80±0.02 | 0.78±0.01 | 0.84±0.02 | 0.87±0.01*§ |
| LVAs (mm2) | 0.71±0.10 | 0.75±0.01 | 0.79±0.02 | 0.88±0.04*† | 1.0±0.09*†§ |
| LVAd(mm2) | 3.62±0.03 | 3.95±0.16 | 4.07±0.13 | 4.35±0.18* | 4.41±0.2*† |
| LV mass (mg/10 g) | 21.2±4.1 | 22.7±0.5 | 20.9±0.8 | 27.4±1.5*†§ | 27.5±1.4*†§ |
| EF (%) | 80.5±1.3 | 80.9±0.5 | 80.7±0.5 | 79.7±0.7 | 77.5±1.1 |
| ICF (%) | 4.7±0.3 | 5.9±0.4* | 4.7±0.4† | 5.7±0.5* | 6.1±0.2*§ |
| MCSA (mm2) | 141±3 | 151±2* | 142±3† | 150±2* | 148±5*§ |
ovx: ovariectomy; E2: 17β-estradiol; L: low dose (0.02 μg/day); M: moderate dose (0.42 μg/d); H: high dose (4.2 μg/d). SBP: systolic blood pressure; HR: heart rate; iP: left ventricular instant pressure; LVDs and LVDd: LV systolicand diastolic dimensions; PWTd: diastolic posterior wall thickness; LVAsand LVAd: LV systolic and diastolic area; EF: ejection fraction; ICF: interstitial collagen fraction; MCSA: myocyte cross-sectional area.
p < 0.05 vssham -ovx;
p < 0.05 vs ovx +placebo;
p < 0.05 vs ovx +L.
Dose-Effect of Estrogen on Renal Function and Histology
Kidney weight and renal function
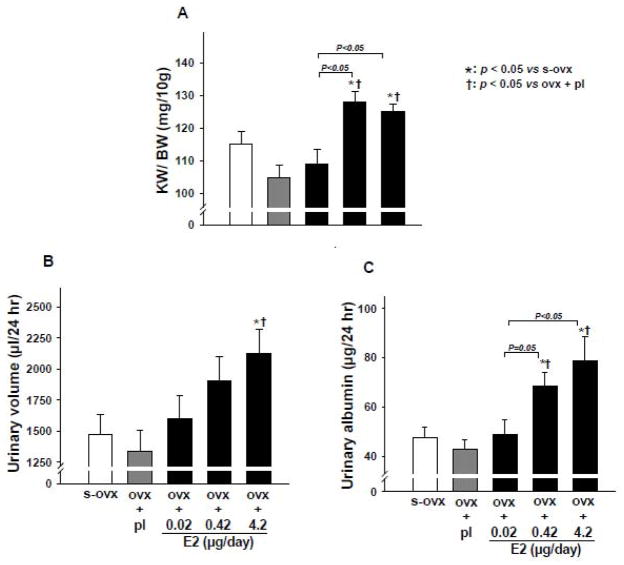
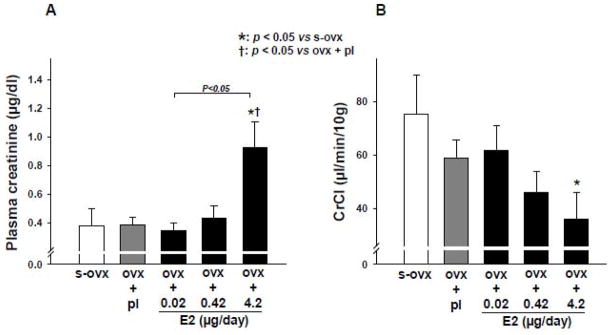
We found that ovx alone or combined with E2-L had no effect on kidney weight, whereas E2-M and E2-H significantly increased the ratio of kidney to body weight (Figure 2A). Urine volume was not affected by ovx per se or ovx plus E2-L or E2-M but was significantly increased by E2-H compared to sham ovx controls (Figure 2B); however, 24-hr urinary albumin excretion was increased significantly in both E2-M and E2-H groups (Figure 2C). Plasma creatinine remained unchanged with ovx alone or ovx + E2-L or E2-M but increased significantly with E2-H (Figure 3A). CrCl tended to decreased with E2-M compared to s-ovx, although the difference was not statistically significant. E2-H significantly reduced CrCl (Figure 3B).
Figure 2.
Dose-effect of 17β-estradiol (E2) replacement on kidney weight corrected by body weight (KW/BW) (A), 24-hr urine volume (B), and 24-hr urinary albumin excretion (C) in ovariectomized (ovx) mice. s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose.
Figure 3.
Dose-effect of 17β-estradiol (E2) replacement on plasma creatinine (A) and creatinine clearance (CrCl) (B) in ovariectomized (ovx) mice. s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose.
Tubular dilatation and glomerular matrix formation
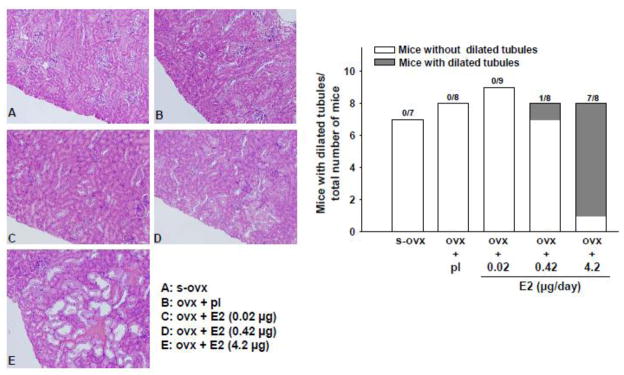
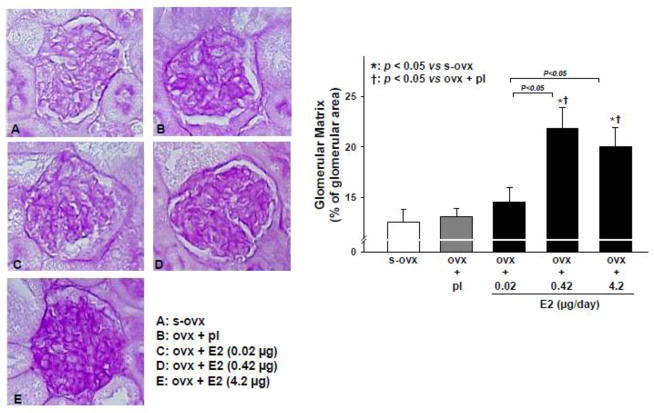
Ovx alone or combined with E2-L had no effect on the renal tubules. However, 1 out of 8 mice given E2-M developed tubular dilatation, while 7 out of 8 mice in the E2-H group had severe dilatation and many tubules contained waxy casts (Figure 4). On PAS staining, ovx alone or combined with E2-L had no effect on the glomerular matrix; however, both E2-M and E2-H increased matrix formation significantly and similarly(Figure 5).
Figure 4.
Dose-effect of 17β-estradiol (E2) replacement on renal tubule dilatation in ovariectomized (ovx) mice. Left panels: representative images showing E2-induced tubular dilatation (seen mostly with high-dose E2) (E). Right panel: number of mice exhibiting tubular dilatation in each treatment group. s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose.
Figure 5.
Dose-effect of 17β-estradiol (E2) replacement on glomerulosclerosis in ovariectomized (ovx) mice. Left panels: representative images showing E2-induced increase in glomerular matrix (dark purple-stained area seen with periodic acid-Schiff staining). Right panel: quantitative analysis of E2-induced glomerulosclerosis. s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose.
Tubulointerstitial injury
Representative images of tubulointerstitial injury caused by E2-H and semi-quantitative injury scores are shown in Figure 6. Ovx per se increased tubulointerstitial injury, influenced mainly by increased calcium deposition scores. This injury was partly reversed by E2-L but unaffected by E2-M. In contrast, E2-H worsened renal injury, as evidenced by a greater distance between neighboring tubules, an increased number of dilated tubules, flattened epithelial cells with loss of the brush border, dead cells, cell fragments and/or casts in the lumen along with interstitial fibrosisand calcium phosphate deposition.
Figure 6.
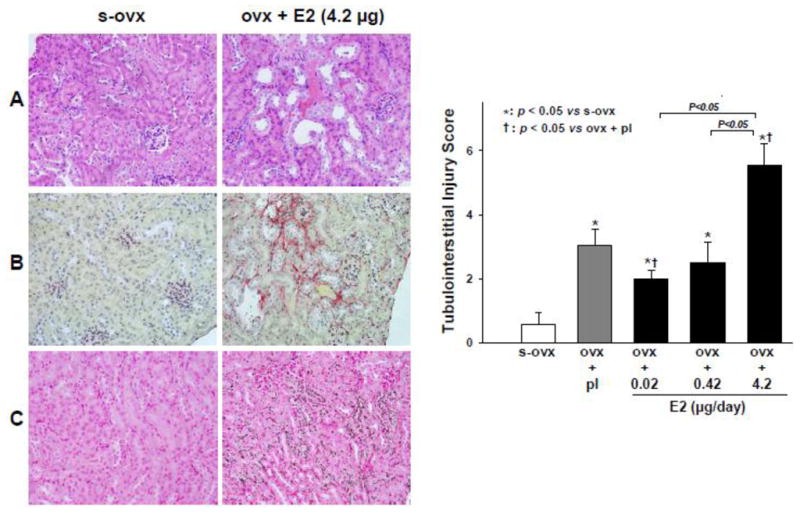
Dose-effect of 17β-estradiol (E2) replacement on tubulointerstitial injury score. Left panels: representative images ofhigh -dose E2-induced tubulointerstitialinjury; A: dilated tubules combined with flattened epithelial cells, loss of the brush border, dead cells and/or cell fragments and casts in the lumen as well as increased distance between two neighboring tubules; B: interstitial fibrosis (picrosirius red staining); and C: renal calcium deposition (von Kassa staining, showing dark calcium crystals using a dark filed and polarized light). s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose.
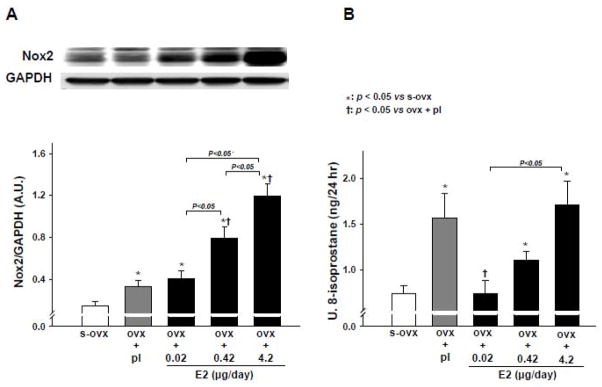
Nox2 protein expression and urinary 8-isoprostane excretion
Ovx increased Nox2 protein expression, which was not affected by E2-L but was enhanced by E2-M and E2-H in a dose-dependent manner (Figure 7A). Urinary 8-isoprostane excretion was significantly increased by ovx, but E2-L reversed it to sham-ovx levels. Both E2-M and E2-H groups exhibited significantly higher levels of 8-isoprostane than sham-ovx controls (Figure 7B).
Figure 7.
Dose-effect of 17β-estradiol (E2) replacement on Nox2 protein expression (A) and urinary 8-isoprostane excretion (B) in ovariectomized (ovx) mice. s-ovx: sham ovariectomy; pl: placebo; 0.02 μg/day: E2-low dose; 0.42 μg/day: E2-moderate dose; 4.2 μg/day: E2-high dose.
DISCUSSION
Using a mouse model of surgically induced menopause (ovx), we examined the dose-dependent effectsof estrogen replacement on the heart and kidney. We found that in mice with an intact heart E2 did not affect systolic blood pressure or contractile function at any of the doses we tested, although LV chamber dimension and wall thickness were slightly but significantly increased by moderate (E2-M) and high doses (E2-H). However, in the kidney dose-dependent detrimental effects of E2 (especially E2-H) on morphology and function became apparent, including: 1) increased kidney weight and hydronephrosis; 2) increased plasma creatinine and albuminuria; 3) increased glomerular matrix formation and interstitial fibrosis; 4) tubular dilatation and tubulointerstitial injury as estimated by semi-quantitative scoring; and 5) increased oxidative stress as indicated by Nox2 protein expression and urinary 8-iosprostane excretion. We believe this is the first reported evidence that E2 causes dose-dependent renal damage in ovariectomized micewith an intact heart, which may contributeto its adverse cardiac effect.
Women have a lower incidence and prevalence of renal disease and slower progression from chronic renal disease to end-stage renal failure (Silbiger and Neugarten 2008; Neugarten et al. 2000; Eriksen and Ingebretsen 2006). This gender advantage for women becomes less or disappears with increased age and reduced estrogen levels after menopause. Animal studies also showed that females are protected from hypertension and renal injury and this protection diminishes or disappears when they are ovariectomized to surgically induce menopause (Reckelhoff and Maric 2010; Sartori-Valinotti et al. 2007; Zhang et al. 2007; Sullivan et al. 2010). These results suggest that ovarian hormones, most likely estrogen, protect females against cardio-renal disease. However, the outcome of hormonal replacement therapy (HRT) on the kidney remains controversial. Agarwal et al (Agarwal et al. 2005) and Karl et al (Karl et al. 2006) found that HRT was associated with reduced urinary albumin excretion and glomerulosclerosis in postmenopausal women; yet Manning et al reported that HRT did not improve microalbuminuria in postmenopausal diabetic women (Manning et al. 2003), whereas others showed that both HRT and contraceptives increased the risk of microalbuminuria (Monster et al. 2001; Ribstein et al. 1999). In animal studies, E2 was reported to either improve renal function and reduce tubulointerstitial fibrosis (Mankhey et al. 2005; Mankhey et al. 2007) or worsen glomerulosclerosis and accelerate renal injury in obese Zucker rats (Gades et al. 1998; Stevenson et al. 2000). The reasons for these conflicting results remain unclear. Since all of these studies involved a single-dose regimen, one cannot be sure whether dosage affects the outcome of E2 treatment. Our present data provide evidence that whether E2 is protective or harmful to the kidneylargely depends on the dosage.
Using a mouse model of surgically induced menopause along with MI, we previously found that moderate and high doses of E2 not only increased mortality and deteriorated cardiac remodeling and function but also caused kidney and liver damage as evidenced by severe hydronephrosis, liver enlargement and ascites (Zhan et al. 2008). Since that study involved mice with MI, we could not be sure whether such renal damage was due to heart failure or a direct adverse effect of E2 on the kidney that in turn contributed to deterioration of cardiac dysfunction and remodeling post-MI. To clarify this, we used ovariectomized mice without MI to see if the toxic effects of E2 on the kidney occur prior to heart dysfunction. We found that ovx per se caused a slight but significant increase in interstitial collagen deposition and myocyte size and that these effects were reversed by a low dose of E2 but augmented by moderate and high doses. Despite those morphological changes, no dosage of E2 had any significant impact on cardiac hemodynamics and function. However, in the kidney moderate or high doses of E2 not only caused structural damage but also reduced renal function as evidenced by hydronephrosis, tubular dilatation and increased glomerular matrix deposition as well as increased plasma creatinine and albuminuria, whereas these effects were not seen with a low dose. Since E2-induced renal damage developed before any noticeable adverse effects on cardiac function, our data suggest that the renal damage was not secondary to cardiac dysfunction; rather, these renal toxic effects may have contributed to and even enhanced cardiac damage.
The precise mechanisms responsible for these detrimental renal effects of E2 are not known. In diabetic animals, E2 reportedly increases plasma creatinine and triglyceride-rich lipoproteins, and it has been suggested that their binding to glomerular cells initiates or accelerates glomerulosclerosis (Gades et al. 1998; Stevenson et al. 2000). In the present study, we found that a high dose of E2 increased plasma creatinine and this was accompanied by heightened albuminuria and glomerular matrix formation as well as reduced creatinine clearance. Unfortunately, we did not measure lipoproteins and do not know whether E2-induced renal damage was at least partially due to an increase in triglyceride-rich lipoproteins. This warrants further investigation in the future.
In addition to glomerular injury, E2 also caused urine retention, tubular dilatation, hydronephrosis, and hydroureter, mostly at high doses. E2-induced urine retention, cystitis and hydronephrosis have been reported by others (Pearse et al. 2009; Gakhar et al. 2009; Levin-Allerhand et al. 2003). The severe tubular dilatation and hydronephrosis could be due to increased pressure and backward flow resulting from urine retention. E2 has been shown to enhance β-adrenergic receptor-mediated relaxation of the detrusor muscle in the bladder (Yono et al. 2000). Chronic E2 treatment also reportedly inhibits micturition (Buhl et al. 1985) and produces thickening of the urethral epithelium, even to the point of hermetically sealing the urethra (Blakeman et al. 2000). All of these conditions can cause urine retention and increase bladder pressure, contributing to the development of hydronephrosis observed with E2 treatment. Clinically, E2 has been used to treat urinary incontinence (UI) in elderly women (Fantl et al. 1994; Fantl 1994), but the recent clinical trials HERS (Steinauer et al. 2005) and WHI (Hendrix et al. 2005) failed to shown that HRT has a beneficial effect on UI. Since E2-induced urine retention and hydronephrosis appear to be dose-dependent as evidenced by the present study and others (Pearse et al. 2009; Gakhar et al. 2009; Ba and Friedman 2004), it is reasonable to assume that urine retention and increased pressure in the bladder may contribute to the disappointing results of E2 or HRT used to treat UI inpost -menopausal women, which could bedose -related.
Generation of reactive oxygen species (ROS) that leads to increased oxidative stress plays an important pathophysiological role in cardio-renal disease (Sullivan et al. 2010; Sartori-Valinotti et al. 2007; Ji et al. 2007). ROS can serve as both intercellular and intracellular second messengers, altering the signaling molecules involved in cell growth/proliferation, apoptosis and release of pro-inflammatory mediators and leading to tissue and end-organ damage (Takimoto and Kass 2007; Touyz 2004). Studies have shown that E2 inhibits NADPH oxidase (Nox2, a major source of ROS generation), reduces ROS production and increases NO availability, thereby slowing or reversing progression of renal injury (Javeshghani et al. 2009; Ji et al. 2007; Blush et al. 2004; Zhang et al. 2007). In the present study we showed that ovx by itself increased renal Nox2 protein expression and urinary 8-isoprostane excretion while the increase in 8-isoprostane was reversed by a low dose of E2, supporting the concept that E2 reduces oxidative stress. However, Nox2 and 8-isoprostane excretion were heightened by E2-M and -H, which correlated with renal damage and as well as cardiac structural and morphological changes such as increased myocyte size and cardiac massinduced by those doses of E2, although these adverse cardio-renal effects were independent of blood pressure. While we do not know precisely how E2 at high doses enhances oxidative stress dose-dependently, we believe these data may have significant implications for increased cardiovascular events in women on HRT. The potential toxic effects of high doses of E2 on the kidney could very well contribute to the unfavorable outcome of HRTon the heart, which warrants further investigation.
In summary, our data suggest that E2 replacement that increases plasma estrogen uterine weight far beyond physiological levels is harmful to the heart but can even more severely damage the kidney. At lower doses that partially restored uterine weight, E2 did not adversely affect the kidney but in fact tended to offer cardio-renal protection in ovariectomized mice as indicated by decreased myocardial interstitial collagen deposition and myocyte hypertrophy as well as reduced renal injury and oxidative stress. Thus before we can be sure whether E2 replacement is detrimental or beneficial, we first need to establish a safe and effectivedo sage.
Acknowledgments
This work was supported by National Institutes of Health Grants HL -078951 (XPY) and Henry Ford Health System institutional funds(XPY).
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KF, Jr, Sueta CA, Gheorghiade M, O’Connor CM, Schwartz TA, Koch GG, Uretsky B, Swedberg K, McKenna W, Soler-Soler J, Califf RM for the FIRST Investigators. Gender differences in survival in advanced heart failure. Insights from the FIRST study. Circulation. 1999;99 (14):1816–1821. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- Agarwal M, Selvan V, Freedman BI, Liu Y, Wagenknecht LE. The relationship between albuminuria and hormone therapy in postmenopausal women. American Journal of Kidney Disease. 2005;45 (6):1019–1025. doi: 10.1053/j.ajkd.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium. 2004;35 (3):229–237. doi: 10.1016/j.ceca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Beer S, Reincke M, Kral M, Callies F, Stromer H, Dienesch C, Steinhauer S, Ertl G, Allolio B, Neubauer S. High-dose 17beta-estradiol treatment prevents development of heart failure post-myocardial infarction in the rat. Basic Research of Cardiology. 2007;102 (1):9–18. doi: 10.1007/s00395-006-0608-1. [DOI] [PubMed] [Google Scholar]
- Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU International. 2000;86 (1):32–38. doi: 10.1046/j.1464-410x.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- Blum A, Koh K, Cannon RO., III CME Paper: Hormone replacement therapy for prevention or treatment of atherosclerosis in postmenopausal women: promises, controversies, and clinical trials. The American Journal of Geriatric Cardiology. 2000;9 (2):81–88. doi: 10.1111/j.1076-7460.2000.80013.x. [DOI] [PubMed] [Google Scholar]
- Blush J, Lei J, Ju W, Silbiger S, Pullman J, Neugarten J. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney International. 2004;66 (6):2148–2154. doi: 10.1111/j.1523-1755.2004.66005.x. [DOI] [PubMed] [Google Scholar]
- Brett KM, Madans JH. Long-term survival after coronary heart disease. Comparisons between men and women in a national sample. Annals of Epidemiology. 1995;5 (1):25–32. doi: 10.1016/1047-2797(94)00037-t. [DOI] [PubMed] [Google Scholar]
- Buhl AE, Yuan YD, Cornette JC, Frielink RD, Knight KA, Ruppel PL, Kimball FA. Steroid-induced urogenital tract changes and urine retention in laboratory rodents. Journal of Urology. 1985;134 (6):1262–1267. doi: 10.1016/s0022-5347(17)47708-1. [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Sankey SS, Yu A-L, Menon S, Yang X-P. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. American Journal of PhysiologyHeart and Circulatory Physiology. 2003;284 (5):H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Tao Z, Menon S, Yang X-P. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sciences. 2004;75 (18):2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Cingolani OH, Yang X-P, Cavasin MA, Carretero OA. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension. 2003;41 (2):249–254. doi: 10.1161/01.hyp.0000052832.96564.0b. [DOI] [PubMed] [Google Scholar]
- Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. Cardiovascular disease in women. Circulation. 1993;88(4 Pt 1):1999–2009. doi: 10.1161/01.cir.88.4.1999. [DOI] [PubMed] [Google Scholar]
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney International. 2006;69 (2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- Fantl JA. The lower urinary tract in women--effect of aging and menopause on continence. Experimental Gerontology. 1994;29 (3–4):417–422. doi: 10.1016/0531-5565(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Fantl JA, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. First report of the Hormones and Urogenital Therapy Committee. Obstetrics and Gynecology. 1994;83 (1):12–18. [PubMed] [Google Scholar]
- Gades MD, Stern JS, van Goor H, Nguyen D, Johnson PR, Kaysen GA. Estrogen accelerates the development of renal disease in female obese Zucker rats. Kidney International. 1998;53 (1):130–135. doi: 10.1046/j.1523-1755.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- Gakhar G, Wight-Carter M, Andrews G, Olson S, Nguyen TA. Hydronephrosis and urine retention in estrogen-implanted athymic nude mice. Veterinary Pathology. 2009;46 (3):505–508. doi: 10.1354/vp.08-VP-0180-N-BC. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. New England Journal of Medicine. 2003;348 (7):645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine. 2000;133 (12):933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Raij L. Nitric oxide synthase activity and renal injury in genetic hypertension. Hypertension. 1998;31 (1 Pt 2):266–270. doi: 10.1161/01.hyp.31.1.266. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, Aragaki A, Naughton MJ, Wallace RB, McNeeley SG. Effects of estrogen with and without progestin on urinary incontinence. Journal of the American Medical Association. 2005;293 (8):935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- Hodis HN. Assessing benefits and risks of hormone therapy in 2008: new evidence, especially with regard to the heart. Cleveland Clinic Journal of Medicine. 2008;75(Suppl 4):S3–12. doi: 10.3949/ccjm.75.suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Hügel S, Reincke M, Strömer H, Winning J, Horn M, Dienesch C, Mora P, Schmidt HHHW, Allolio B, Neubauer S. Evidence against a role of physiological concentrations of estrogen in post-myocardial infarction remodeling. Journal of the American College of Cardiology. 1999;34 (5):1427–1434. doi: 10.1016/s0735-1097(99)00368-x. [DOI] [PubMed] [Google Scholar]
- Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, Vittinghoff E, Hunninghake D for the HERS Research Group. Noncardiovascular disease outcomes during 6.8 years of hormone therapy. Heart and Estrogen/progestin Replacement Study follow-up (HERS II) Journal of the American Medical Association. 2002;288(1):58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- Isles CG, Hole DJ, Hawthorne VM, Lever AF. Relation between coronary risk and coronary mortality in women of the Renfrew and Paisley survey: comparison with men. Lancet. 1992;339 (8795):702–706. doi: 10.1016/0140-6736(92)90599-x. [DOI] [PubMed] [Google Scholar]
- Javeshghani D, Schiffrin EL, Sairam MR, Touyz RM. Potentiation of vascular oxidative stress and nitric oxide-mediated endothelial dysfunction by high-fat diet in a mouse model of estrogen deficiency and hyperandrogenemia. Journal of the American Society of Hypertension. 2009;3 (5):295–305. doi: 10.1016/j.jash.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ji H, Zheng W, Menini S, Pesce C, Kim J, Wu X, Mulroney SE, Sandberg K. Female protection in progressive renal disease is associated with estradiol attenuation of superoxide production. Gender Medicine. 2007;4 (1):56–71. doi: 10.1016/s1550-8579(07)80009-x. [DOI] [PubMed] [Google Scholar]
- Karl M, Berho M, Pignac-Kobinger J, Striker GE, Elliot SJ. Differential effects of continuous and intermittent 17beta-estradiol replacement and tamoxifen therapy on the prevention of glomerulosclerosis: modulation of the mesangial cell phenotype in vivo. American Journal of Pathology. 2006;169 (2):351–361. doi: 10.2353/ajpath.2006.051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. American Heart Journal. 1986;111 (2):383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- Levin-Allerhand JA, Sokol K, Smith JD. Safe and effective method for chronic 17beta-estradiol administration to mice. Contemporary Topics in Laboratory Animal Science. 2003;42 (6):33–35. [PubMed] [Google Scholar]
- Liao T-D, Yang X-P, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin-II-induced hypertension. Hypertension. 2008;52 (2):256–263. doi: 10.1161/HYPERTENSIONAHA.108.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. American Journal of Physiology Renal Physiology. 2005;288 (2):F399–F405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- Mankhey RW, Wells CC, Bhatti F, Maric C. 17beta-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. American Journal of PhysiologyRegulatory, Integrative and Comparative Physiology. 2007;292 (2):R769 –R777. doi: 10.1152/ajpregu.00375.2006. [DOI] [PubMed] [Google Scholar]
- Manning PJ, Sutherland WH, Allum AR, de Jong SA, Jones SD. HRT does not improve urinary albumin excretion in postmenopausal diabetic women. Diabetes Research and Clinical Practice. 2003;60 (1):33–39. doi: 10.1016/s0168-8227(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Archives of Internal Medicine. 2001;161 (16):2000–2005. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. Journal ofthe American Society of Nephrology. 2000;11(2):319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- Pearse G, Frith J, Randall KJ, Klinowska T. Urinary retention and cystitis associated with subcutaneous estradiol pellets in female nude mice. Toxicological Pathology. 2009;37 (2):227–234. doi: 10.1177/0192623308329281. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Maric C. Sex and gender differences in cardiovascular-renal physiology and pathophysiology. Steroids. 2010;75 (11):745–746. doi: 10.1016/j.steroids.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Ribstein J, Halimi JM, du CG, Mimran A. Renal characteristics and effect of angiotensin suppression in oral contraceptive users. Hypertension. 1999;33 (1):90–95. doi: 10.1161/01.hyp.33.1.90. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. Journal of the American Medical Association. 2002;288 (3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Sanada M, Higashi Y, Nakagawa K, Tsuda M, Kodama I, Kimura M, Chayama K, Ohama K. A comparison of low-dose and standard-dose oral estrogen on forearm endothelial function in early postmenopausal women. The Journal of Clinical Endocrinology and Metabolism. 2003;88 (3):1303–1309. doi: 10.1210/jc.2002-021147. [DOI] [PubMed] [Google Scholar]
- Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clinical and Experimental Pharmacology and Physiology. 2007;34 (9):938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin F2α. Hypertension. 1999;33 (1 Pt 2):424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- Schnatz PF. Hormonal therapy: does it increase or decrease cardiovascular risk? Obstetrical and Gynecological Survey. 2006;61 (10):673–681. doi: 10.1097/01.ogx.0000238674.98471.bb. [DOI] [PubMed] [Google Scholar]
- Silbiger S, Neugarten J. Gender and human chronic renal disease. Gender Medicine. 2008;5(Suppl A):S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. New England Journal of Medicine. 1991;325 (11):756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Steinauer JE, Waetjen LE, Vittinghoff E, Subak LL, Hulley SB, Grady D, Lin F, Brown JS. Postmenopausal hormone therapy: does it cause incontinence? Obstetrics and Gynecology. 2005;106 (5 Pt 1):940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson FT, Wheeldon CM, Gades MD, Kaysen GA, Stern JS, van Goor H. Estrogen worsens incipient hypertriglyceridemic glomerular injury in the obese Zucker rat. Kidney International. 2000;57 (5):1927–1935. doi: 10.1046/j.1523-1755.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2010;298 (1):R61–R69. doi: 10.1152/ajpregu.00526.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Carretero OA, Xu J, Rhaleb N-E, Wang F, Lin C, Yang JJ, Pagano PJ, Yang X-P. Lack of inducible NO synthase reduces oxidative stress and enhances cardiac response to isoproterenol in mice with deoxycorticosterone acetate-salt hypertension. Hypertension. 2005;46 (6):1355–1361. doi: 10.1161/01.HYP.0000192651.06674.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49 (2):241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Brazilian Journal of Medical and Biological Research. 2004;37 (8):1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, Grohe C, Mendelsohn ME, Karas RH. 17-beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. Journal of the American College of Cardiology. 2003;41 (11):2084–2092. doi: 10.1016/s0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- Villa P, Suriano R, Ricciardi L, Tagliaferri V, De CS, De FP, Colacurci N, Lanzone A. Low-dose estrogen and drospirenone combination: effects on glycoinsulinemic metabolism and other cardiovascular risk factors in healthy postmenopausal women. Fertility and sterility. 2010 doi: 10.1016/j.fertnstert.2010.07.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Xu J, Carretero OA, Cavasin MA, Lin C, Shesely EG, Yang JJ, Reudelhuber TL, Yang X-P. Role of cardiac overexpression of angiotensin II in the regulation of cardiac function and remodeling post-myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology. 2007;293 (3):H1900–H1907. doi: 10.1152/ajpheart.00379.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-P, Liu YH, Rhaleb N-E, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. American Journal of PhysiologyHeart and Circulatory Physiology. 1999;277 (5 Pt 2):H1967 –H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- Yono M, Yoshida M, Takahashi W, Inadome A, Seshita H, Miyamoto Y, Ueda S. Effects of ovarian hormones on beta-adrenergic receptor-mediated relaxation in the female rabbit bladder. Urological Research. 2000;28 (1):38–45. doi: 10.1007/s002400050008. [DOI] [PubMed] [Google Scholar]
- Zhan E, Keimig T, Xu J, Peterson E, Ding J, Wang F, Yang X-P. Dose-dependent cardiac effect of oestrogen replacement in mice post-myocardial infarction. Experimental Physiology. 2008;93 (8):982–993. doi: 10.1113/expphysiol.2008.042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fujii S, Kosaka H. Effect of oestrogen on reactive oxygen species production in the aortas of ovariectomized Dahl salt-sensitive rats. Journal of Hypertension. 2007;25 (2):407–414. doi: 10.1097/HJH.0b013e328010beee. [DOI] [PubMed] [Google Scholar]