Abstract
Site-specific recombinases catalyze recombination between specific targeting sites to delete, insert, invert, or exchange DNA with high fidelity. In addition to the widely used Cre and Flp recombinases, the phiC31 integrase system from Streptomyces phage may also be used for these genetic manipulations in eukaryotic cells. Unlike Cre and Flp, phiC31 recognizes two heterotypic sites, attB and attP, for recombination. While the phiC31 system has been recently applied in mouse and human cell lines and in Drosophila, it has not been demonstrated whether it can also catalyze efficient DNA recombination in zebrafish. Here we show that phiC31 integrase efficiently induces site-specific deletion of episomal targets as well as chromosomal targets in zebrafish embryos. Thus, the phiC31 system can be used in zebrafish for genetic manipulations, expanding the repertoire of available tools for genetic manipulation in this vertebrate model.
Keywords: Phic31 integrase, Site-specific recombination, Zebrafish
Introduction
Site-specific recombinases have been used extensively in model systems to carry out targeted recombination to facilitate genetic analysis (Bischof and Basler 2008; Branda and Dymecki 2004). These recombinases are generally classified into two families, tyrosine recombinases and serine recombinases, based on the catalytic mechanism. In eukaryotic cells, two tyrosine recombinases, Cre recombinase from the phage P1 and the FLP recombinase from Saccharomyces cerevisiae are commonly used for site-specific recombination. Many other phage integrases have been widely used in prokaryotes for site-specific recombination. Some of these phage integrases, such as the tyrosine integrase for E. coli phage lambda, require accessory proteins and have limited application in eukaryotes. But some phage integrases, such as the serine integrase for Streptomyces phage phiC31, do not require accessory factors and are potentially useful in eukaryotes (Groth and Calos 2004). Indeed, the phiC31 system has been used for genetic manipulation in both mice and flies (Belteki et al. 2003; Bischof et al. 2007; Groth et al. 2004). PhiC31 integrase (Int-phiC31) catalyzes recombination between an attP and an attB site (Groth and Calos 2004). These heterotypic target sites are advantageous for certain genetic manipulations such as site-specific integration and stbale inversion, since the reaction is irreversible (Groth and Calos 2004). In flies, the phage Int-phiC31 is sufficiently active to mediate targeted integration at a reasonable frequency (Groth et al. 2004; Fish et al. 2007; Bischof et al. 2007; Bischof and Basler 2008). It has also been used for targeting transgenes at engineered chromosomal loci by attP and attB guided exchange of genomic DNA in the germ line (Bateman et al. 2006; Bateman and Wu 2008). Similar applications in mouse ES cells have been reported (Belteki et al. 2003). Zebrafish has emerged as a major non-mammalian vertebrate genetic model for many aspects of biomedical research because of its high fecundity and low maintenance cost. Many commonly used genetic techniques, including site-specific recombination using Cre and Flp, are being adopted in zebrafish (Dong and Stuart 2004; Langenau et al. 2005; Boniface et al. 2009; Hans et al. 2009). The application of the phiC31 system in zebrafish has not been reported but could be used to expand the options for genetic manipulation in zebrafish. Here we demonstrate that injection of Int-phiC31 RNA can induce site-specific deletion both in newly injected and stably integrated transgenes.
Materials and methods
Fish husbandry
Zebrafish were raised in Aquatic Habitats systems (Apopka, FL) on a 14–10 h light dark cycle as described previously (Boniface et al. 2009). Embryos were reared in 0.3 × Danieau’s solution at 28°C until 5 day-postfertilization (dpf).
Int-phiC31 activity assay
The minimal attP and attB was synthesized by annealing two oligonucleotides followed by filling-in the single stranded portion by Klenow fragment using standard methods. The resulting fragment was digested with XbaI and cloned into pBlueScript II KS(−) in the XbaI site. The two oligonucleotides are: attB,5′-AACTCTAGAGGTGCCAGGGCGTGCCCTTGGGCTCCCCGGGCGCGGCTAGCGATATCGATGAATTC-3′ (underlined is the minimal attB site); attP: 5′-AGTTCTAGACCCCCAACTGAGAGAACTCAAAGGTTACCCCAGTTGGGGGAATTCATCGATATCGCTAGC-3′ (underlined is the minimal attP site). The mCitrine coding sequence from pmCitrine-N1 was inserted between attP and attB site and the resultant cassette was inserted in between the hybrid efl1a/b-actin2 promoter (eab2) (Boniface et al. 2009) and mCherry gene to generate the Int-phiC31 activity reporter Tg(eab2:attP-mCitrine-attBmCherry). To assay Int-phiC31 integrase activity on episomal DNA in zebrafish embryos, about 1 nl sterile isotonic saline solution containing a mixture of Tg(eab2:attP-mCitrine-attB-mCherry) DNA (30 ng/µl) with or without synthetic phiC31 RNA (30 ng/µl) was injected into fertilized eggs of wild type zebrafish. The embryos were checked for expression of fluorescent proteins under a fluorescence microscope 2 days after injection. The zebrafish images were acquired using a Zeiss M2Bio or an Axiovert 200 microscope equipped with a color CCD camera controlled by AxioVison (Zeiss) and processed in Photoshop (Adobe).
To assay phiC31 activity on integrated DNA in zebrafish embryos, about 1 nl sterile isotonic saline solution containing synthetic Int-phiC31 RNA (30, 60, and 100 ng/µl) was injected into fertilized eggs of wild type fish crossed with the transgenic line.
(14-3-3εEt) with an insertion of Tg(UAS:[mCherry-T-mCitrine]; attB-hsp70 l:Gal4VP16-attP) near the 14-3-3ε gene (Maddison et al. 2009). The [] denotes that the intervening sequence can be inverted by FlEx (Boniface et al. 2009). The injected embryos were examined and images processed as above.
PCR and sequencing
Genomic DNA was extracted from individual injected embryos at 5 dpf. PCR was done in a Bio-Rad thermocycler using standard PCR parameters (95°C for 2 min, then followed by 30 cycles of the denaturing at 95°C for 30 s, annealing at 58°C for 40 s and extension at 72°C for 2 min). The primer sequences are as follows:
β-actin2 F 5′-GATAGCCGGCATGGGAAGTTC-3′. mCherry R 5′-CTTCAAGTAGTCGGGGATGTCG-3′. mCitrine R 5′-CTGAACTTGTGGCCGTTTACGTC-3′. Tol2 3’TIR R 5′-GTATCTGGCTAGAATCTTACTTGAG-3′.
The PCR product was run in 0.8% agarose gel and the gel was imaged using a Kodak Image system. PCR products were isolated from the gels, purified and cloned into pCR4-Topo vector and sequenced.
Results
Int-phiC31 induces intramolecular recombination between target sites in episomal DNA in zebrafish embryos
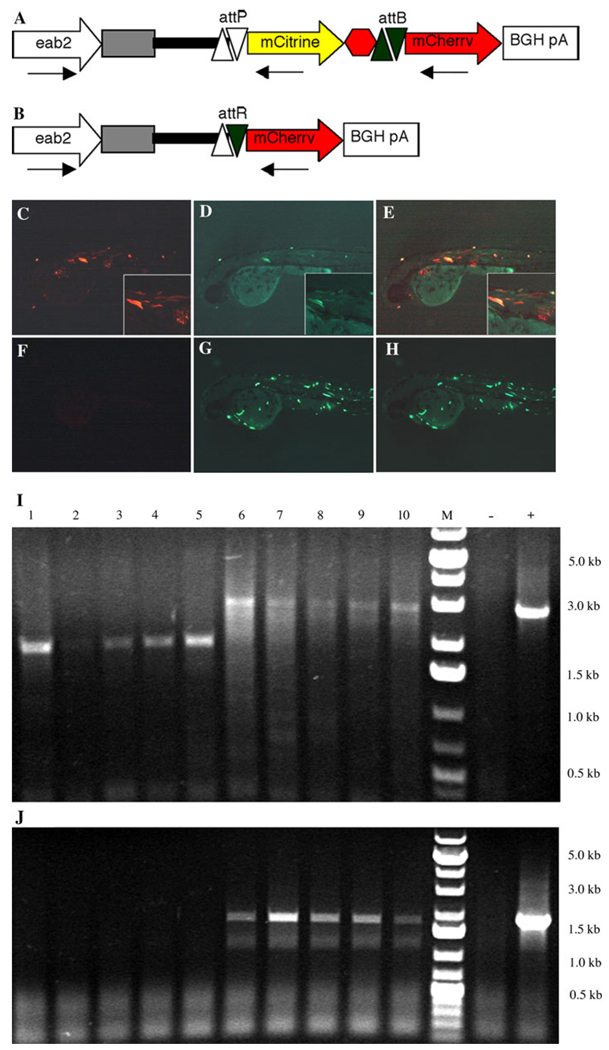
We first tested whether Int-phiC31 can delete sequences flanked by the minimal attP/attB in zebrafish. We constructed a deletion reporter gene Tg(eab2:attP-mCitrine-attB-mCherry) (Fig. 1a) that switches from mCitrine to mCherry expression after Int-phiC31-mediated deletion (Fig. 1b). The reporter gene construct was injected into one-cell stage embryos with or without synthetic Int-phiC31 RNA. With Int-phiC31 RNA co-injection, strong mCherry expression was detected in the injected embryos (Fig. 1c), suggesting recombination between the attP and attB sites. There was also weak but persistent mCitrine expression, which overlapped extensively with mCherry expression (Fig. 1d, e). Without IntphiC31 RNA, strong mCitrine expression was detected in the injected embryos (Fig. 1g, f), but mCherry expression was undetectable (Fig. 1f). To determine whether the residual mCitrine expression in the Int-phiC31-injected embryos is due to incomplete Int-phiC31-mediated recombination, the total DNA from 5 day-old individual embryos was analyzed by PCR. As shown in Fig. 1i, co-injection of the reporter and Int-phiC31 RNA resulted in the deletion of a 1 kb fragment between the promoter and mCherry. Sequencing of the PCR product confirmed that it is derived from attB–attP recombination. The PCR product from the original vector was undetectable (Fig. 1j), suggesting that the recombination was very efficient and the majority of the injected vector has been recombined. Nevertheless, there may be residual original vector below the detection levels that partially contribute to the observed mCitrine expression. As expected, in embryos injected with the vector alone, the original DNA, but not the recombinant, was detected (Fig. 1i, j). This data suggested that Int-phiC31 is highly efficient in zebrafish.
Fig. 1.
PhiC31 integrase induces intramolecular recombination between target sites in episomal DNA in zebrafish. a Schematic of the reporter construct Tg(eab2:attP-mCitrine-attB-mCherry) containing a chimeric eab2 promoter (open arrow), the non-coding 1st exon (grey box) and 1st intron (black line) of β-actin2 followed by an attP site (open triangles), mCitrine cDNA (yellow arrow), 3xSV40 Poly A (red hexagon), an attB site (black triangles) and mCherry cDNA (red arrow). Arrows represent the location of PCR primers. b Schematic of the reporter construct after phiC31 mediated deletion. c–e Embryos co-injected with the reporter and Int-phiC31 RNA have strong mCherry expression (c) and weak mCitrine expression (d). The mCitrine expression is co-localized with mCherry expression (e). Insets are higher magnification of selected regions. f–h Embryos injected with the reporter alone show strong mCitrine expression (g) and no mCherry expression (f), merged image in (h). i, j PCR analysis of the injected embryos. Lanes 1–5 DNA from individual embryos injected with reporter plasmid and Int-phiC31 RNA, Lanes 6–10 DNA from individual embryos injected with reporter plasmid only, M, 1 kb DNA ladder;-, DNA from uninjected embryos; +, plasmid control. (i) PCR using reverse primer within mCherry. The 3.0 kb band is the intact transgene and the 2.0 kb band is the recombinant. (j) PCR using reverse primer within mCitrine. Upon recombination no product is detectable
Int-phiC31 integrase induces intramolecular recombination between integrated target sites in zebrafish
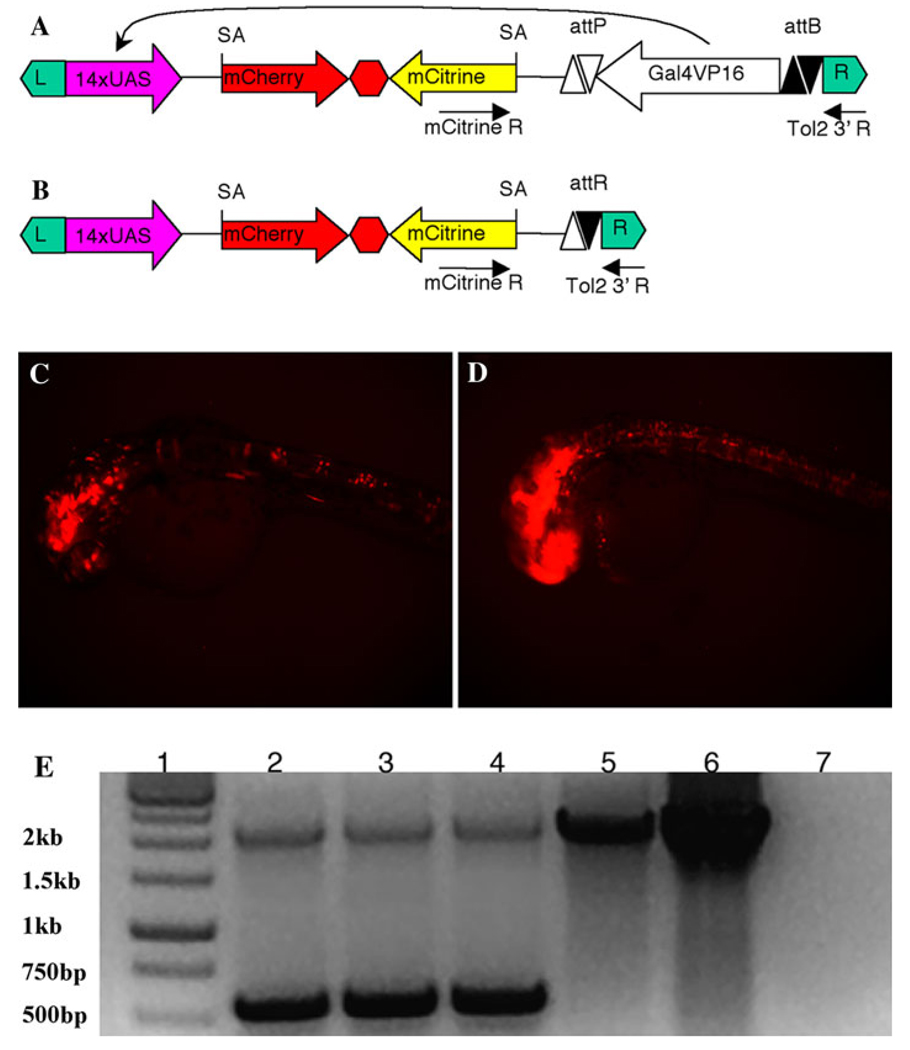
To test whether Int-phiC31 works on a stably integrated transgene in zebrafish, we used a previously described enhancer trap line (14-3-3εEt) with an insertion of Tg(UAS:[mCherry-T-mCitrine]; attPhsp70 l:Gal4VP16-attB) near the 14-3-3ε gene (Maddison et al. 2009). The enhancer line was generated using Tol2 transgenesis to deliver a cassette consisting of the hsp70 l basal promoter driving Gal4-VP16 and UAS-driven mCherry and displays strong mCherry throughout the CNS beginning at 12 somites. The hsp70 l basal promoter and the Gal4VP16 coding sequence in the enhancer trap vector is flanked by phiC31 attB and attP sites (Fig. 2a). Activity of Int-phiC31 should eliminate Gal4-VP16 expression, resulting in reduced mCherry expression (Fig. 2b). To test this, out-crossed 14-3-3εEt embryos were each injected with 30 pg Int-phiC31 mRNA. Two days after injection, the embryos injected with Int-phiC31 RNA had reduced mCherry expression (Fig. 2c) compared to embryos injected with isotonic saline (Fig. 2d). To determine whether increased Int-phiC31 RNA may increase the deletion efficiency, we injected 60 or 100 pg of Int-phiC31 RNA to each outcrossed embryo. However, higher amounts of Int-phiC31 RNA did not result in complete removal of mCherry expression (data not shown). To determine whether the persistence of the mCherry signal is due to incomplete recombination, DNA from the injected embryos was analyzed by PCR to determine the structure of the transgene locus. In embryos injected with Int-phiC31 RNA, both the 2 kb product from the original transgene and a 500 bp PCR product were detected, although the smaller product appeared much more abundant (Fig. 2e). Sequencing of the smaller PCR product verified that it was the Int-phiC31 recombinant. However, increased Int-phiC31 RNA did not significantly decrease the 2 kb product from the original transgene. This suggests genomic DNA may be more refractory to recombination than episomal plasmid DNA.
Fig. 2.
PhiC31 integrase induces intramolecular recombination between integrated target sites in zebrafish. a Schematic of the gene/enhancer trap vector Tg(UAS:[mCherry-T-mCitrine]; attP-hsp70 l:Gal4VP16-attB). The transgene is flanked by Tol2 terminal inverted repeats (green boxes, L, left side; R, right side). The transgene itself consists of 14 repeats of upstream activation sequence (UAS) (pink arrow), mCherry cDNA (red arrow), 3xSV40 poly A (red hexagon), mCitrine cDNA (yellow arrow). The enhancer trap driver (open arrow) consists of Gal4VP16 and basal promoter of hsp701 and is flanked by an attP site (open triangles) and an attB site (black triangles). Arrows represent the location of PCR primers. SA stands for splice acceptor. Curved arrow indicates activation of UAS-driven mcherry by Gal4-VP16 binds. b Schematic of the vector after phiC31 mediated deletion of Gal4-VP16. c, d Expression of mCherry at 2dpf in the gene/enhancer trap line when injected with 30 pg Int-phiC31 RNA (c) or isotonic saline (d). e PCR analysis of the injected embryos. Lane 1, 1 kb DNA ladder. Lane 2, embryos injected 30 pg of phiC31 mRNA. Lane 3, embryos injected 60 pg of phiC31 mRNA. Lane 4, embryos injected 100 pg of phiC31 mRNA. Lane 5, embryos injected isotonic saline solution. Lane 6, vector DNA. Lane 7, dH2O control. The 2 kb amplicon is from the original transgene and the 500 bp amplicon is from the recombinant
Discussion
We demonstrate here that the phiC31 system works in zebrafish and the recombination efficiency by Int-phiC31 is reasonably high. The efficiency is higher in episomal targets than in chromosomal targets. For episomal targets, recombination efficiency is such that the original construct is hardly detectable. Nevertheless, the product (mCitrine) from the original vector is still detectable as a fluorescent protein. Some of the mCitrine signal may be from expression prior to the recombination and persists due to the long half-life of fluorescent proteins in zebrafish. However, the weak mCitrine signal is still visible in larvae at 5 dpf suggesting that, although undetectable by PCR, there is still residual original vector in the Int-phiC31 RNA injected embryos. The reason for the lower recombination efficiency on chromosomal targets is not known. It may be related to the lower accessibility of the targets. Nevertheless, recombination seemed to have occurred in the majority of the targets in both episomal and chromosomal DNA. With the improved Int-phiC31 (Keravala et al. 2009), it may be possible to achieve even higher recombination efficiency.
The demonstration of high recombination efficiency of Int-phiC31 in zebrafish should encourage researchers to use the phiC31 system for genetic manipulations. A major difference between Int-phiC31 and other commonly used recombinases, such as Cre and Flp, is that the sequences of the two target sites are very different. Consequently, recombination is irreversible. This is advantageous in at least two applications, targeted integration and stable inversion. Targeted integration of transgenes minimizes positional effects on transgene expression. In Drosophila, Int-phiC31 based targeted integration has already become a routine transgenesis method (Bischof and Basler 2008; Fish et al. 2007). The Int-phiC31 has been shown to be capable of inserting very large DNA constructs (of up to 130 kb) into various attP lines (Venken et al. 2006). Targeted integration has not been reported in zebrafish. Invertible gene trap vectors have been used to generate conditional mutations in mouse ES cells (Schnütgen et al. 2005; Xin et al. 2005). Given the efficiency of gene trap mutagenesis in zebrafish (Kawakami et al. 2004), similar approach may be used in zebrafish. Current vectors for conditional gene trap relies on incompatible Cre or Flp target sites. But the incompatibility of these sites is not absolute and the phiC31 system may be a more attractive alternative.
In summary, the phiC31 system works efficiently in zebrafish. Coupled with the efficiency of Tol2 transgenesis system (Kawakami et al. 2004), many attP and attB sites can be implanted in the zebrafish genome with desired configurations and would provide ample opportunity for more sophisticated genetic manipulations in zebrafish.
Acknowledgments
We thank Pinxun Yu for excellent fish care, and other members of the Chen lab for discussions. This work is supported by a NIH grant R01 EY016092 to WC.
Contributor Information
Jianjun Lu, Vollum Institute, OHSU, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA.
Lisette A. Maddison, Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine, Nashville, TN 37232, USA
Wenbiao Chen, Email: wenbiao.chen@vanderbilt.edu, Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine, Nashville, TN 37232, USA.
References
- Bateman JR, Wu CT. A simple polymerase chain reaction-based method for the construction of recombinase-mediated cassette exchange donor vectors. Genetics. 2008;180:1763–1766. doi: 10.1534/genetics.108.094508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman JR, Lee AM, Wu CT. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics. 2006;173:769–777. doi: 10.1534/genetics.106.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belteki G, Gertsenstein M, Ow DW, Nagy A. Site-specific cassette exchange and germline transmission with mouse ES cells expressing phiC31 integrase. Nat Biotechnol. 2003;21:321–324. doi: 10.1038/nbt787. [DOI] [PubMed] [Google Scholar]
- Bischof J, Basler K. Recombinases and their use in gene activation, gene inactivation, and transgenesis. Methods Mol Biol. 2008;420:175–195. doi: 10.1007/978-1-59745-583-1_10. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface EJ, Lu J, Victoroff T, Zhu M, Chen W. FlEx-based transgenic reporter lines for visualization of Cre and Flp activity in live zebrafish. Genesis. 2009;47:484–491. doi: 10.1002/dvg.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Dong J, Stuart GW. Transgene manipulation in zebrafish by using recombinases. Methods Cell Biol. 2004;77:363–379. doi: 10.1016/s0091-679x(04)77020-x. [DOI] [PubMed] [Google Scholar]
- Fish MP, Groth AC, Calos MP, Nusse R. Creating transgenic Drosophila by microinjecting the site-specific phiC31 integrase mRNA and a transgene-containing donor plasmid. Nat Protoc. 2007;2:2325–2331. doi: 10.1038/nprot.2007.328. [DOI] [PubMed] [Google Scholar]
- Groth AC, Calos MP. Phage integrases: biology and applications. J Mol Biol. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Kaslin J, Freudenreich D, Brand M. Temporally-controlled site-specific recombination in zebrafish. PLoS ONE. 2009;4:e4640. doi: 10.1371/journal.pone.0004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Keravala A, Lee S, Thyagarajan B, Olivares EC, Gabrovsky VE, Woodard LE, Calos MP. Mutational derivatives of PhiC31 integrase with increased efficiency and specificity. Mol Ther. 2009;17:112–120. doi: 10.1038/mt.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau DM, Feng H, Berghmans S, Kanki JP, Kutok JL, Look AT. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison LA, Lu J, Victoroff T, Scott E, Baier H, Chen W. A gain-of-function screen in zebrafish identifies a guanylate cyclase with a role in neuronal degeneration. Mol Genet Genomics. 2009;281(5):551–563. doi: 10.1007/s00438-009-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnütgen F, De-Zolt S, Van Sloun P, Hollatz M, Floss T, Hansen J, Altschmied J, Seisenberger C, Ghyselinck NB, Ruiz P, Chambon P, Wurst W, von Melchner H. Genomewide production of multipurpose alleles for the functional analysis of the mouse genome. Proc Natl Acad Sci USA. 2005;102:7221–7226. doi: 10.1073/pnas.0502273102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- Xin HB, Deng KY, Shui B, Qu S, Sun Q, Lee J, Greene KS, Wilson J, Yu Y, Feldman M, Kotlikoff MI. Gene trap and gene inversion methods for conditional gene inactivation in the mouse. Nucleic Acids Res. 2005;33:e14. doi: 10.1093/nar/gni016. [DOI] [PMC free article] [PubMed] [Google Scholar]