Abstract
Following traumatic brain injury (TBI) there is significant neuropathology which includes mitochondrial dysfunction, loss of cortical grey matter, microglial activation, and cognitive impairment. Previous evidence has shown that activation of the peroxisome proliferator-activated receptors (PPARs) provide neuroprotection following traumatic brain and spinal injuries. In the current study we hypothesized that treatment with the PPAR ligand Pioglitazone would promote neuroprotection following a rat controlled cortical impact model of TBI. Animals received a unilateral 1.5mm controlled cortical impact followed by administration of pioglitazone at 10mg/kg beginning 15 minutes after the injury and subsequently every 24hrs for five days. Beginning one day after the injury there was significant impairment in mitochondrial bioenergetic function which was attenuated by treatment with Pioglitazone at 15 minutes and 24 hours (p<0.05). In an additional set of animals, cognitive function was assessed using the Morris Water Maze (MWM) and it was observed that over the course of four days of testing the injury produced a significant increase in both latency (p<0.05) and distance (p<0.05) to the platform. Animals treated with Pioglitazone performed similarly to sham animals and did not exhibit any impairment in MWM performance. Sixteen days after the injury tissue sections through the lesion site were quantified to determine the size of the cortical lesion. Vehicle treated animals had an average lesion size of 5.09±0.73mm3 and treatment with Pioglitazone significantly reduced the lesion size by 55% to 2.27±0.27mm3 (p<0.01). Co-administration of the antagonist T0070907 with Pioglitazone blocked the protective effect seen with administration of Pioglitazone by itself. Following the injury there was a significant increase in the number of activated microglia in the area of the cortex adjacent to the site of the lesion (p<0.05). Treatment with Pioglitazone prevented the increase in the number of activated microglia and no difference was observed between sham and Pioglitazone treated animals. From these studies we conclude that following TBI Pioglitazone is capable ameliorating multiple aspect of neuropathology. These studies provide further support for the use of PPAR ligands, specifically Pioglitazone, for neuroprotection.
2.1 Introduction
Traumatic brain injury (TBI) pathology results from both a primary injury and a secondary injury cascade. The primary injury is due to biomechanical damage which results in the shearing and compression of neuronal, glial, and vascular tissue. The cascade of secondary injury damage, which occurs in the hours and days following the initial insult, is due to activation of pathophysiological cascades, consisting of complex biochemical and cellular pathways that influence progression of the injury, such as alterations in excitatory amino acids (Yamamoto et al., 1999, Rose et al., 2002), increased reactive oxygen species (ROS) production (Marklund et al., 2001, Hall et al., 2004, Tavazzi et al., 2005), disruption of calcium homeostasis (Mattson and Scheff, 1994, Xiong et al., 1997, Sullivan et al., 1999b), post-traumatic neuroinflammation (Morganti-Kossmann et al., 2001, Vlodavsky et al., 2006) and mitochondrial dysfunction (Azbill et al., 1997, Xiong et al., 1997, Sullivan et al., 1998, Sullivan et al., 1999a, Sullivan et al., 1999b). As a result of these secondary injury processes, there are significant reductions in ATP levels, increases in lipid peroxidation, release of cytochrome c and activation of apoptotic pathways (Sullivan et al., 1998, Sullivan et al., 2002), all of which can lead to the initiation of cell death pathways. Mitochondria are a major component of this secondary injury pathway because they function as a highly sensitive gatekeeper of cell death mechanisms and as the primary energy producer for the cell. As such, mitochondria play a pivotal role in cerebral energy metabolism, intracellular calcium homeostasis, and ROS generation and detoxification.
Following TBI, a significant disruption of mitochondrial homeostasis has been documented that results in a decline in cellular bioenergetics, increased mitochondrial ROS production and a decline in synaptic equilibrium (Azbill et al., 1997, Xiong et al., 1997, Sullivan et al., 1998, Sullivan et al., 1999a, Sullivan et al., 1999b). Therefore, following TBI, the degree of mitochondrial injury or dysfunction can be an important determinant of cell survival or death (for reviews see (Robertson, 2004, Sullivan et al., 2005, Robertson et al., 2006) and therapeutic treatments designed to protect and stabilize the mitochondria have demonstrated the ability to reduce injury in preclinical studies (Sullivan et al., 2000a, Pandya et al., 2007). Although preclinical research has identified neuroprotective agents which target mitochondrial function, inflammation, and oxidative damage, attempts to move therapies into clinical usage have so far been unsuccessful (Schouten, 2007). Given the complexity of the secondary injury, it has been suggested that drugs which target multiple pathological pathways may yield more effective therapeutic approaches for TBI. The PPARγ agonist Pioglitazone has been shown to reduce inflammation (Besson et al., 2005, Chen et al., 2007, Park et al., 2007, Hyong et al., 2008, Kapadia et al., 2008) and oxidative damage (Chen et al., 2007, Yi et al., 2008), attenuate mitochondrial dysfunction (Hunter et al., 2007), and reduce cell death (McTigue et al., 2007, Park et al., 2007) following CNS injury. Pioglitazone's ability to target multiple injury mechanisms may provide it with an advantage over other therapeutics for TBI which target a single secondary injury cascade. The peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily, which regulate gene expression using various ligand-dependent and independent molecular processes. Three different isoforms of the PPARs exist, which are encoded by separate genes: PPAR-γ (NR1C3), PPAR-α (NR1C1), and PPAR-δ (NR1C2, β, or NUC-1) (Dreyer et al., 1992, Michalik and Wahli, 1999, Torra et al., 2001). While these isoforms have similar protein sequence and structure, they differ in their ligand-binding domains and have different ligand specificity, tissue distribution, and biological actions (Guan et al., 2002). The PPARs regulate inflammation mainly through their transrepression capabilities although the transactivation of certain target genes can occur. Several inflammatory signaling systems may be affected by PPAR-mediated transrepression such as nuclear factor-kappa B (NFκB), activator protein-1 (AP-1), signal transducer and activator of transcription (STAT), or nuclear factor of activated T cells (NFAT) (Ricote et al., 1998, Delerive et al., 2001, Park et al., 2003, Bernardo and Minghetti, 2006). These pathways immunoregulate macrophages, microglia, dendritic cells, endothelial cells, B cells, and T cells (for reviews see (Clark, 2002, Daynes and Jones, 2002, Hunter, 2007)). Treatment with Pioglitazone following LPS induced brain inflammation has shown the ability to prevent both mitochondrial impairment and neuronal cell loss (Hunter et al., 2007). The therapeutic use of various PPARs has shown benefit in multiple CNS injury models including spinal cord injury (SCI) (McTigue et al., 2007, Park et al., 2007), traumatic brain injury (TBI) (Besson et al., 2005, Chen et al., 2007, Chen et al., 2008, Yi et al., 2008), and stroke (Collino et al., 2006, Allahtavakoli et al., 2009). Of the three known PPAR isoforms, PPARα and PPARγ have been the most well studied in CNS injury and have been shown to reduce lesion size both in SCI (McTigue et al., 2007, Park et al., 2007) and TBI (Yi et al., 2008), reduce inflammation (Besson et al., 2005, Chen et al., 2007, Park et al., 2007, Hyong et al., 2008, Kapadia et al., 2008), minimize oxidative damage (Chen et al., 2007, Yi et al., 2008), spare neurons (McTigue et al., 2007, Park et al., 2007), and preserve behavioral function (Chen et al., 2007, McTigue et al., 2007, Park et al., 2007, Chen et al., 2008).
The PPARγ agonists Pioglitazone and Rosiglitazone are both FDA approved drugs for diabetes treatment (for review see (Sood et al., 2000)) and have been utilized as therapeutics in animal models of CNS injury (Besson et al., 2005, Kiaei et al., 2005, Schutz et al., 2005, Collino et al., 2006, Chen et al., 2007, McTigue et al., 2007, Park et al., 2007, Chen et al., 2008, Feng et al., 2008, Hyong et al., 2008, Sun et al., 2008, Yi et al., 2008, Allahtavakoli et al., 2009). Rosiglitazone has been previously shown to have a higher binding affinity for the PPARγ receptor (Young et al., 1998), however, Pioglitazone has been shown to more readily cross the blood brain barrier (BBB) (Berger and Moller, 2002) as well as partially activate the PPARα receptor (Sakamoto et al., 2000). Pioglitazone's increased brain penetration and activation of two separate PPAR pathways may yield a greater therapeutic potential for the treatment of TBI (for review see (Kapadia et al., 2008)). Currently evidence exists showing that activation of either the PPARα (Chen et al., 2007, Chen et al., 2008) or PPARγ (Yi et al., 2008) pathways are protective in models of TBI, however, no studies currently exist showing the effect of Pioglitazone following TBI. Because of the success of PPARγ agonists in multiple models of CNS injury and their offer of a broad range of potentially protective properties, we hypothesize that PPARγ activation by Pioglitazone will be beneficial in an animal model of controlled cortical impact (CCI) that has hallmarks of human TBI. The current project addresses the hypothesis that Pioglitazone will offer significant neuroprotection leading to maintenance of mitochondrial function, sparing of cortical tissue, attenuation of inflammation, and preservation of cognitive function following TBI.
3. Results
3.1 Pioglitazone protects mitochondria from injury-induced mitochondrial dysfunction
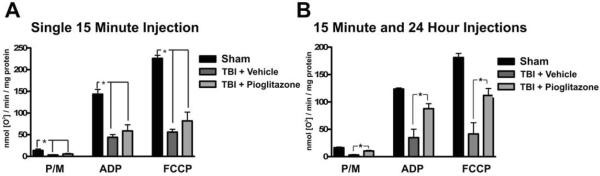
Following TBI there is significant damage to the mitochondria resulting in impaired in bioenergetic function. In order to elucidate the effect of Pioglitazone on mitochondrial bioenergetic function, two different treatment paradigms for Pioglitazone administration were utilized. In the first set of animals, Pioglitazone was administered 15min after the injury (10mg/kg) and animals were sacrificed 25hrs after the injury. In the second set of animals Pioglitazone was administered at 15min and 24hrs after injury (10mg/kg/injection) and animals were sacrificed at 25hrs after the injury. Following injury in both sets of animals, reductions in Pyruvate/Malate (PM), ADP, and FCCP (uncoupled) respiration rates were seen in vehicle treated animals (Figure 1, *=p<0.01). With only a single 15min injection there was a slight but non-significant increase in respiration rates following Pioglitazone treatment (Figure 1A); however, when Pioglitazone was given at both 15min and 24hrs after the injury a significant increase in mitochondrial function was observed (*=p<0.01, Figure 1B), indicating that under these conditions Pioglitazone treatment leads to preservation of the mitochondria's ability to produce ATP.
Figure 1. Pioglitazone treatment attenuates mitochondrial dysfunction after TBI.
In order to assess the ability of Pioglitazone to prevent the mitochondrial dysfunction which occurs with this model of TBI, mitochondrial bioenergetic function was analyzed 1 day following a controlled cortical impact TBI. A. Following a single injection of Pioglitazone (10mg/kg) 15 minutes after the injury, no improvements in mitochondrial function were observed. B. When Pioglitazone was administered at both at 15 minutes and 24 hours after the injury (10mg/kg/injection) a significant increase in mitochondrial bioenergetics was observed. These studies indicate that treatment with Pioglitazone at 15 minutes and 24 hours is capable of preventing mitochondrial dysfunction following TBI.
(* p<0.01 by one-way ANOVA with SNK post-test).
3.2 Pioglitazone treatment improves Morris Water Maze performance following TBI
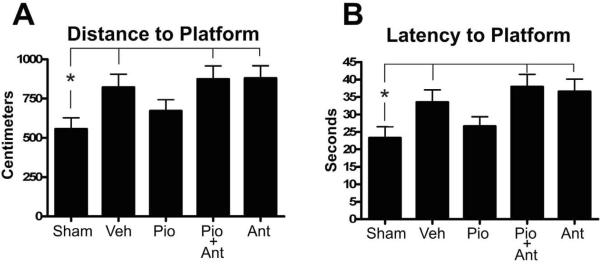
Cognitive impairment is a significant pathological outcome with both human and rodent cortical impact TBI. In order to assess the ability of Pioglitazone to reduce cognitive impairment following injury, animals were administered vehicle, Pioglitazone, or Pioglitazone plus the PPARγ antagonist T0070907 with an initial injection at 15 min post-injury and subsequent injections at 24h, 48h, 72h and 96h after the first injection. Pioglitazone and T0070907 were administered at 10 mg/kg at every dose. MWM assessments were assessed 10 days post-injury and consisted of 4 days of trials. Following repeated measures analysis of the MWM results, a significant effect of day (p<0.0001) and a trend towards effect of treatment (latency p=0.0693; distance p=0.0648) were observed. To further elucidate the effect of Pioglitazone on MWM behavior and increase statistical power, groups were collapsed across days. When averaged across all testing days, the latency and distance to the platform showed significant effects. Injured vehicle-treated animals showed impairments in both distance (Figure 2A, *=p<0.05) and latency (Figure 2B, *=p<0.05) to the platform and treatment with Pioglitazone significantly improved behavior. Treatment with the PPARγ antagonist T0070907 and Pioglitazone together prevented the beneficial effects of Pioglitazone following injury (Figure 2 p<0.05 Sham vs. Pio+Ant) since animals given the antagonist with Pioglitazone performed similarly to injured vehicle-treated animals, indicating direct involvement of the PPARγ receptor. No significant differences in swim speed were observed among the treatment groups, suggesting that the differences in MWM performance were not related to motor deficits.
Figure 2. Pioglitazone treatment reduces cognitive impairment following traumatic brain injury.
With this model of TBI there are impairments in Morris Water Maze (MWM) performance following the injury. Pioglitazone was administered for 5 days following the injury in order to determine if treatment with Pioglitazone is capable of preventing cognitive impairment with the MWM test. Treatment with Pioglitazone produced a significant reduction in both the distance and latency to find the platform following TBI. Additionally, animals which were also treated with the PPARγ antagonist T0070907 exhibited no preservation of function indicating that PPARγ receptor activation is required for Pioglitazone's ability to preserve cognitive functioning. (* p<0.05 by one-way ANOVA).
3.3 Pioglitazone treatment reduces cortical damage following TBI
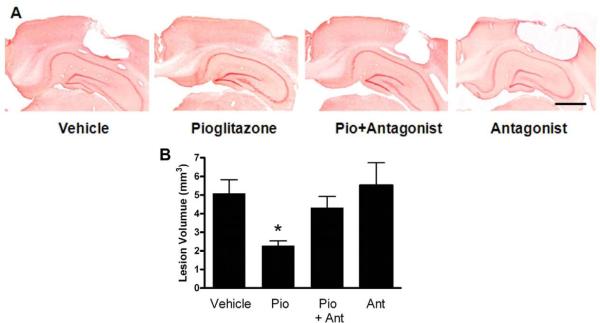
Quantification of the cortical lesion was utilized to assess the efficacy of Pioglitazone to reduce the cortical tissue loss which occurs following CCI brain injury. Administration of Pioglitazone and the antagonist T0070907 was conducted in the same manner as for the behavioral analysis, with injections of 10mg/kg beginning 15 minutes after the injury and continuing at 24h, 48h, 72h and 96h after the first injection. A moderate CCI injury (1.5 mm) resulted in a significant loss of cortical tissue (Figure 3). At 16 days post-injury, the cortical contusion volume in the vehicle-treated control group was measured to be 5.09±0.73mm3 (Figure 3B). Treatment with Pioglitazone resulted in reduction of the lesion size by 55% to 2.27±0.27mm3 which was significantly smaller compared to the vehicle treated TBI group (Figure 3B, *=p<0.05). Co-treatment of Pioglitazone with the PPARγ antagonist T0070907 abolished the effect seen with Pioglitazone treatment alone. Animals that were treated with Pioglitazone and the antagonist had an average lesion size of 4.32±0.60 mm3 which was larger than the lesion size in the Pioglitazone group (Figure 3B, *=p<0.05 Pio vs. Pio+Ant), suggesting that the PPARγ receptor activation is involved in Pioglitazone's ability to spare cortical tissue. Treatment with the PPARγ antagonist alone did not result in a significant increase the size of the cortical lesion compared to vehicle treated animals (Figure 3B).
Figure 3. Pioglitazone treatment reduces cortical tissue loss.
Following a cortical impact brain injury there is significant loss of cortical tissue which occurs in the days and weeks following the injury, Pioglitazone was administered for 5 days following a unilateral cortical contusion in order to access the ability of Pioglitazone to reduce cortical damage. In these studies Pioglitazone treatment reduced the size of the lesion which occurs in this CCI rat model of TBI. These studies also indicate that activation of the PPARγ receptor is required for this aspect of Pioglitazone's neuroprotective function since treatment with the PPARγ antagonist blocked the therapeutic effect. A. Representative coronal sections stained with neutral red for animals treated with vehicle, Pioglitazone, Pioglitazone and T0070907, or T0070907 alone at 16 days post-injury. Scale bar=1000 μm B. Lesion volume quantification showed a significantly reduced lesion volume in Pioglitazone-treated CCI animals when compared with the vehicle group and antagonist treatment prevented this effect (*=p<0.05, one-way ANOVA with SNK post-test).
3.4 Pioglitazone attenuates the neuroinflammatory response after TBI
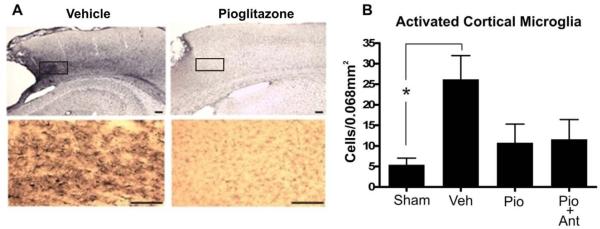
Following TBI there is a significant increase in microglial activation and inflammation and Pioglitazone was administered in attempts to reduce post-injury inflammation. At 16 days after TBI, Pioglitazone-treated rats showed significantly fewer OX42-positive cells in the injured cortex adjacent to the injury site, suggesting a reduction in both microglial activation and macrophage infiltration. The effect of Pioglitazone on inhibiting the inflammatory process was assessed by stereological quantification of the activated microglia. OX-42 immunoreactive cells exhibited two morphologies: resting microglia with small cell body and multiple thin processes and activated microglia with enlarged amoeboid cell body and pyknic processes. Following injury there was a significant increase in the number of activated microglia (p<0.05 Figure 4B). In the injured cortex, the total number of activated microglia was reduced in the Pioglitazone-treated group compared vehicle treatment (Figure 4B). Compared with animals which received a sham injury, animals treated with Pioglitazone did not show a significant increase in the number OX-42 positive cells. Treatment with the PPARγ antagonist T0070907 did not prevent the effect of Pioglitazone on preventing the increase in inflammation, indicating that the effect was not dependent upon the PPARγ receptor.
Figure 4. Pioglitazone reduces post-injury microglial activation following TBI.
With this cortical contusion model of TBI there is significant inflammation which occurs following the injury. Pioglitazone was administered for 5 days following the injury in order to assess its ability to reduce inflammation following TBI. In this model, Pioglitazone attenuated the neuroinflammatory response in the injured animals. These studies also show that Pioglitazone does not require the PPARγ receptor in order to reduce inflammation following injury since treatment with the PPARγ inhibitor T0070907 did not block Pioglitazone's neuroprotective effects. A. Immunohistochemistry for OX-42 was shown in cerebral cortex adjacent to the cortical lesion. The bottom images are high magnification views of the boxed regions. Scale bar = 100μm. B. Total number of activated microglia was counted in the cortex and a reduction in the number of activated microglia was found in Pioglitazone treated animals compared to vehicle treated animals. Compared with sham animals, Pioglitazone treated animals exhibited no significant increase in activated microglia. (* p<0.05 by one-way ANOVA with SNK post-test).
4.1 Discussion
Previous reports from our group have shown that following TBI there is significant impairment of cortical mitochondria which begins acutely and substantially damages the mitochondria over the course of the first day following the injury (Pandya et al., 2007, Gilmer et al., 2009, Pandya et al., 2009). Interventions that are capable of protecting mitochondrial function following TBI have been demonstrated to reduce cortical damage and behavioral impairment (Scheff and Sullivan, 1999, Sullivan et al., 2000b, Pandya et al., 2007, Pandya et al., 2009). In the present studies, mitochondrial function was analyzed one day following the injury. We chose this time point based on previous experiments which have shown that at this time point there is significant mitochondrial dysfunction in our model of cortical impact injury (Pandya et al., 2009). We found that with Pioglitazone treatment there are improvements in mitochondria function which are observed in the Complex-I dependant, Pyruvate/Malate-driven, rates of oxygen consumption as well as the State III (ADP-present) and maximal (uncoupled) respiration rates, a measure of electron transport capacity/reserve (Figure 1B). From the mitochondrial experiments it can be concluded that animals treated with Pioglitazone maintain more functional mitochondria which are capable of producing more ATP and therefore providing the brain with the much needed energy source required for normal function as well as post-injury protective and reparative mechanisms.
The mitochondrial data from these experiments also suggests that at least part of Pioglitazone's ability to improve mitochondrial function involves a direct effect on the mitochondria since a single 15min post injury injection only produced a non-significant trend towards improved mitochondrial bioenergetics (Figure 1A) while a subsequent injection of Pioglitazone 1hr prior to analyzing the tissue resulted in a dramatic increase in mitochondrial bioenergetic function (Figure 1B). Since the improvement in mitochondrial function was only observed after giving the second injection, the mitochondrial effect is most likely not due to only an up regulation of protein expression. Previously published in vitro experiments have shown that treatment with Pioglitazone can up regulate mitochondrial electron transport proteins, however, these changes take at least 2 days to produce significant increases in protein levels (Miglio et al., 2009). Up regulation of mitochondrial protein expression is not likely the cause for the improvements in mitochondrial function seen in these experiments after the first 24 hours of Pioglitazone treatment, especially considering the rapid change in mitochondrial function observed 1 hour following the second Pioglitazone treatment. Treatment with Pioglitazone will likely produce even greater improvements in mitochondrial function at time points beyond 24hrs when significant increases in mitochondrial proteins are likely occur. The rapid increase in mitochondrial function seen after the second injection of Pioglitazone may involve the mitochondrial outer membrane bound protein MitoNEET. Previously it has been shown that Pioglitazone binds to MitoNEET and stabilizes its conformational structure (Paddock et al., 2007) and that knocking-out the MitoNEET protein results in reductions in mitochondrial electron transport chain function(Wiley et al., 2007). In addition to Pioglitazone's ability to rapidly increase mitochondrial function as seen in these studies, considering that Pioglitazone treatment leads to reductions in oxidative damage (Chen et al., 2007, Yi et al., 2008) and inflammation (Besson et al., 2005, Chen et al., 2007, Park et al., 2007, Hyong et al., 2008, Kapadia et al., 2008) following injury, Pioglitazone treatment will promote a less hostile cellular environment which will lead to reductions in secondary injury cascades that cause further mitochondrial dysfunction and propagate bioenergetic impairments.
To further understand the effects of Pioglitazone following TBI, we wanted to determine if Pioglitazone was capable of reducing lesion volume and improving cognition. PPAR agonists with their anti-inflammatory (Besson et al., 2005, Chen et al., 2007, Park et al., 2007, Hyong et al., 2008, Kapadia et al., 2008) and anti-oxidant (Chen et al., 2007, Yi et al., 2008) properties, have been examined for protective properties in several models of CNS injury and disease (Sundararajan et al., 2006), including spinal cord injury. Following spinal cord injury Pioglitazone offers protection against the induction of inflammatory genes, astrogliosis, and microgliosis even if treatment is delayed for up to two hours after the injury (Park et al., 2007). Previously it has been shown that Pioglitazone protects against intrastriatal lipopolysaccharide (LPS)-induced neurodegeneration by suppressing the inflammatory response, reducing oxidative damage and preventing mitochondrial dysfunction (Hunter et al., 2008, Xing et al., 2008). The neuroprotective actions of Pioglitazone may be a general characteristic of PPARγ activation, as this phenomenon has been noted in other models of CNS injury. For instance, Pioglitazone treatment in murine models of Parkinson's disease promoted neuronal sparing within the substantia nigra (Breidert et al., 2002, Dehmer et al., 2004). Potent neuroprotection of motor neurons was also induced by Pioglitazone in transgenic mouse models of amyotrophic lateral sclerosis (Kiaei, 2008). Additionally, several studies have detected enhanced neuroprotection and decreased lesion sizes in animal models of stroke and intracerebral hemorrhage (Ou et al., 2006, Victor et al., 2006). To determine if any measurable effect on cognition or lesion volume was the result of activation of the PPARγ pathway, the selective PPARγ antagonist T0070907 was co-administered with Pioglitazone. It has previously been shown that following TBI the injury causes cortical damage which correlates well with cognitive dysfunction as measured using the Morris Water Maze (MWM) (Marklund et al., 2001). From these studies it was determined that Pioglitazone is capable preserving cognitive function, as determined by MWM assessment. It was observed at 15 days post injury that animals treated with Pioglitazone exhibited a decreased latency time and distance to the platform compared to animals treated with either vehicle or animals treated with Pioglitazone and the PPARγ antagonist T0070907 together (Figure 2). Since the PPARγ antagonist was able to block the protective effects of Pioglitazone on MWM function, activation of the PPARγ receptor is further implicated as a beneficial therapeutic strategy for TBI. Additionally, treatment with Pioglitazone resulted in a reduction of the size of the cortical lesion at 16 days post injury with the size of the cortical cavity being reduced from 5.09±0.73mm3 (vehicle treated animals) to 2.27±0.27mm3 (Pioglitazone treated animals) (Figure 3). Similar to the MWM testing, administration of the PPARγ antagonist T0070907 almost completely blocked the neuroprotective benefit of Pioglitazone on sparing cortical tissue, indicating that activation of the PPARγ pathway is critical to attenuating cortical tissue loss as well as cognitive deficits. As has been observed in other CNS injury models, these studies show that following TBI Pioglitazone is capable of reducing neuronal cell loss and improving behavioral outcomes.
To further elucidate the benefits of Pioglitazone following TBI, tissue was analyzed for assessment of inflammation following injury. It has been established that TBI is accompanied by a dramatic inflammatory response, which escalates over the first week post-injury and is thought to contribute to the secondary pathology of TBI. Multiple studies link agonism of PPARγ to the attenuation of inflammation (Jiang et al., 1998, Ricote et al., 1998, Drew et al., 2006), such that PPARγ activation influences the development and intensity of the inflammatory response. With the realization that inflammation plays a role in several neurodegenerative diseases, researchers have searched for a role of PPARγ in neurodegeneration. PPARγ activation regulates inflammation by decreasing the expression of a variety of pro-inflammatory genes such as COX-2, iNOS, and several cytokines (Jiang et al., 1998, Ricote et al., 1998, Kitamura et al., 1999, Bernardo et al., 2000) that have all been associated with inflammation-induced neurodegeneration (Banati et al., 1993, Przedborski et al., 1996, Minghetti and Levi, 1998, Liberatore et al., 1999, Heneka et al., 2000, Arimoto and Bing, 2003, Vijitruth, 2006). Since evidence shows that PPARγ is expressed throughout the brain (Moreno et al., 2004) in neurons (Braissant et al., 1996) and glia (Cullingford et al., 1998, Heneka et al., 1999, Bernardo et al., 2000, Cristiano et al., 2001), it is possible that PPARγ agonism will inhibit neuroinflammation and neurodegeneration in multiple injury and disease states. Because of its wide range of potential therapeutic efficacy, several clinical trials using synthetic PPAR agonists have begun for the treatment of diseases involving aberrant or chronic immune/inflammatory responses (Pershadsingh et al., 2004, Risner et al., 2006).
As has been previously shown for other PPARα (Chen et al., 2007, Chen et al., 2008) and PPARγ(Yi et al., 2008) agonists, treatment with Pioglitazone resulted in a significant reduction in inflammation following TBI in the present study. Following treatment with Pioglitazone, reductions in the number of activated microglia was observed in the area of the cortex adjacent to the site of the cortical lesion (Figure 4). In contrast to our data indicating that the effect of Pioglitazone on the cortical lesion and cognitive function is dependent on the PPARγ receptor, treatment with the PPARγ antagonist along with Pioglitazone did not prevent the reduction in activated microglia in these experiments. These studies indicate that Pioglitazone's ability to reduce inflammation following TBI is not dependent upon PPARγ receptor activation. Additionally, since mitochondrial dysfunction can lead to cell death and cause increases in inflammation, it is expected that the protective effects of Pioglitazone on mitochondrial function (Figure 1B) are likely to lead to further reductions in post-injury increases in inflammation. Regardless of the exact mechanism(s) by which Pioglitazone leads to reductions in inflammation, the evidence from this study and studies in SCI (Park et al., 2007) indicate that Pioglitazone treatment leads to significant reductions in inflammation following injury.
5.1 Conclusions
These experiments are the first to show a therapeutic effect of Pioglitazone following TBI and they fit well with what has been previous shown regarding the use of PPAR agonists in CNS injury. As has been observed in SCI (McTigue et al., 2007), Pioglitazone is capable of reducing the tissue loss and improving behavioral outcome following injury. These experiments also produce similar findings to studies that have utilized either PPARα or PPARγ agonists following TBI that produce reductions in inflammation (Chen et al., 2007, Yi et al., 2008), reduction in lesion volume (Yi et al., 2008), and behavioral improvement (Chen et al., 2007, Chen et al., 2008). This is the first report to our knowledge of any PPAR agonist showing protection of mitochondrial function following TBI. In conclusion, these experiments show that following TBI Pioglitazone is capable of protecting mitochondria, reducing inflammation, minimizing the cortical lesion, and improving cognitive function. Given the results of these experiments and considering that Pioglitazone has been shown to have better BBB permeability than the other PPAR agonist Rosiglitazone (Berger and Moller, 2002), as well as partially activate the PPARα pathway (Sakamoto et al., 2000), which has been shown to be efficacious following TBI (Chen et al., 2007, Chen et al., 2008), we feel that Pioglitazone will be a more effective treatment for TBI than other PPAR agonists previously utilized for the treatment of TBI.
6 Materials and Methods
6.1 Controlled Cortical Impact Brain Injury
Studies were approved by the University of Kentucky Institutional Animal Care and Usage Committee. Fifty male Sprague Dawley rats weighing 250 grams (Harlan Laboratory, IN) were utilized for these experiments. Rats were anesthetized with 2% isofluorane and placed in a Kopf stereotaxic frame for positioning under a pneumatic impactor (Precision Science Instruments). A 6 mm craniotomy was performed, with a hand trephine, lateral to the central fissure on the left side of the skull centered between lambda and bregma. Animals in injury groups received a unilateral injury directly to the surface of the brain. The injury parameters consisted of a 1.5mm deep contusion at 3.5 meters/second for 500ms. Sham animals received a craniotomy but did not receive an impact to the brain. Following the injury a piece of Surgicel (Johnson&Johnson) sized to fit into the craniotomy was placed directly on the brain. The skull cap was replaced and secured in place with dental acrylic. Once the acrylic was allowed time to harden, the scalp incision was closed with surgical staples. Animals were removed from isofluorane and placed in a clean cage and temperature was maintained at 37°C with the use of a heating pad.
6.2 Pioglitazone Treatment
Pioglitazone and the antagonist T0070907 were prepared by adding 1mg of the compound to 25uL of 100% Ethanol, 1uL 38% HCl, and 25uL of 0.9% Saline. This mixture was vortexed briefly and then 200uL of 0.9% Saline was added. This mixture was made fresh for each set of injections. Pioglitazone or the PPARγ antagonist T0070907 were administered at 10mg/kg/injection. The vehicle administration group was administered the same solution used to dissolve the Pioglitazone and antagonist. All injections were administered intraperitoneally. For mitochondrial analysis experiments treatments were started 15 minutes following the injury and depending upon the study a second injection was administered 24 hours later. For the behavioral and histological experiments treatment was begun 15 minutes after the injury and continued with subsequent injections at 24h, 48h, 72h and 96h after the first injection.
6.3 Histological Analysis
Animals were anesthetized and transcardially perfused with saline followed by 4% paraformaldehyde. The brain were removed and placed in 4% paraformaldehyde and 30% sucrose in PBS for 24hrs. After 24hrs the brains were transferred to a 30% sucrose PBS buffer without paraformaldehyde. Coronal sections 35μm thick were cut with a freezing microtome throughout the rostral caudal extent of the damaged cortex. Sections were stained with neutral red and subjected to image analysis for lesion volume assessment. Quantitative assessment of cortical damage employed an unbiased stereological protocol using the Cavalieri method as previously described (Sullivan et al., 2000b, Sullivan et al., 2002). All slides were assessed blindly with respect to treatment group. For immunohistochemistry, sections containing regions of interest were incubated overnight at 4°C with a primary antibody. The primary antibody for OX-42 (monoclonal, 1:2000, PharMingen, San Diego, CA) was used to detect microglia. After washes and incubation with an appropriate secondary antibody (Vector Laboratories, Burlingame, CA), immunoreactive cells were visualized by the avidin-biotin immunoperoxidase method (ABC kits, Vector Laboratories) with chromogen 3, 3'-diaminobenzidine tetrahydrochloride (Sigma). For each animal, sections at an interval of 770μm throughout the injured cortex were utilized for microglia counting. Five sections were selected for each animal and five non-overlapping adjacent fields of each section were selected which surrounded the edge of the cortical lesion. A Nikon Eclipse 80i light microscope with an attached Nikon microscope camera was used to obtain data images using a 200× objective. The images were captured and imported into Simple PCI image processing software (Compix Inc.) for quantification of activated microglia based upon cellular morphology since OX-42 immunoreactive cells exhibited two morphologies: resting microglia with small cell body and multiple thin processes and activated microglia with enlarged amoeboid cell body and pyknic processes. Six animals per group were utilized for both lesion volume analysis and quantification of activated microglial cells.
6.4 Mitochondrial isolation and respiration analysis
Cortical mitochondria were isolated using differential centrifugation, nitrogen disruption, and a Ficoll gradient. Animals were asphyxiated with CO2 and rapidly decapitated. A cortical punch encompassing the injury site was removed and immediately placed in ice-cold isolation buffer (215mM Mannitol, 75mM Sucrose, 0.1% BSA, 1mM EGTA, and 20mM Hepes at pH 7.2). Samples were homogenized and centrifuged at 1.3 rcf for 3 minutes, Following the first spin the supernatant was placed in a fresh tube and the pellet was resuspended in isolation buffer and spun at 1.3rcf for 3min. The supernatant from the first and second spins were collected in separate tubes and spun at 13,000rcf for 10 minutes. The pellets from both tubes was combined, resuspended in 400ul isolation buffer and placed in a nitrogen bomb at 1,200psi for 10min. The pressure in the nitrogen bomb was rapidly released and the sample was placed as the top layer on a Ficoll separation column which consisted of a 10% Ficoll layer and a 7.5% Ficoll layer. The Ficoll column with sample was centrifuged at 32,000rpm for 30min at 4°C using a Beckman SW 55Ti rotor and ultra-centrifuge. The final mitochondrial pellet was resuspended in isolation buffer without EGTA to yield a final concentration of approximately 10mg/ml, and stored immediately on ice. To normalize the results, the protein concentrations were determined with all the samples on the same plate using the BCA protein assay kit and measuring absorbance at 560nm with a Biotek Synergy HT plate reader (Winooski, Vermont). Mitochondrial oxygen consumption was measured using a Clark-type electrode (Hansatech Instruments, Norfolk, England) in a continuously stirred, sealed chamber at 37°C as previously described (Sullivan et al., 2003). Isolated mitochondrial protein (100μg) was suspended in respiration buffer (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, 2 mM MgCl, 2.5 mM KH2PO4 at pH 7.2) in a final chamber volume of 0.25 mL. Mitochondrial bioenergetic analysis was measured by the sequential administration of substrates: 5mM Pyruvate, 2.5mM Malate, 150nM ADP, 1uM Oligomycin, and 1uM FCCP. Three to four animals per group were utilized for all mitochondrial studies.
6.5 Morris Water Maze Behavioral Assessment
Water maze testing was begun 10 days post-surgery and consisted of four trials per day for four consecutive days. The maze consisted of a dark black circular pool (170 cm diameter, 56 cm high) filled with water (27°C) to a depth of 30 cm. A clear circular Plexiglas platform 13 cm in diameter was placed 2 cm below the surface and served as the goal platform. A video camera placed directly above the center of the pool recorded swimming performance. Each video record was processed by a video motion analyzer (Videomex V; Columbus Instruments, Columbus, OH). The swim speed was measured for each animal to ensure that changes observed were not secondary to impairments in motor function. For each animal, goal latency and distance traversed to the platform were measured for each of the four daily trials and averaged. Four to six animals per group were utilized for Morris Water Maze testing.
Research Highlights.
Pioglitazone attenuates mitochondrial dysfunction following TBI
Pioglitazone attenuates cognitive dysfunction via a PPARγ mediated pathway
Pioglitazone attenuates cortical tissue loss via a PPARγ mediated pathway
Pioglitazone attenuates microglial activation via a PPARγ independent pathway
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.1 References
- Allahtavakoli M, Moloudi R, Arababadi MK, Shamsizadeh A, Javanmardi K. Delayed post ischemic treatment with Rosiglitazone attenuates infarct volume, neurological deficits and neutrophilia after embolic stroke in rat. Brain research. 2009;1271:121–127. doi: 10.1016/j.brainres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. doi: 10.1016/s0969-9961(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain research. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12:2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett. 2005;388:7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Beziaud T, Plotkine M, Marchand-Leroux C. Combination therapy with fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, and simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, on experimental traumatic brain injury. J Pharmacol Exp Ther. 2008;326:966–974. doi: 10.1124/jpet.108.140368. [DOI] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma. 2007;24:1119–1131. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Cristiano L, Bernardo A, Ceru MP. Peroxisome proliferator-activated receptors (PPARs) and peroxisomes in rat cortical and cerebellar astrocytes. J Neurocytol. 2001;30:671–683. doi: 10.1023/a:1016525716209. [DOI] [PubMed] [Google Scholar]
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70:1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Feng D, Zhang Y, Chen G. Cortical expression of peroxisome proliferator-activated receptor-alpha after human brain contusion. J Int Med Res. 2008;36:783–791. doi: 10.1177/147323000803600421. [DOI] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, Sullivan PG, Miller K, Scheff S. Early mitochondrial dysfunction following cortical contusion injury. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Breyer MD. The Role of PPARs in the Transcriptional Control of Cellular Processes. Drug News Perspect. 2002;15:147–154. doi: 10.1358/dnp.2002.15.3.840011. [DOI] [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Feinstein DL, Galea E, Gleichmann M, Wullner U, Klockgether T. Peroxisome proliferator-activated receptor gamma agonists protect cerebellar granule cells from cytokine-induced apoptotic cell death by inhibition of inducible nitric oxide synthase. J Neuroimmunol. 1999;100:156–168. doi: 10.1016/s0165-5728(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000;20:6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Choi DY, Ross SA, Bing G. Protective properties afforded by pioglitazone against intrastriatal LPS in Sprague-Dawley rats. Neurosci Lett. 2008;432:198–201. doi: 10.1016/j.neulet.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Hunter RL, B G. Agonism of peroxisome proliferator receptor–gamma may have therapeutic potential for neuroinflammation and Parkinson's disease. Current Neuropharmacology. 2007 doi: 10.2174/157015907780077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, Tang J. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain research. 2008;1215:218–224. doi: 10.1016/j.brainres.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaei M. Peroxisome Proliferator-Activated Receptor-gamma in Amyotrophic Lateral Sclerosis and Huntington's Disease. PPAR Res. 2008;2008:418765. doi: 10.1155/2008/418765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Kakimura J, Matsuoka Y, Nomura Y, Gebicke-Haerter PJ, Taniguchi T. Activators of peroxisome proliferator-activated receptor-gamma (PPARgamma) inhibit inducible nitric oxide synthase expression but increase heme oxygenase-1 expression in rat glial cells. Neurosci Lett. 1999;262:129–132. doi: 10.1016/s0304-3940(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Marklund N, Clausen F, McIntosh TK, Hillered L. Free radical scavenger posttreatment improves functional and morphological outcome after fluid percussion injury in the rat. J Neurotrauma. 2001;18:821–832. doi: 10.1089/089771501316919184. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Scheff SW. Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma. 1994;11:3–33. doi: 10.1089/neu.1994.11.3. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol. 2007;205:396–406. doi: 10.1016/j.expneurol.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik L, Wahli W. Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Curr Opin Biotechnol. 1999;10:564–570. doi: 10.1016/s0958-1669(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Miglio G, Rosa AC, Rattazzi L, Collino M, Lombardi G, Fantozzi R. PPARgamma stimulation promotes mitochondrial biogenesis and prevents glucose deprivation-induced neuronal cell loss. Neurochem Int. 2009;55:496–504. doi: 10.1016/j.neuint.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/s0301-0082(97)00052-x. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock (Augusta, Ga. 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, Aronowski J. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPARgamma) and 15d-prostaglandin J2--mediated protection of brain after experimental cerebral ischemia in rat. Brain research. 2006;1096:196–203. doi: 10.1016/j.brainres.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Paddock ML, Wiley SE, Axelrod HL, Cohen AE, Roy M, Abresch EC, Capraro D, Murphy AN, Nechushtai R, Dixon JE, Jennings PA. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci U S A. 2007;104:14342–14347. doi: 10.1073/pnas.0707189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Sullivan PG. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp Neurol. 2009;218:381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Pershadsingh HA, Heneka MT, Saini R, Amin NM, Broeske DJ, Feinstein DL. Effect of pioglitazone treatment in a patient with secondary multiple sclerosis. J Neuroinflammation. 2004;1:3. doi: 10.1186/1742-2094-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Robertson CL. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. Journal of bioenergetics and biomembranes. 2004;36:363–368. doi: 10.1023/B:JOBB.0000041769.06954.e4. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Soane L, Siegel ZT, Fiskum G. The potential role of mitochondria in pediatric traumatic brain injury. Developmental neuroscience. 2006;28:432–446. doi: 10.1159/000094169. [DOI] [PubMed] [Google Scholar]
- Rose ME, Huerbin MB, Melick J, Marion DW, Palmer AM, Schiding JK, Kochanek PM, Graham SH. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain research. 2002;935:40–46. doi: 10.1016/s0006-8993(02)02445-9. [DOI] [PubMed] [Google Scholar]
- Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, Sawada H. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochemical and biophysical research communications. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Schouten JW. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Current opinion in critical care. 2007;13:134–142. doi: 10.1097/MCC.0b013e3280895d5c. [DOI] [PubMed] [Google Scholar]
- Schutz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schurmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood V, Colleran K, Burge MR. Thiazolidinediones: a comparative review of approved uses. Diabetes Technol Ther. 2000;2:429–440. doi: 10.1089/15209150050194297. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999a;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Dube C, Dorenbos K, Steward O, Baram TZ. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000a;48:723–729. [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Bussen WL, Scheff SW. Cytochrome c release and caspase activation after traumatic brain injury. Brain research. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Mattson MP, Scheff SW. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. Journal of neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson M, Scheff SW. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp Neurol. 2000b;161:631–637. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A Attenuates Acute Mitochondrial Dysfunction following Traumatic Brain Injury. Exp Neurol. 1999b;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Sun H, Huang Y, Yu X, Li Y, Yang J, Li R, Deng Y, Zhao G. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. Int J Dev Neurosci. 2008;26:505–515. doi: 10.1016/j.ijdevneu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int. 2006;49:136–144. doi: 10.1016/j.neuint.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, Marmarou A, Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery. 2005;56:582–589. doi: 10.1227/01.neu.0000156715.04900.e6. discussion 582–589. [DOI] [PubMed] [Google Scholar]
- Torra IP, Chinetti G, Duval C, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors: from transcriptional control to clinical practice. Curr Opin Lipidol. 2001;12:245–254. doi: 10.1097/00041433-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Vijitruth R, Liu M, Choi DY, Nguyen X, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondrary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. Journal of Neuroinflammation. 2006;3:1742–2094. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky E, Palzur E, Soustiel JF. Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathology and applied neurobiology. 2006;32:40–50. doi: 10.1111/j.1365-2990.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci U S A. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Xin T, Hunter RL, Bing G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J Neuroinflammation. 2008;5:4. doi: 10.1186/1742-2094-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Rossi S, Stiefel M, Doppenberg E, Zauner A, Bullock R, Marmarou A. CSF and ECF glutamate concentrations in head injured patients. Acta neurochirurgica. 1999;75:17–19. doi: 10.1007/978-3-7091-6415-0_4. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain research. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PW, Buckle DR, Cantello BC, Chapman H, Clapham JC, Coyle PJ, Haigh D, Hindley RM, Holder JC, Kallender H, Latter AJ, Lawrie KW, Mossakowska D, Murphy GJ, Roxbee Cox L, Smith SA. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther. 1998;284:751–759. [PubMed] [Google Scholar]