Abstract
Renin-angiotensin system inhibitors significantly reduce the incidence of arrhythmias. However, the underlying mechanism(s) is not well understood. We aim to test the hypothesis that Ang II induces early afterdepolarizations (EADs) and triggered activities (TAs) via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-ROS-calmodulin kinase II (CaMKII) pathway. ROS production was analyzed in the isolated rabbit myocytes loaded with ROS dye. Ang II (1–2 µM) increased ROS fluorescence in myocytes, which was abolished by Ang II type 1 receptor blocker losartan, NADPH oxidase inhibitor apocynin, and antioxidant MnTMPyP, respectively. Action potentials were recorded using the perforated patch-clamp technique. EADs emerged in 27 out of 41 (66%) cells at 15.8 ± 1.6 min after Ang II (1~2 µM) perfusion. Ang II-induced EADs were eliminated by losartan, apocynin, or trolox. The CaMK II inhibitor KN-93 (n=6) and inhibitory peptide (AIP) (n=4) also suppressed Ang II-induced EADs, whereas the inactive analogue KN-92 did not. Nifedipine, a blocker of L-type Ca current (ICa,L), or ranolazine, an inhibitor of late Na current (INa), abolished Ang II-induced EADs. The effects of Ang II on major membrane currents were evaluated using voltage clamp. While Ang II at same concentrations had no significant effect on total outward K+ current, it enhanced ICa.L and late INa, which were attenuated by losartan, apocynin, trolox, or KN-93. We conclude that Ang II induces EADs via intracellular ROS production through NADPH oxidase, activation of CaMKII, and enhancement of ICa,L and late INa. These results provide evidence supporting a link between renin-angiotensin system and cardiac arrhythmias.
Keywords: Angiotensin II, early afterdepolarizations, triggered activities, reactive oxygen species, CaMKII, L-type calcium channel, sodium channel
1. Introduction
Reactive oxygen species (ROS), including superoxide anions, hydroxyl radicals, and hydrogen peroxide (H2O2), are suggested to be arrhythmogenic factors. Our recent study [1] has shown that exogenous addition of H2O2 to ventricular myocytes induces early afterdepolarizations (EADs), delayed afterdepolarizations (DADs) and triggered activities (TAs) via activation of Ca/Calmodulin kinase II (CaMK II), which in turn enhances L-type calcium current (ICa,L) and late sodium current (INa). However, it remains to be examined whether endogenously generated ROS under certain pathological conditions are sufficient to stimulate CaMKII and generate EADs and DADs.
It has been demonstrated that various membrane receptors (e.g. Angiotensin II type 1 receptor (AT1R), endothelin type A receptor (ETAR), and transforming growth factor β receptors (TGFβR) mediate generation of superoxide and H2O2 via the reduction of O2 by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex in non-cardiac cells [2–4]. Recent studies have also found a similar NADPH oxidase system in cardiac myocytes [5–7]. Angiotensin II (Ang II) activates NADPH oxidase, via AT1R leading to increased generation of ROS and myocardial injury [8–9].
Ang II is an endogenous peptide hormone playing a critical role in the pathophysiological modulation of cardiovascular disorders, such as hypertension, ischemic heart disease, cardiac hypertrophy, and heart failure [10–11]. Recent clinical studies has revealed that angiotensin-converting enzyme (ACE) inhibitors and Ang II receptor blockers are important therapeutic agents in the treatment of atrial and ventricular arrhythmias [12–13], suggesting Ang II is arrhythmogenic under pathophysiological conditions including heart failure and cardiac ischemic-reperfusion. This notion was further supported by experimental data [14–15]. While electrical (ion channels) and structural (fibrosis, dilatation and hypertrophy) remodeling and neurohumoral activation have been suggested to account for the arrhythmogenic effect of Ang II [15–16], little is known about the role of Ang II in inducing arrhythmias via NADPH oxidase-ROS pathway. The present study was designed to assess the hypothesis that Ang II is proarrhythmic by inducing EADs and TAs via NADPH oxidase, ROS generation, activation of CaMK II, and activation of ICa,L and late sodium current (INa).
2. Materials and Methods
An expanded Methods section is available in the Online Data Supplement. All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Medicine and Dentistry of New Jersey-New Jersey Medical School. All experiments were performed at 35 to 37°C.
2.1 Cell isolation
Ventricular myocytes were enzymatically isolated from the hearts of New Zealand white rabbits (Male, 2–3 kg) as described previously [1].
2.2. Intracellular calcium ion (Cai) measurement
Myocytes were loaded with the Ca2+ indicator Fluo-4 AM. The Cai fluorescence signals were measured using a charge-coupled device (CCD) camera system.
2.3. Electrophysiological recording
Myocytes were patch-clamped using the perforated patch-clamp technique in the whole-cell configuration. Action potentials (APs) were recorded under current clamp mode, and whole cell currents (ICa,L, late INa, total IK) were recorded under voltage clamp mode with a MultiClamp 700A patch-clamp amplifier controlled by a personal computer using a Digidata 1322A acquisition board driven by pCLAMP 10 software (Molecular Devices, Sunnyvale, CA).
2.4. Detection of intracellular ROS
Myocytes were incubated with 5 µM C-DCDHF-DA-AM. ROS fluorescence was measured by using a charge-coupled device camera.
2.5. CaMKII assay
CaMKII activity was measured using SignaTECT Calcium/Calmodulin-Dependent Protein kinase Assay system (Promega) following manufacturer’s instructions.
2.6. Statistical analysis
Data are presented as mean ± SEM. Statistical significance was assessed using paired, unpaired Student’s t tests or ANOVA analysis, with P<0.05 considered significant.
3. Results
3.1. Ang II-induced ROS production in rabbit ventricular myocytes
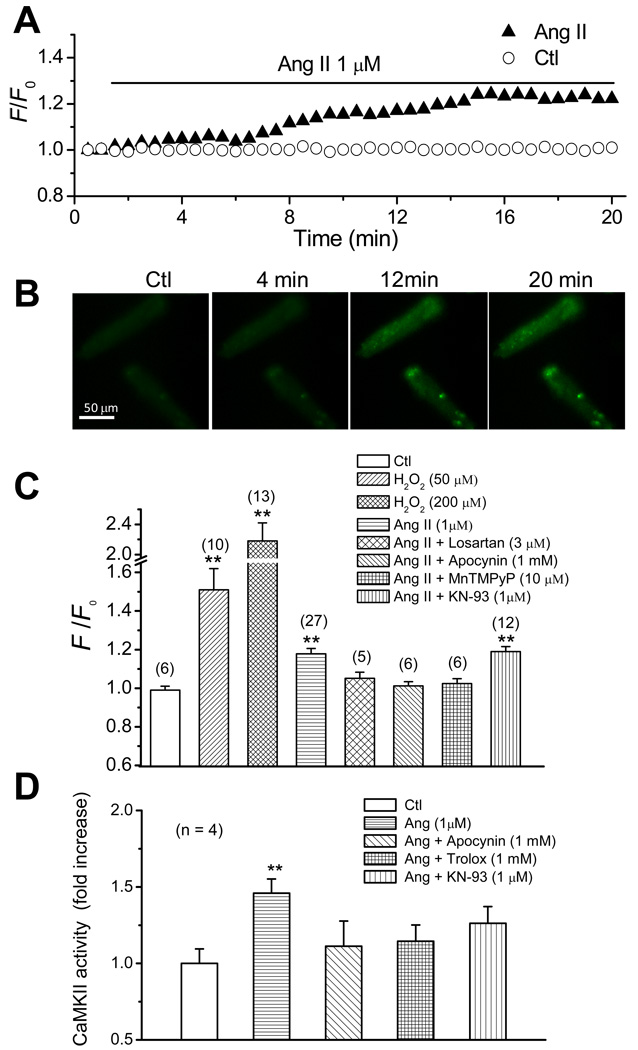
We first investigated the effect of Ang II on ROS production in isolated rabbit ventricular myocytes by monitoring fluorescence intensity of ROS-sensitive dye C-DCDHF-DA. While H2DCFDA being widely used to detect hydrogen peroxide [17], it actually detects a broader range of ROS [18]. Thus, we used H2DCFDA as an indicator of general oxidative stress level. In vehicle-treated myocytes, DCF fluorescence remained stable during the monitoring period (Fig. 1A). The effects of exogenous H2O2 at 50 and 200 µM were evaluated as a positive control. A rapid and dramatic increase in DCF fluorescence intensity was observed in the myocytes after H2O2 treatment. The F/F0 of DCF fluorescence intensity reached the value of 2.18 ± 0.24 and 1.51 ± 0.11 at 5~7 min of exposure to 200 and 50 µM H2O2, respectively (Fig. 1C). Ang II (1 µM) enhanced DCF fluorescence intensity mildly compared to direct application of H2O2. As shown in Fig. 1A, the elevation of intensity initiated at 3–4 min and reached a stable state at 15–20 min after Ang II application. Representative DCF fluorescence images in the presence or absence of Ang II are shown in Fig. 1B, indicating marked fluorescence increases at 12, 20 min after Ang II application. Note that Ang II-induced ROS production appeared higher in some localized cytosolic region (figure 1B). Although we do not have a ready explanation for this phenomenon, it may share a common mechanism of “metabolic sink” accounting for regional polarized mitochondria and high level of ROS during ischemia/reperfusion suggested by O'Rourke’s group [19]. The F/F0 of DCF fluorescence intensity was measured at 18–20 min after Ang II treatment and showed significant increase (1.18 ± 0.03) compared to control group (0.99 ± 0.02) (Fig. 1C). However, Ang II failed to enhance DCF fluorescence intensity in the presence of the AT1R antagonist losartan (3–5 µM), the NADPH oxidase inhibitor apocynin (1 mM), or manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP, 10 µM) (a SOD/catalase mimetic, which has been considered as a general ROS scavenger [20–21]). Losartan, Apocynin and MnTMPyP had no effect on the DCF fluorescence intensity in the absence of Ang II. These results suggest that Ang II causes ROS production via AT1R and NADPH oxidase in rabbit ventricular myocytes. Ang II was still able to increase ROS production in the presence of CaMKII inhibitor, KN93 (1 µM), suggesting that CaMKII activation is downstream of ROS production.
Fig. 1.
Ang-II induced ROS production in isolated rabbit ventricular myocytes. (A). Time course of DCF intensity in cells treated with vehicle (open circles) or 1 µM Ang II (filled triangles). (B). Images showing DCF intensity of myocytes at control, and 4, 12, 20 mins after treatment with 1 µM Ang II. (C). Histograms illustrating the effect of 1 µM Ang II treatment (18–20 min) on DCF fluorescence intensity in the absence and presence of losartan, apocynin, MnTMPyP, and KN-93 (measured at 2–5 min after application). H2O2 (50 and 200 µM)-induced increase of DCF intensity is shown as a positive control. Note the break on the Y-axis. (D). Histograms illustrating the effect of Ang II on CaMKII activity in the absence and presence of apocynin, Trolox, or KN-93. **P < 0.01 compared to control. Numbers in parentheses indicate the number of cells in each group.
Since CaMKII can be activated by oxidation by ROS [8–9], we next determined if Ang II could also activate CaMKII via ROS production. As shown in Fig. 1D, Ang II treatment (1 µM, for ~ 60 min) significantly increased the CaMKII activity in isolated myocytes, however, it failed to activate CaMKII in the presence of apocynin (1mM), Trolox (1 mM), or KN-93 (1 µM).
3.2. Induction of EADs by Ang II in rabbit ventricular myocytes
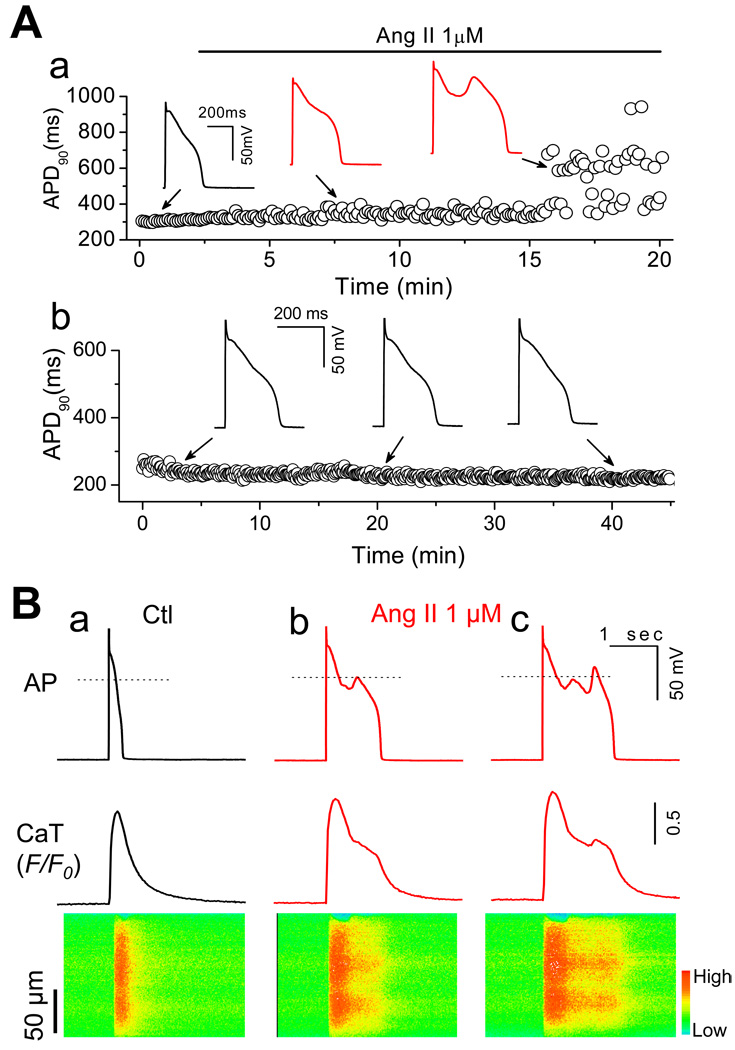
In the following experiments, we assessed whether Ang II could induce EADs via NADPH oxidase-ROS-CaMKII-ICa,L/INa pathway. APs were recorded from isolated rabbit ventricular myocytes using perforated whole-cell patch-clamp technique under current clamp mode. In order to reliably induce EADs, the cells were paced at a PCL of 6 sec based on our previous studies [1, 22]. After action potential duration (APD) and morphology reached steady state, cells were perfused with 1 to 2 µM Ang II for up to 40 min or until EADs appeared. As shown in Fig. 2A, the APD was initially prolonged gradually after application of Ang II (e.g. APD was prolonged from 286.3±32.9 to 475.5±95.3 ms (n= 8) at 10 min of exposure to Ang II). EADs were induced in 27 out of 41 cells after an average exposure time of 15.8 ± 1.6 min (n=27). EADs could be irregular, single, or multiple with an oscillating membrane potential before repolarization (see figures 2–6). DADs were also observed in 3 out of 41 cells exposed to 1 µM Ang II (data not shown). EADs were also induced in 3 out of 7 cells by 100 nM Ang II and in 5 out of 9 cell by 50 µM H2O2, while control experiments in parallel showed that neither EADs nor DADs occurred in the absence of Ang II up to 40 min (Fig.2-Ab) (n=6),
Fig. 2.
Early afterdepolarizations (EADs) and intracellular calcium (Cai) alteration induced by Ang II. (A-a). APs recorded under perforated whole-cell configuration before and during Ang II perfusion. Values of consecutive APD90 are plotted over time. EADs were induced at 15.8 ± 1.6 min after exposure to Ang II. (A-b). A representative AP recording from a cell perfused with control perfusate for > 40 min. No EADs were observed. (B). Cai Transients recorded under control and during Ang II-Induced EADs. a. AP, whole-cell Cai transient, and a line-scan image along the long axis of the myocyte before Ang II treatment. b & c. Same following exposure to 1µM Ang II for ~20 min. EADs result in persistent elevation (b) and additional release in Cai (c).
Fig. 6.
Involvement of late INa in Ang II-induced EADs. (A). The specific INa blocker TTX (10 µM) further shortened APD after EADs were eliminated by nifedipine. (B). The selective inhibitor of late INa, ranolazine (Ran), also abolished EADs induced by Ang II. (C). Representative INa traces under control condition (Ctl), in the presence of Ang II, and Ang II + KN-93. (D). A bar graph summarizing 1 µM Ang II-induced increase of late INa, which is significantly suppressed by 1mM trolox, 1 µM apocynin, or 1 µM KN-93, respectively.
3.3. Ang II-induced Cai abnormality in adult rabbit ventricular myocytes
The effect of Ang II on Cai handling correlated to EADs was also investigated. As shown in Fig. 2B, we simultaneously recorded APs (top panel), Cai transients (middle panel) and line scan images (bottom panel) in Fluo-4 AM-loaded myocytes. The effect of Ang II (1 µM) on Cai transients amplitude was evaluated. Ang II did not cause significant increase in Ca transient amplitude before EAD appeared (F/F0: 1.89 ± 0.14 at 5 min after Ang II, vs. 1.94 ± 0.11 at control, P > 0.05, n = 5), while it enhanced Ca transient amplitude after EADs were induced (F/F0: 2.04 ± 0.11, n = 5, P < 0.05). Increased Ca2+ entry though ICa,L or the reverse mode of NCX has been proposed as the underlying mechanism(s) [23]. During Ang II-induced EADs, Cai remained elevated as a plateau (middle panel in Fig. 2B-b) or increased as a second peak (middle panel in Fig. 2B-c). The peak of second EAD in the AP (Fig, 2B-c) constantly preceded the second Ca transient peak following the long elevated state, suggesting an additional SR Ca release attributable to reactivation of ICa.L.
3.4. Cellular signaling pathways involved in Ang II-induced EADs
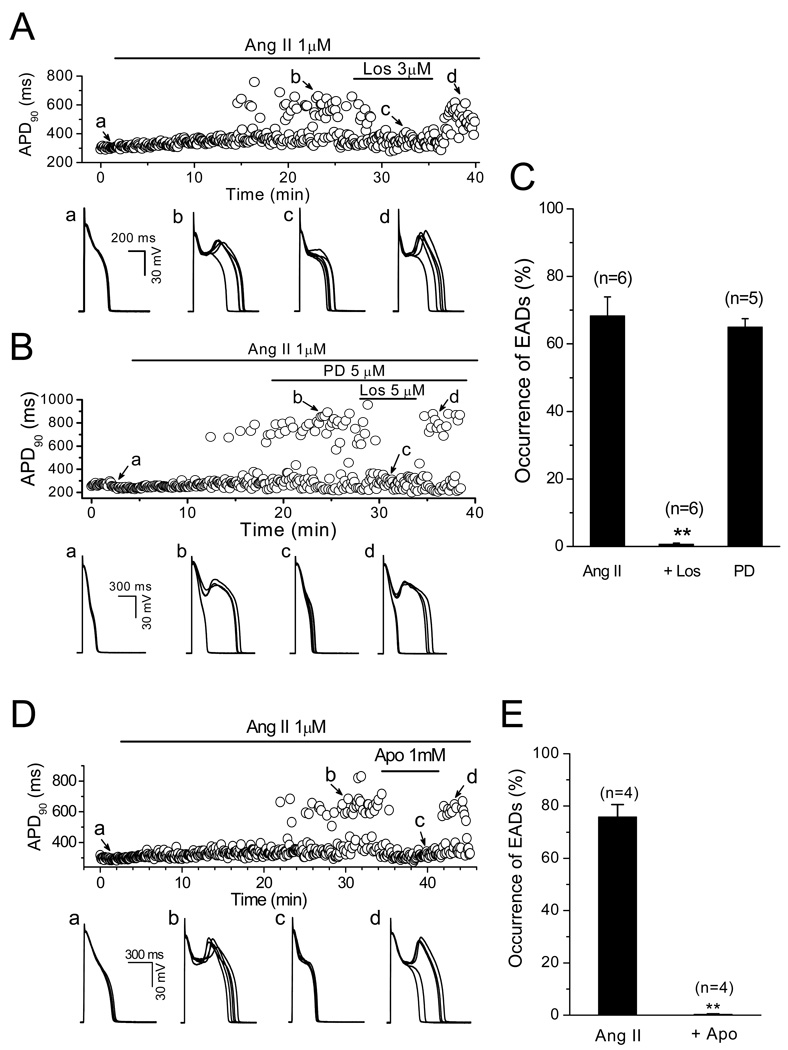
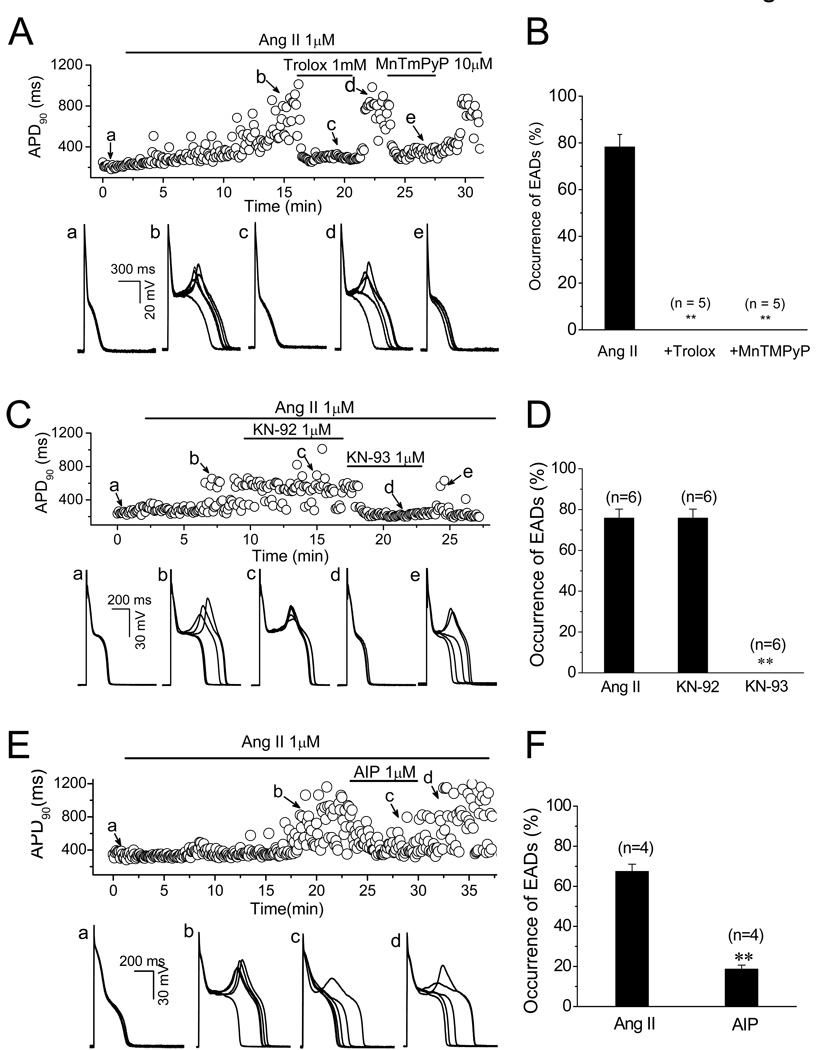
Ang II interacts with at least two receptors: type 1 Ang II receptor (AT1R) and type 2 Ang II receptor (AT2R) [24]. In order to identify the Ang II receptor subtype which mediated the induction of EADs, either AT1R antagonist losartan (Los, 3–5 µM) or AT2 receptor antagonist PD-123319 (PD, 2–5 µM) was administrated after EADs were constantly induced by 1 µM Ang II. As shown in Fig. 3A–C, Los suppressed Ang II-induced EADs in a reversible manner. The probability of EAD occurrence was reduced from 68.3 ± 5.6% (Ang II) to 0.7±0.3% (Ang II + Los) (n=6, P<0.01), while PD had no effect, suggesting the activation of AT1R was responsible for the induction of EADs by Ang II. Consistent with its effects on Ang II-elicited ROS generation, apocynin (0.5 or 1 mM), an NADPH oxidase inhibitor, also suppressed the rate of EADs induced by Ang II (from 75.8±4.7% to 0.3 ± 0.2 %, n=4, P<0.01) (Fig. 3D & E).
Fig. 3.
Ang II-induced EADs are mediated by Ang II type 1 receptor (AT1R) and NADPH oxidase. (A). Time course of APD90 in a myocyte treated with Ang II and AT1R blocker Losartan (Los). Five consecutive APs recorded at points a–d are displayed underneath. (B). Time course of APD90 in a myocyte treated with Ang II and AT2R blocker PD-123319 (PD) and AT1R blocker Losartan (Los). Five consecutive APs recorded at points a–d are displayed underneath. (C). Summarized histogram shows probabilities of EAD occurrence in the presence of 1 µM Ang II, 1 µM Ang II + 3 µM Los, and 1 µM Ang II + 5 µM PD. (D). Time course of APD90 in a myocyte treated with Ang II and NADPH oxidase inhibitor apocynin (Apo). Five consecutive APs recorded at points a–d each are shown beneath. (E). Summarized histogram shows probabilities of EAD occurrence in the presence of 1 µM Ang II and Ang II+ 1 µM Apo, respectively. .
In addition, Ang II-induced EADs were eliminated by antioxidants trolox (0.5 or 1 mM) or MnTMPyP (10 µM). EAD occurrence probability was reduced from 78.3 ± 5.3% to 0% by both regents (n=5, P<0.01) (Fig. 4A & B). These results support the hypothesis that the activation of AT1R, and in turn the generation of NADPH oxidase-derived ROS are involved in the EAD formation by Ang II.
Fig. 4.
Suppression of Ang II-induced EADs by antioxidant and CaMKII inhibitor. (A). Time course of APD90 in a myocyte treated with Ang II and antioxidants trolox (1 mM) and MnTMPyP (10 µM). Five consecutive APs recorded at points a–e are shown underneath. (B). Summarized histogram shows probabilities of EAD occurrence in the presence of 1 µM Ang II, 1 µM Ang II+ 1mM trolox, and 1 µM Ang II +10 µM MnTMPyP, respectively. (C). Time course of APD90 in a myocyte treated with Ang II, KN-93 (CaMK II inhibitor), and KN-92 (an inactive analog of KN-93). Five consecutive APs recorded at points a–e each are shown underneath. (D). Summarized histogram shows probabilities of EAD occurrence in the presence of 1 µM Ang II, 1 µM Ang II+1 µM KN-92, and 1 µM Ang II+1 µM KN-93, respectively. (E). Time course of APD90 in a myocyte treated with Ang II, and AIP. Five consecutive APs recorded at points a–d each are shown underneath. (F). Summarized histogram shows probabilities of EAD occurrence in the presence of 1 µM Ang II, and 1 µM Ang II+1 µM AIP, respectively.
It has been shown that binding of Ca2+/CaM is required to expose the redox sites in the regulatory CaMKII domain in order for oxidation to persistently activate CaMKII [8, 25]. We therefore investigated the Ca2+ dependence of Ang II-induced EADs. In myocytes preloaded with BAPTA-AM (4 µM) for 30 min, Ang II did not induce EADs even at a higher concentration (4 µM) in 5 cells, although APDs were markedly prolonged (e.g. the average APD was prolonged from 345.8±38.4 to 673.7±55 ms (n=6) at 10 min of exposure to Ang II in the presence of BAPTA), indicating Ang II-induced EADs were Cai dependent, presumably through Ca2+/CaM. Chelating of Ca2+ by BAPTA may reduce Ca-sensitive inactivation of ICa, L, which may contribute to the prolongation of APD in the presence of BAPTA. Next, we examined the effect of direct CaMKII inhibition on EADs generation by Ang II. As shown in Fig. 4C & D, CaMKII inhibitor KN-93 (1 µM) completely suppressed EADs induced by Ang II (n=4, EAD occurrence probability from 75.8 ± 4.4% to 0%), while KN-92, an inactive analogue of KN-93, had neither preventive nor inhibitory effect on Ang II-induced EADs. To further exclude nonspecific effects of KN-93 [26], we also examined the effect of a membrane permeable CaMKII selective inhibitory peptide, autocamtide-2 related inhibitory peptide (AIP). AIP (1 µM) was perfused after EADs were induced by Ang II (1 µM). As shown in Fig 4-E &F, Ang II-induced EADs were significantly suppressed by AIP; the EAD occurrence probability was suppressed from 68.3 ± 5.6% to 18.8 ± 2.0 % (n=4). These results suggest that Ca-dependent CaMKII activation is responsible for Ang II-induced EADs.
3.5. Contribution of ICa,L in Ang II-induced EADs
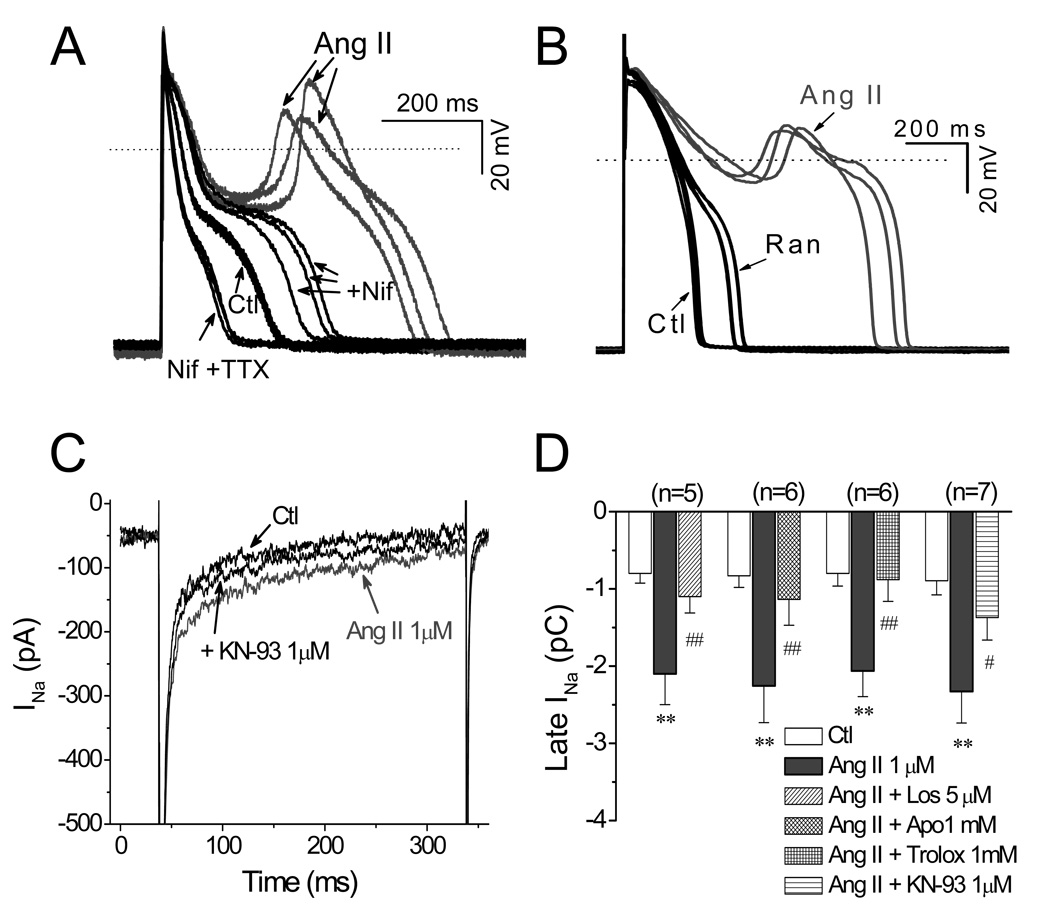
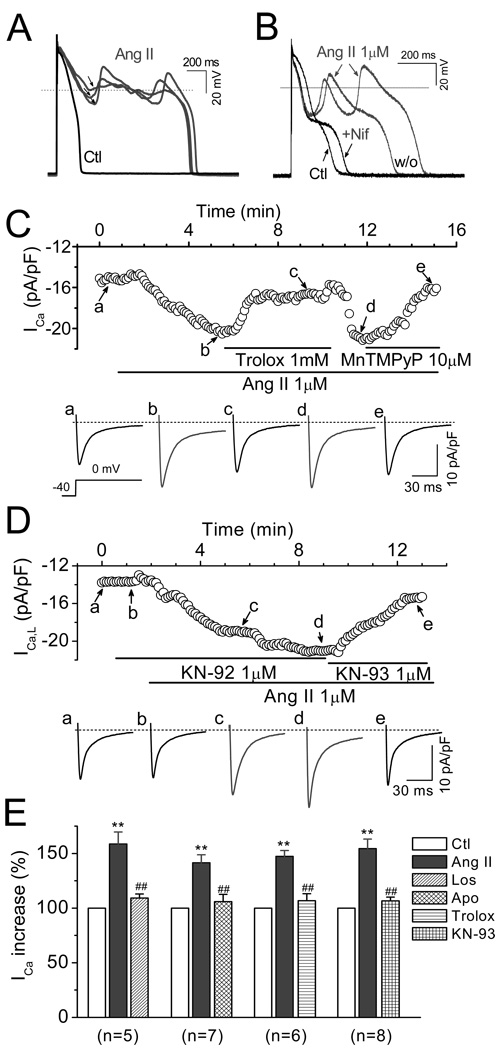
CaMKII modulates multiple sarcolemmal channels [27], among which ICa.L and late INa are known to be key factors in ROS-induced EAD formation [1, 28–29]. We next assessed the involvement of ICa.L and late INa in Ang II-induced EADs. We observed that the amplitude of EADs induced by Ang II depended on their takeoff potentials, i.e. the more negative the takeoff potential, the larger the EAD amplitude (Fig. 5A). This relationship is reminiscent of the voltage dependence of ICa.L reactivation and the ICa,L window currents [30], which resembles the effects of H2O2 in our previous work. Supporting this notion, Nifedipine (10 µM), a selective ICa.L blocker abolished EADs (Fig. 5B). To confirm that the activation of ICa,L plays a key role in Ang II-induced EADs, we directly examined the effects of Ang II on ICa,L by using voltage clamp recording. As shown in Fig. 5 C–D, Ang II (1 µM) increased the amplitude of ICa.L at 2–3 min after exposure and reached the steady state at 6–8 min. The peak amplitude of ICa.L (at 0 mV) was pronouncedly increased by 48.9 ± 5.5 % from 8.3 ±1.4 to 12.2 ± 2.3 pA/pF (n=26, P < 0.01). The Ang II-induced increases of ICa.L were significantly attenuated by 3 µM Los, 1 mM apocynin, 1 mM trolox, or 1 µM KN-93, respectively (as summarized in Fig. 5E). Consistent with previous reports [31–32], we did not observe significant effects of losartan, apocynin, or trolox (at the same concentrations) on basal ICa,L. Although both KN-93 and KN-92 have been reported to suppress basal ICa,L in a CaMKII-independent manner [26], our results showed only KN-93 eliminated EADs, presumably due to its additional CaMKII inhibitory effect. This conclusion was further supported by the inhibitory effect of the selective CaMKII inhibitory peptide, AIP.
Fig. 5.
Involvement of ICa.L in Ang II-induced EADs. (A). APs recorded under control condition (Ctl) and after EADs were induced by Ang II perfusion. The amplitude of Ang II-induced EADs depended on their take-off potentials (arrows). (B). The ICa,L blocker nifedipine (Nif, 10 µM) reversibly suppressed the EADs upstroke induced by Ang II. (C). Time course of peak of ICa,L in a myocyte treated with Ang II, and antioxidants trolox or MnTMPyP (MnT). Representative traces of ICa,L corresponding to points a–e are shown. (D). Time course of peak ICa,L in a myocyte treated with Ang II, and KN-92 or KN-93. (E). Summary of the activation of peak ICa,L by Ang II (1 µM), and antagonistic effect of losartan (5 µM), apocynin (1 mM), trolox (1mM), and KN-93 (1 µM). **P<0.01 vs. control; ##P< 0.05 vs. Ang II. .
3.6. Contribution of late INa in Ang II-induced EADs
Although Ang II-induced EAD upstrokes were eliminated by nifedipine, the APD remained prolonged, presumably due to the activation of late INa, since the selective Na+ channels blocker tetrodotoxin (TTX, 10 µM) shortened the APD ( Fig. 6A). In addition, ranolazine (2 µM), a more selective blocker of late INa, also suppressed Ang II-induced EADs, suggesting that late INa also plays a key role in EADs generation by Ang II. Supporting this idea, Fig. 6C and 6D show that late INa was significantly enhanced from −0.84 ± 0.16 pC to −2.22 ± 0.40 pC (n=20, P<0.01) when the increase of late INa reached the steady state at 10–15 min after application of Ang II (1 µM). Ang II-induced enhancement of late INa was significantly attenuated by 1 mM apocynin, (to −1.14±0.33 pC, n=6, P<0.01), 1 mM trolox (to −0.88±0.3 pC, n =6, P<0.01) or 1 µM KN-93 (to −1.37±0.29 pC, n=7, P<0.05), respectively (Fig. 6D).
3.7. Less involvement of outward K+ currents in Ang II-induced EADs
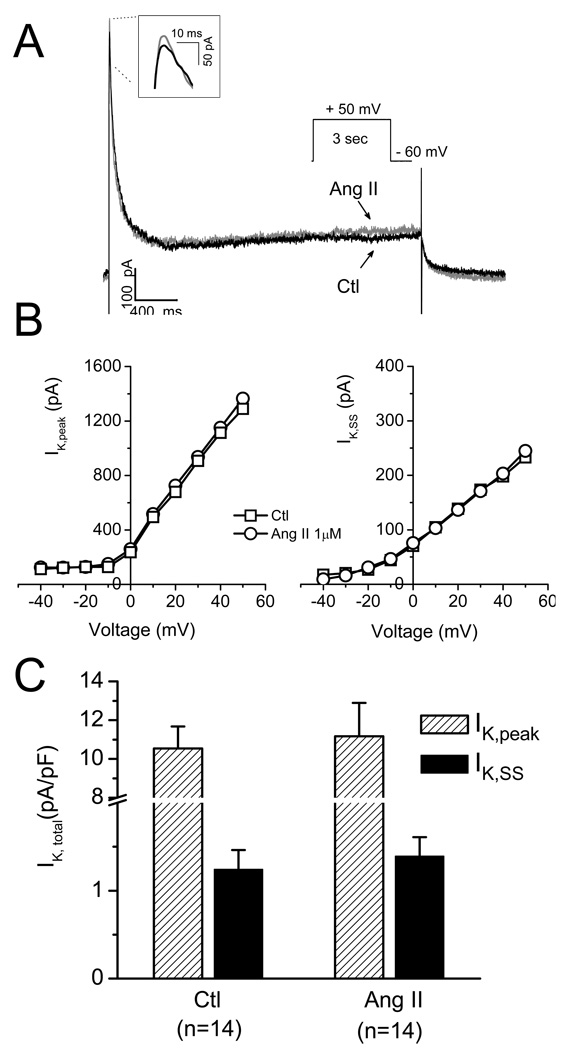
Since outward K+ currents may also play roles in APD alternation and EAD genesis, we next evaluated the effects of Ang II on total outward K+ currents. As shown in Fig. 7, neither peak (IK, peak) (ctl: 10.5 ± 1.1 vs. Ang II: 11.2 ± 1.7 pA/pF, n = 14; P > 0.05) nor steady state (IK,ss) (ctl: 1.2 ± 0.2 vs. Ang II: 1.4 ± 0.3 pA/pF, n = 14; P > 0.05) outward potassium currents were significantly altered by Ang II at the same concentration that induced EADs under current clamp condition. We also tested the effects of aforementioned regulatory agents and found that none of losartan, Apocinin, trolox, and KN-93 affected total IK under our experimental condition, excluding the possibility that these agents may suppress Ang II-induced EADs via alternation in IK.
Fig. 7.
Less effect of Ang II on total outward K+ current. (A). Representative traces of the total K+ current in the absence (Ctl) and presence of Ang II (1µM). (B). Current-voltage curves of the total peak currents (IK,peak, left) and stead-state currents (IK, SS, right). (C). Summarized data showing no significant effects of 1 µM Ang II on the total outward IK,peak and IK, SS.
4. Discussion
We have recently shown that exogenously applied ROS (H2O2) induced EADs, DADs and TAs via CaMKII signaling pathway, which may cause arrhythmias under pathological conditions such as heart failure and ischemia-reperfusion. The activation of the rennin-angiotensin system (RAS) has been implicated in arrhythmias associated with heart failure and ischemia-reperfusion since inhibitors of this system reduce the incidence of sudden death. However, direct evidence linking AT1R to NADPH oxidase-dependent ROS production and induction of EADs is missing. In the present study, we found that: 1) Ang II induces EADs in isolated ventricular myocytes; 2) the activation of AT1R, and NADPH oxidase causes ROS production which is responsible for the generation of EADs; 3) CaMKII activation via ROS plays a key role in Ang II-induced EADs; 4) the activation of ICa.L and late INa by Ang II contributes to the inward currents accounting for the EAD generation. In summary, the present study provides direct evidence that endogenous ROS, derived via the NADPH oxidase, mediate generation of arrhythmogenic EADs in response to angiotensin II. These results also provide mechanistic basis for the clinical approaches for treatment of arrhythmias by angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, or NADPH oxidase inhibitors [13, 33].
Activation of various membrane receptors (such as AT1R, ETAR, and TGFβR) have been shown to trigger a common signaling cascade [3]. Thus, it is very likely that NADPH oxidase-ROS-CaMKII pathway could represent a common mechanism accounting for arrhythmias induced by the agonists of above receptors. Supporting this assumption, endothelin-1 has been reported to cause ventricular tachyarrhythmias via prolongation of APD and formation of EADs [34–36], promote mitochondrial ROS production triggered by NADPH oxidase [37], and activates ICa,L by CaMKII [38]. Further studies are needed to provide direct link between these individual steps.
4.1. Ang II-induced endogenous generation of ROS in myocytes via AT1R and NADPH oxidase
The NADPH oxidase family plays a central role in generation of ROS in cardiovascular disorders [39–40]. It catalyzes the formation of superoxide oxygen, which in turn is reduced to H2O2 by superoxide dismutases. In the present study, we have clearly demonstrated the correlation between ROS production by NADPH oxidase and EAD induction by Ang II via AT1R. It is obvious that Ang II elevated the intracellular ROS level in a slower time course and to a lesser extent compared to exogenous application of H2O2 (50 or 200 µM) (Fig. 1), suggesting Ang II-induced endogenous ROS production involves intracellular signal pathways and enzyme reactions so that the ROS increase in a mild and slow manner. In addition to the ROS generation directly via NADPH oxidase, the ROS-induced ROS release from mitochondria may be involved in Ang II-induced ROS formation in cardiac myocytes [41–42].
4.2. Afterdepolarizations induced by endogenously generated ROS via Ang II: comparison with exogenously administrated H2O2
Although the effects of Ang II on membrane ion currents and AP have been well investigated, it is not known if Ang II can induce EADs via NADPH oxidase-ROS pathway in the cardiac myocytes. In our current study, we observed EADs occurred with a slow time course (at 15.8 ± 1.6 min) after Ang II perfusion, which is consistent with that for ROS production by Ang II. It should be noticed that the incidence rate of EAD or DAD was lower and the perfusion time to induce EADs was longer by Ang II than by direct H2O2 treatment. These results suggest that endogenously generated ROS by receptor stimulation (AT1R in our case) showed lower inducibility of EADs, consistent with the relatively mild increase of intracellular ROS level by Ang II compared to H2O2 as measured with ROS fluorescence dye (Fig. 1C).
4.3. Downstream of ROS — Similar cellular and ionic mechanisms underlying Ang II- and exogenous H2O2-induced EADs
Our results demonstrate that Ang II and exogenous H2O2 share the same downstream targets including CaMKII, ICa,L and INa. CaMKII is a multifunctional protein kinase expressed abundantly in the heart. Previous studies have implicated that ROS-activated CaMKII may be arrhythmogenic due to alternation of cardiac repolarization and Ca2+ handling and induction of EADs and DADs [1, 8, 43]. Our present finding that the CaMKII inhibitors AIP and KN-93 (but not its inactive analogue KN-92) suppressed Ang II-induced EADs suggests that CaMKII activation is the common mechanism for both Ang II- and H2O2- induced EADs. CaMKII further activates ICa,L and late INa, which account for the inward membrane current causing EADs. However, the overall contribution of K currents to Ang II-induced EADs is neglectable under our experimental conditions. It has been reported that Ang II promotes Ca influx by activating the reverse mode of Na-Ca exchange current (NCX) [44–45]. However, this effect seemed unlikely to contribute to the EAD induction, since the reverse mode of NCX would generate an outward current.
We have shown in our previous study that direct application of exogenous H2O2 causes Ca2+ overload and generates spontaneous Ca2+ release and Ca2+ waves, which in turn induces DADs via Na+-Ca2+ exchange current [1]. These effects may result either from direct modification of Cai-cycling proteins, including ryanodine receptors [46] and SERCA2a [47] by ROS, or secondarily from APD prolongation [1]. It should be noticed that Ang II treatment seldom induced DADs (only in 3/41 cells), suggesting that endogenously generated ROS cause less intracellular Ca2+ overload compared to exogenous ROS (H2O2) application.
4.4. Possible involvement of other signal transduction pathways
Ang II receptors couple to various signal transduction pathways that involve G-proteins, intracellular second messengers, and protein kinases. [24, 48], [49]. The detailed mechanism(s) of NADPH activation has not been completely understood yet. Some studies have shown that NADPH oxidase activation requires upstream PKC in various tissues, such as brain, vascular smooth muscle and mesangial cells [4, 50]. PKC may also activate membrane ion channels, including ICa,L, via direct phosphorylation [51]. However, we did not observe an inhibitory effect of selective PKC blockers (chelerythrine or GF 109203X) on Ang II-induced EADs, suggesting PKC may not be involved in current signaling pathway. In fact, a novel signal transduction pathway of Ang II-induced ROS production in cardiomyocytes was reported by Nishida et al, who demonstrated that AT1R stimulation by Ang II activates Gα12/13 proteins, which in turn cause Rho/ROCK-mediated Rac1 activation. Rac, one of the small GTP-binding proteins, promotes the production of ROS by activating NADPH oxidase [52].
4.5. Arrhythmogenesis by Ang II and clinical relevance
Our results underscore the propensity for Ang II to induce EADs and cause cardiac arrhythmias via triggered activity mechanism. The possible role of renin-angiotensin system activation in the genesis of atrial and ventricular arrhythmias suggests that ACE inhibitors and Ang II receptor blockers may serve as effective therapeutic agents in the prevention and treatment of arrhythmias [13]. This postulation is supported by both experimental and clinical studies [12, 16]. The anti-arrhythmic effects of ACE inhibitors and AT1R blockers may be attributed to the suppression of both long-term structural remodeling (e.g prevention of fibrosis) and short-term electrical remodeling (direct modulation of ion-channel function) [16].
Similar to H2O2 [1, 22], Ang II-induced EADs were slow-rate-dependent and were observed at PCL > 2 sec. Accordingly, Ang II-induced ventricular arrhythmias may more readily manifest in the clinical setting of bradycardia [53], such as sinus pauses (sinus-node dysfunction), atrialventricular conduction disturbances, atrial fibrillation or long QT syndromes.
Ang II can be produced locally in the myocardium as an autocrine regulator. Ang II levels in cardiac tissue are much higher than those in plasma and can reach up to ~20 nM [54]. It is also well known that Ang II levels can increase under pathological condition such as hypertrophy, heart failure and ischemic heart disease [10]. For example, the Ang II concentration in the medium of serum-deprived cardiomyocytes increases near 100-fold upon stretch [55]. In addition, the density of Ang II receptors is also upregulated during pathological conditions such as ischemia-reperfusion [56–57]. Therefore the cardiomyocytes in vivo may be challenged by a wide range of Ang II level, and the Ang II concentrations (0.1–2 µM) used in the present study are likely within pathophysiologically relevant range.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009 Jan 2;104(1):79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babior BM. NADPH oxidase: an update. Blood. 1999 Mar 1;93(5):1464–1476. [PubMed] [Google Scholar]
- 3.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003 Aug;14(8) Suppl 3:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 4.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009 Apr 29;302(2):148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000 Mar 17;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 6.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004 May;90(5):491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009 Jul;47(1):15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008 May 2;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, et al. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009 Dec 4;105(12):1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 10.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999 Oct 1;85(7):643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- 11.Gavras I, Gavras H. Angiotensin II as a cardiovascular risk factor. J Hum Hypertens. 2002 May;16 Suppl 2:S2–S6. doi: 10.1038/sj.jhh.1001392. [DOI] [PubMed] [Google Scholar]
- 12.Garg S, Narula J, Marelli C, Cesario D. Role of angiotensin receptor blockers in the prevention and treatment of arrhythmias. Am J Cardiol. 2006 Mar 15;97(6):921–925. doi: 10.1016/j.amjcard.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Makkar KM, Sanoski CA, Spinler SA. Role of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and aldosterone antagonists in the prevention of atrial and ventricular arrhythmias. Pharmacotherapy. 2009 Jan;29(1):31–48. doi: 10.1592/phco.29.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, et al. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1242–H1253. doi: 10.1152/ajpheart.01400.2006. [DOI] [PubMed] [Google Scholar]
- 15.Delpon E, Caballero R, Gomez R, Nunez L, Tamargo J. Angiotensin II, angiotensin II antagonists and spironolactone and their modulation of cardiac repolarization. Trends Pharmacol Sci. 2005 Mar;26(3):155–161. doi: 10.1016/j.tips.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006 Mar;27(5):512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 17.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 18.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2',7'-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999 Jul;27(1–2):146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 19.Aon MA, Cortassa S, Akar FG, Brown DA, Zhou L, O'Rourke B. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol. 2009 Oct;41(10):1940–1948. doi: 10.1016/j.biocel.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997 Nov 15;347(2):256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001 Aug 31;89(5):453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 22.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, et al. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):2983–2988. doi: 10.1073/pnas.0809148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vila Petroff MG, Mattiazzi AR. Angiotensin II and cardiac excitation-contraction coupling: questions and controversies. Heart Lung Circ. 2001;10(2):90–98. doi: 10.1046/j.1444-2892.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- 24.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- 25.Anderson ME. CaMKII and a failing strategy for growth in heart. J Clin Invest. 2009 May;119(5):1082–1085. doi: 10.1172/JCI39262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006 Jul 14;345(4):1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 27.Bers DM, Grandi E. Calcium/Calmodulin-dependent Kinase II Regulation of Cardiac Ion Channels. J Cardiovasc Pharmacol. 2009 May 16; doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006 Jul;318(1):214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 29.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997 May 1;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989 May;64(5):977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 31.Brown ST, Scragg JL, Boyle JP, Hudasek K, Peers C, Fearon IM. Hypoxic augmentation of Ca2+ channel currents requires a functional electron transport chain. J Biol Chem. 2005 Jun 10;280(23):21706–21712. doi: 10.1074/jbc.M503144200. [DOI] [PubMed] [Google Scholar]
- 32.Tsai CT, Wang DL, Chen WP, Hwang JJ, Hsieh CS, Hsu KL, et al. Angiotensin II increases expression of α1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007 May 25;100(10):1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 33.Sovari AA, Morita N, Karagueuzian HS. Apocynin: a potent NADPH oxidase inhibitor for the management of atrial fibrillation. Redox Rep. 2008;13(6):242–245. doi: 10.1179/135100008X309000. [DOI] [PubMed] [Google Scholar]
- 34.Merkely B, Geller L, Toth M, Kiss O, Kekesi V, Solti F, et al. Mechanism of endothelin-induced malignant ventricular arrhythmias in dogs. J Cardiovasc Pharmacol. 1998;31 Suppl 1:S437–S439. doi: 10.1097/00005344-199800001-00125. [DOI] [PubMed] [Google Scholar]
- 35.Merkely B, Kiss O, Vago H, Zima E, Szabo T, Geller L. Arrhythmogenic action of endothelin-1. Cardiovasc Res. 2000 Nov;48(2):357–358. doi: 10.1016/s0008-6363(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 36.Yorikane R, Shiga H, Miyake S, Koike H. Evidence for direct arrhythmogenic action of endothelin. Biochem Biophys Res Commun. 1990 Nov 30;173(1):457–462. doi: 10.1016/s0006-291x(05)81080-0. [DOI] [PubMed] [Google Scholar]
- 37.Deng W, Baki L, Baumgarten CM. Endothelin signalling regulates volume-sensitive Cl− current via NADPH oxidase and mitochondrial reactive oxygen species. Cardiovasc Res. 2010 Jun 5; doi: 10.1093/cvr/cvq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komukai K, J OU, Morimoto S, Kawai M, Hongo K, Yoshimura M, et al. Role of Ca2+/calmodulin-dependent protein kinase II in the regulation of the cardiac L-type Ca2+ current during endothelin-1 stimulation. Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H1902–H1907. doi: 10.1152/ajpheart.01141.2009. [DOI] [PubMed] [Google Scholar]
- 39.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003 Jun;4(2):51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 40.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in α1-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002 Apr;282(4):C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 41.Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension. 2005 May;45(5):847–848. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, et al. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008 Aug;10(8):1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 43.Song YH, Cho H, Ryu SY, Yoon JY, Park SH, Noh CI, et al. L-type Ca2+ channel facilitation mediated by H2O2-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol. 2010 Apr;48(4):773–780. doi: 10.1016/j.yjmcc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Fujita S, Endoh M. Influence of a Na+-H+ exchange inhibitor ethylisopropylamiloride, a Na+-Ca2+ exchange inhibitor KB-R7943 and their combination on the increases in contractility and Ca2+ transient induced by angiotensin II in isolated adult rabbit ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1999 Nov;360(5):575–584. doi: 10.1007/s002109900123. [DOI] [PubMed] [Google Scholar]
- 45.Petroff MG, Aiello EA, Palomeque J, Salas MA, Mattiazzi A. Subcellular mechanisms of the positive inotropic effect of angiotensin II in cat myocardium. J Physiol. 2000 Nov 15;529(Pt 1):189–203. doi: 10.1111/j.1469-7793.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zissimopoulos S, Lai FA. Redox regulation of the ryanodine receptor/calcium release channel. Biochem Soc Trans. 2006 Nov;34(Pt 5):919–921. doi: 10.1042/BST0340919. [DOI] [PubMed] [Google Scholar]
- 47.Morris TE, Sulakhe PV. Sarcoplasmic reticulum Ca2+-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med. 1997;22(1–2):37–47. doi: 10.1016/s0891-5849(96)00238-9. [DOI] [PubMed] [Google Scholar]
- 48.Goette A, Lendeckel U. Electrophysiological effects of angiotensin II. Part I: signal transduction and basic electrophysiological mechanisms. Europace. 2008 Feb;10(2):238–241. doi: 10.1093/europace/eum283. [DOI] [PubMed] [Google Scholar]
- 49.Saito Y, Berk BC. Angiotensin II-mediated signal transduction pathways. Curr Hypertens Rep. 2002 Apr;4(2):167–171. doi: 10.1007/s11906-002-0042-1. [DOI] [PubMed] [Google Scholar]
- 50.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002 Sep 6;91(5):406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 51.Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca2+ current by a Ca2+- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol. 2001 Apr;280(4):H1528–H1536. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- 52.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, et al. Gα 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem. 2005 May 6;280(18):18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 53.Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med. 2000 Mar 9;342(10):703–709. doi: 10.1056/NEJM200003093421006. [DOI] [PubMed] [Google Scholar]
- 54.Danser AH, Saris JJ, Schuijt MP, van Kats JP. Is there a local renin-angiotensin system in the heart? Cardiovasc Res. 1999 Nov;44(2):252–265. doi: 10.1016/s0008-6363(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 55.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993 Dec 3;75(5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 56.Yang BC, Phillips MI, Ambuehl PE, Shen LP, Mehta P, Mehta JL. Increase in angiotensin II type 1 receptor expression immediately after ischemia-reperfusion in isolated rat hearts. Circulation. 1997 Aug 5;96(3):922–926. doi: 10.1161/01.cir.96.3.922. [DOI] [PubMed] [Google Scholar]
- 57.Yang BC, Phillips MI, Mohuczy D, Meng H, Shen L, Mehta P, et al. Increased angiotensin II type 1 receptor expression in hypercholesterolemic atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998 Sep;18(9):1433–1439. doi: 10.1161/01.atv.18.9.1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.