Abstract
To demonstrate the ability to assess long-term hypothalamic-pituitary-adrenocortical (HPA) axis activity in polar bears (Ursus maritimus), a pilot study was conducted in which cortisol concentrations was analyzed in hair from 7 female (3–19 years) and 10 male (6-19 years) East Greenland polar bears sampled 1994–2006. Hair was chosen as matrix as it is non-invasive, seasonally harmonized, and has been validated as an index of long-term changes in cortisol levels. Samples were categorized according to contamination: Eight were clean (2 females, 6 males), 5 had been contaminated with bear blood (2 F, 3 M), and 4 with bear fat (3 F, 1 M). There was no significant difference in cortisol concentration between the three categories after external contamination was removed. However, contaminated hair samples should be cleaned before cortisol determination. Average hair cortisol concentration was 8.90 pg/mg (range: 5.5 to 16.4 pg/mg). There was no significant correlation between cortisol concentration and age (p = 0.81) or sampling year (p = 0.11). However, females had higher mean cortisol concentration than males (females mean: 11.0 pg/mg, males: 7.3 pg/mg; p = 0.01). The study showed that polar bear hair contains measurable amounts of cortisol and that cortisol in hair may be used in studies of long-term stress in polar bears.
Keywords: Contaminants, cortisol, hair, hormone, polar bear, stress, Ursus maritimus
Introduction
Stress in animals has been measured as catecholamine or corticosteroid hormone concentration in matrices such a blood, feces, urine, feathers, eggs and saliva (Larter & Nagy 2001; Van der Staay et al. 2007; Bortolotti et al. 2008; Downing & Bryden 2008; Saco et al. 2008; Lupica & Turner 2009; Okuliarová et al. 2010; Saeb et al. 2010). More recently, analyses of hair (Koren et al. 2002; Davenport et al. 2006; Dettmer et al. 2009; Gow et al. 2010), and liver and gonad tissue (Flores-Valverde & Hill 2008) have also been included. Faecal, egg, and especially feather and hair samples have the advantage that they do not show short-term hormonal fluctuations, but rather express chronic stress (Koren et al. 2002; Davenport et al, 2006; Bortolotti et al. 2008; Saco et al. 2008; Okuliarová et al. 2010).
Stress studies involving measurement of hair cortisol have usually been conducted under controlled laboratory conditions (Davenport et al. 2006; Dettmer et al. 2009), meaning easy access to clean and standardized samples. This, however, is not always the case when working with samples obtained from wildlife such as polar bears. For example, East Greenland polar bear hair samples that are collected by the indigenous people during their hunt are often contaminated by polar bear blood and fat (subcutaneous adipose tissue). Recent studies examined cortisol in blood plasma from polar bears that were live captured at Svalbard during 1995–1998 (Tryland et al. 2002; Haave et al. 2003; Oskam et al. 2004). In these studies relationships between cortisol concentrations and a number of organic pollutants in blood plasma were found, although the authors also pointed out that stress induced by the chase, darting, and anesthetization of the polar bears may have influenced cortisol levels.
Acute stress can obviously lead to artificially high baseline cortisol levels in blood plasma samples. Also, blood cortisol samples obtained from sedated animals may vary due to the difficulty in maintaining consistent levels of sedation over time and between individuals (Montfort et al.1993). As hair samples are not affected by acute stress, but rather reflect long-term hypothalamic-pituitary-adrenocortical (HPA) system activity, measuring hair cortisol in polar bears would provide an important non-invasive and non-biased matrix for studies of long-term stress. Hair samples would also be much easier to work with logistically than blood samples, as they require nothing more than a sharp tool and a plastic bag, with no particular handling or storage requirements, on the way from field to laboratory.
The aim of the present pilot study was to investigate whether it was possible to remove the external source of cortisol (here: bear blood or fat) while retaining hormone tightly bound to the matrix of the hair shaft, and to assess the feasibility of extracting measurable cortisol concentrations from polar bear hair.
Materials and methods
Samples
Hair from 17 (7 female and 10 male) East Greenland polar bears was included in the present study. Age determination had been done by counting the cementum growth layer groups (GLG) of the lower right incisor (I3) (Hensel and Sorensen 1980). Samples were collected in East Greenland (app. 61°–82°N, 10°–42°W) during the period 1994–2006. Hair samples are collected routinely for NERI (National Environmental Research Institute, Aarhus University, Denmark) by the subsistence hunters living in Scoresby Sound during their annual catch of polar bears. As the bears are usually flayed in the field, hair samples were often partially cross-contaminated with blood and subcutaneous adipose tissue (fat). Therefore hair samples fell into three categories: clean, blood contaminated, and fat contaminated, depending on their condition. In total there were 8 clean (2 female, 6 male), 5 blood-contaminated (2 female, 3 male), and 4 fat-contaminated (3 female, 1 male) samples. According to our contact person in Scoresby Sound, all hair samples were taken from the chest area of the polar bears. No examination of cortisol content in proximal vs. distal hair was done due to insufficiency of samples and the low levels of cortisol present. However, no differences were found in cortisol concentration of proximal and distal hair segments in rhesus monkeys (Davenport et al. 2006) or across the dogs examined by Bennett & Hayssen (2010). At NERI, the samples were kept in clear plastic bags in the freezer at −20°C. However, the hair samples have all been subjected to varying temperatures, also in the plus range, during transportation.
Cortisol analysis
Hair samples weighing app. 150 to 250 mg were processed and analyzed according to the methods described in Davenport et al. (2006) with modification to assess and remove external sources of cortisol, particularly in the blood- and fat-contaminated samples. Clean samples were subjected to our standard procedure of two 3-min washes, each with 5.0 ml of HPLC-grade isopropanol (Fisher Scientific, Pittsburgh, USA). Contaminated samples were subjected to either two or three additional washes (see Table 1). In all cases, an aliquot of each wash was dried down under a stream of nitrogen gas and reconstituted in assay buffer for subsequent determination of cortisol content of the wash. Washed hair samples were air dried and then ground to a fine powder in an MM 200 ball mill (Retsch, Newtown, USA). Approximately 50 mg of powdered hair was extracted for 24 h with HPLC-grade methanol (Fisher Scientific), dried down, reconstituted in assay buffer, and then analyzed for cortisol using a sensitive and specific enzyme immunoassay (Salimetrics, State College, PA USA). All samples were analyzed in a single assay with an intra-assay coefficient of variation of 3.0%. Inter-assay coefficients of variation averaged less than 7%.
Table 1.
Results from the analyses of hair cortisol concentration in 17 East Greenland polar bears (Ursus maritimus).
| A | B | C | D | E1 | E2 | E3 | E4 | E5 | F |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Year of kill | Hair status | pg cort in Wash 1 | pg cort in Wash 2 | pg cort in Wash 3 | pg cort in Wash 4 | pg cort in Wash 5 | pg cort/mg hair |
| F | 16 | 2000 | Clean | 108 | 40 | n/a | n/a | n/a | 12 |
| F | 3 | 1999 | Clean | ND | 24 | n/a | n/a | n/a | 9.7 |
| M | 8 | 2001 | Clean | ND | ND | n/a | n/a | n/a | 6.5 |
| M | 8 | 2006 | Clean | ND | ND | n/a | n/a | n/a | 7 |
| M | 17 | 2006 | Clean | 132 | ND | n/a | n/a | n/a | 8.6 |
| M | n/a | 2006 | Clean | 128 | ND | n/a | n/a | n/a | 6.3 |
| M | 19 | 2006 | Clean | 112 | 4 | n/a | n/a | n/a | 5.8 |
| M | n/a | 1995 | Clean | 180 | 16 | n/a | n/a | n/a | 19.9 |
| M | 8 | 2000 | Fat | 180 | 272 | 28 | ND | n/a | 9.8 |
| F | 19 | 2000 | Fat | 336 | 380 | 432 | ND | n/a | 10.8 |
| F | 5 | 1999 | Fat | 640 | 528 | 148 | ND | n/a | 8 |
| F | < 5 | 1994 | Fat | 460 | 396 | 220 | ND | n/a | 8.2 |
| M | 6 | 2001 | Blood | 232 | 20 | ND | ND | ND | 9 |
| F | 6 | 1999 | Blood | 1240 | 296 | 92 | ND | ND | 16.4 |
| M | 6 | 2006 | Blood | 936 | 612 | 416 | ND | ND | 5.5 |
| M | 12 | 2006 | Blood | 208 | 72 | ND | ND | ND | 6.9 |
| F | 8 | 2006 | Blood | 420 | 128 | 28 | ND | ND | 11.9 |
Column A-C give the individual sex, age, and year of kill, respectively; column D denotes the physical state of the hair (please see text for further explanation); column E gives pg/cortisol pr. mg. hair; column E1–5 refers to the content of cortisol in the washing solution after the entire hair sample was washed in it; column F gives the total cortisol concentration measured in the washed and powdered sample, standardized to be app. 50 mg regardless of the original weight of the hair sample referred to in column E. ND: not detectable; n/a: not available (wash not performed).
Statistical analyses
Pearson’s correlation tests were applied to test the relationship between cortisol and age or year, respectively. Student’s t-test was applied to test the difference in hair cortisol concentrations between males and females, and the differences between the three groups (clean, fat-contaminated, and blood-contaminated). Confidence levels were set to α= 0.05 and all statistical analyses were conducted using R (version 2.10.0).
Results
A single outlier (Table 1: 19.9 pg cortisol/mg hair) fell more than 1.5 times outside the interquartile range above the third quartile in a plot of all cortisol measurements (as determined by R). With sample size being so small, it remains uncertain whether this was in fact an outlier or simply a testimony to natural variation in hair cortisol. Therefore, statistical analyses were conducted both with and without inclusion of the outlier sample.
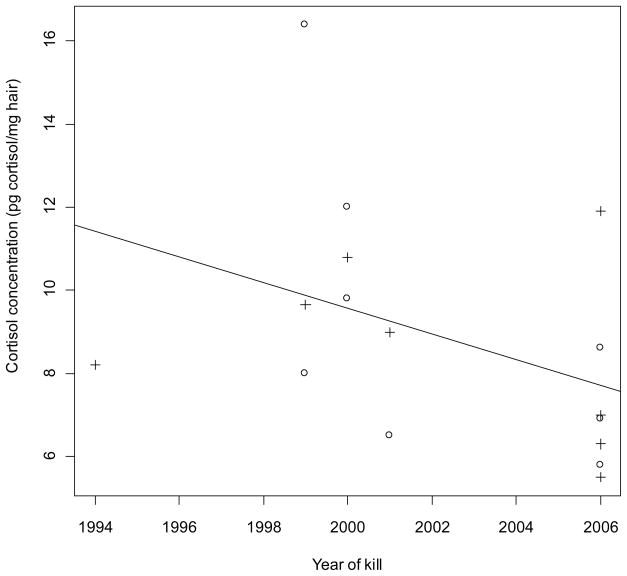
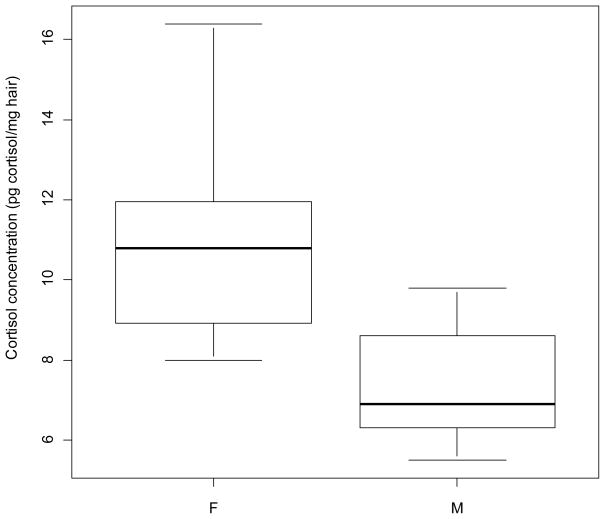
Cortisol results are shown in Table 1. For individuals with known age (n = 14), age range for females was 3–19 years (n = 6, mean = 9.5), and for males 6-19 years (n = 8, mean = 10.5).There were no statistically significant correlations between age and cortisol (p = 0.81) or between year of kill and cortisol (p = 0.11, though without removing the outlier a significant p = 0.02). Without the outlier, r = −0.41 (Fig. 1); with the outlier, r = −0.57 (not shown). Females had a significantly higher cortisol concentration than males (p = 0.01, though without removing the outlier a non-significant p = 0.17). Fig. 2 shows cortisol concentration in female and males (excl. outlier).
Figure 1.
Hair cortisol concentration (pg cortisol/mg hair) vs. year of kill for East Greenland polar bears (Ursus maritimus) collected 1994–2006. p = 0.11, r = −0.41 (excl. outlier; year of kill: 1995. 19.9 pg cortisol/mg hair, see text for further explanation). +: female, o: male.
Figure 2.
Box-and-whisker plot of hair cortisol concentration (pg cortisol/mg hair) in female and male East Greenland polar bears (Ursus maritimus) (excl. outlier. See text for further explanation). Females had significantly higher mean cortisol concentration in hair than males (p = 0.01).
Cross-contamination
Mean cortisol concentration across all samples was 8.5 pg cortisol/mg hair. For fat-contaminated samples the mean was 8.6 pg cortisol/mg hair (range = 6.2 – 10.8), for blood-contaminated samples the mean was 9.9 pg cortisol/mg hair (range = 5.5 – 16.4), and for clean samples the mean was 6.9 pg cortisol/mg hair (range = 3 – 12). If the outlier was included, the clean samples had an average of 8.8 pg cortisol/mg hair. When comparing samples prior to and after the washing procedure fat-contaminated hair samples had an average of 30 % of the sample cortisol washed out, blood-contaminated hair samples had an average reduction of 26 %, and clean hair samples had an average reduction of 5 %.
Student’s t-test was applied to test the difference in hair cortisol concentrations between the three groups (clean, fat-contaminated, and blood-contaminated). All were non-significant (including outlier, 0.73 ≤ p ≥ 0.88; excluding outlier, 0.29 ≤ p ≥ 0.73).
Discussion
Age and sex
No correlation was found between age and hair cortisol concentration. This is consistent with the findings of Tryland et al. (2002), who did not find any significant differences in polar bear blood plasma cortisol levels related to age. Thus, polar bear HPA axis activity may be relatively stable across age. In the present study, females had significantly higher cortisol concentrations than males. This is in accordance with Oskam et al. (2004) who found that adult female polar bears had higher plasma cortisol than adult males (other age groups showed no inter-sex differences). The findings by Handa et al. (1994; review across species) and Dobson & Smith (2000; sheep and dairy cows) were also in accordance with females having higher cortisol concentrations than males. Tryland et al. (2002) however, found no difference in cortisol levels related to sex, which corresponds to our results when the outlier was not removed. Similarly, Chow et al. (2010a,b) found no sex differences in either polar bear or grizzly bear serum total cortisol concentration, whereas serum CBG (corticosteroid binding globulin) expression was significantly higher in adult females compared to males (grizzly bear) and significantly elevated in lactating females relative to males and solitary female bears (polar bear), respectively.
Cross-contamination
Average cortisol concentration did not differ significantly between fat contaminated, blood contaminated and clean hair samples. One extra wash in addition to the standard two washes proved sufficient to remove external contamination, apparently without leaching tightly bound cortisol from the interior of the hair shaft (Table 1). The percentage cortisol in washes showed that in the absence of external contamination, there was relatively little cortisol on the outside of the polar bear hair shaft. More external cortisol was washed out of the fat and blood samples compared to the clean.
Temporal trends
No correlation between year of kill and cortisol was found, although this depends on whether the outlier is taken into account or not. A larger study should be conducted in order to determine whether the relationship is significant or not (whether the outlier is in fact an outlier or whether it reflects a "natural" cortisol level in a polar bear). In any case, there seems to be a declining trend in cortisol during the study period 1994–2006 which is very interesting, as previous mammal studies have shown that environmental pollutants may influence (generally: decrease) the concentration of this stress hormone in blood plasma and serum (Durham & Brouwer 1990; Oskam et al. 2004; Sonne et al. 2008). Three recent studies have examined cortisol and organic pollutants in Svalbard polar bear plasma, covering the years 1995–1998 (Tryland et al. 2002; Haave et al. 2003; Oskam et al. 2004). The cortisol ranges found in the two studies were similar, but the results were inconclusive. Tryland et al. (2002) compared the plasma cortisol measurements with other plasma biochemical parameters, such as calcium, lipase, alkaline phosphatase, and globulin, with the goal of creating baseline measurements. All three articles (Tryland et al. 2002; Haave et al. 2003; Oskam et al. 2004) mention as potential bias in their study the stress brought on the polar bears by the chase, darting, and anesthetization that is the normal procedure during live capture and blood sampling.
Cortisol variation across species and matrices
The range of cortisol concentration found in the present study was fairly broad, with approximately a 5- to 6-fold range from the lowest to the highest measured values. Tryland et al. (2002) and Haave et al. (2003) also found substantial variation in plasma cortisol concentrations between individual Svalbard polar bears. However, the variation in hair cortisol seen in the present study is not unusually large, as Davenport et al. (2006) reported about an 8-fold range of hair cortisol concentrations in a group of captive male rhesus monkeys. Other hair cortisol studies have found rather higher concentrations than the ones measured in polar bear hair in the present study, with a mean of app. 100–170 pg cortisol/mg hair as the lowest baseline (rock hyrax: Koren et al. 2002; rhesus macaques: Davenport et al. 2006 and Dettmer et al. 2009). However, studies on domestic cats and dogs have found hair cortisol values closer to the ones found in polar bear by the present study; a mean of 2.10–3.32 pg/mg (Accorsi et al. 2008) and 10.88–12.63 pg/mg (Bennett & Hayssen 2010).
As discussed previously, the acute stress inflicted on polar bears during capture or hunting can lead to artificially high baseline cortisol levels in blood plasma samples obtained in this way. For example, polar bear plasma cortisol levels measured in samples from tagged bears (Oskam et al. 2004) were 16 times higher than the cortisol levels found in hair in the present study. Two things must be kept in mind though; Oskam et al. (2004) used plasma from all age groups, whereas the present study used mainly adult animals. Furthermore, the blood plasma was sampled in the spring, whereas the hair samples analyzed in the present study were also sampled in the spring, but grown in the fall.
Further perspectives
In the future, it would be interesting to examine whether the variation in cortisol in the East Greenland bears can be related to any other measures we have on the examined individuals, and to determine whether environmental factors such as stress brought on by individual contaminant load, preferably as measured in hair, might contribute to greater HPA axis activity in some of the bears. An extended study with a larger sample size, preferably also including analysis of blood cortisol and CBG activity for the same individuals, would be recommendable as well.
Conclusions
The present study showed that polar bear hair contains relatively low but measurable amounts of cortisol. With respect to external cortisol contamination from blood and adipose tissue, one extra wash in addition to the standard two washes proved sufficient to remove extraneous cortisol without leaching hormone from the interior of the hair shaft. However, although statistically not significant, cortisol values from contaminated specimens were higher than clean samples, which should be taken into account in further comparison studies. The results from this pilot study validate an extended study of polar bear hair cortisol, preferably with a considerably larger sample size, and in connection with other measurements from the same individuals including thyroid hormones, organic pollutants, mercury, etc. A larger sample size would also allow a better temporal analysis of HPA activity. Correlating blood plasma and adipose subcutaneous tissue sample cortisol concentrations with those measured in hair of the same individuals would also be highly recommendable in the future, as a further validation of the usefulness of the non-invasive method represented by hair sampling.
Acknowledgments
Jonas Brønlund and local hunters are acknowledged for organizing the sampling in East Greenland. Financial support was provided by the Danish Cooperation for Environment in the Arctic and the Commission for Scientific Research in Greenland. The hair cortisol assays were supported by US NIH grants RR11122 to MAN and RR00168 to the New England Primate Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Gamberoni M, Tamanini C, Seren E. Cortisol determination in hair and faeces from domestic cats and dogs. General and Comparative Endocrinology. 2008;155:398–402. doi: 10.1016/j.ygcen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bennett A, Hayssen V. Measuring cortisol in hair and saliva from dogs: coat color and pigment differences. Domestic Animal Endocrinology. 2010;39:171–180. doi: 10.1016/j.domaniend.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Bortolotti GR, Marchant TA, Blas J, German T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Functional Ecology. 2008;22(3):494–500. [Google Scholar]
- Chow BA, Hamilton J, Alsop D, Cattet MRL, Stenhouse G, Vijayan MM. Grizzly bear corticosteroid binding globulin: CLoning and serum protein expression. General and Comparative Endocrinology. 2010a doi: 10.1016/j.ygcen.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Chow BA, Hamilton J, Cattet MRL, Stenhouse G, Obbard ME, Vijayan M. Serum corticosteroid binding globulin expression is modulated by fasting in polar bears (Ursus maritimus) Comparative Biochemistry and Physiology. 2010b doi: 10.1016/j.cbpa.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MFSX, Novak MA, Meyer JS, Suomi SJ. Hair cortisol predicts object performance in infant rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2009;51(8):706–713. doi: 10.1002/dev.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson H, Smith RF. What is stress, and how does it affect reproduction. Animal Reproduction Science. 2000;60–61:743–752. doi: 10.1016/s0378-4320(00)00080-4. [DOI] [PubMed] [Google Scholar]
- Downing JA, Bryden WL. Determination of corticosterone concentrations in egg albumen: a non-invasive indicator of stress in laying hens. Physiology & Behavior. 2008;95(3):381–387. doi: 10.1016/j.physbeh.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Durham SK, Brouwer A. 3,4,3’,4’-Tetrachlorobiphenyl distribution and induced effects in the rat adrenal gland. Localization in the zona fasciculata. Laboratory Investigation. 1990;62(2):232–239. [PubMed] [Google Scholar]
- Flores-Valverde AM, Hill EM. Methodology for profiling the steroid metabolome in animal tissues using Ultraperformance Liquid Chromatography-Electrospray-Time-of-Flight Mass Spectrometry. Analytical Chemistry. 2008;80(22):8771–8779. doi: 10.1021/ac8014966. [DOI] [PubMed] [Google Scholar]
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International. 2010;196:32–37. doi: 10.1016/j.forsciint.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Haave M, Ropstad E, Derocher AE, Lie E, Dahl E, Wiig Ø, Skaare JU, Jenssen BM. Polychlorinated biphenyls and reproductive hormones in female polar bears at Svalbard. Environmental Health Perspectives. 2003;111(4):431–436. doi: 10.1289/ehp.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal-steroid hormone receptors and sex-differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hensel RJ, Sorensen FE. Age determination of live polar bears. International Conference on Bear Research and Management. 1980;4:93–100. [Google Scholar]
- Koren L, Mokady O, Karaskov T, Klein J, Koren G, Geffen E. A novel method using hair for determining hormonal levels in wildlife. Animal Behaviour. 2002;63:403–406. [Google Scholar]
- Larter NC, Nagy JA. Overwinter changes in the urine chemistry of muskoxen from Banks Island. Journal of Wildlife Management. 2001;65(2):226–234. [Google Scholar]
- Lupica SJ, Turner JW. Validation of enzyme-linked immunosorbent assay for measurement of faecal cortisol in fish. Aquaculture Research. 2009;40(4):437–441. [Google Scholar]
- Montfort RW, Brown JL, Wildt DE. Episodic and seasonal rhythms of cortisol secretion in male Eld’s deer (Cervus eldi thamin) Journal of Endocrinology. 1993;138:41–49. doi: 10.1677/joe.0.1380041. [DOI] [PubMed] [Google Scholar]
- Okuliarová M, Sárniková B, Rettenbacher S, Skrobánek P, Zeman M. Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. General and Comparative Endocrinology. 2010;165(1):91–96. doi: 10.1016/j.ygcen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Oskam IC, Ropstad E, Lie E, Derocher AE, Wiig O, Dahl E, Larsen S, Skaare JU. Organochlorines affect the steroid hormone cortisol in free-ranging polar bears (Ursus maritimus) at Svalbard, Norway. Journal of Toxicology and Environmental Health, Part A, Current Issues. 2004;67(12):959–977. doi: 10.1080/15287390490443731. [DOI] [PubMed] [Google Scholar]
- Saco Y, Fina M, Gimenez M, Pato R, Piedrafita J, Bassols A. Evaluation of serum cortisol, metabolic parameters, acute phase proteins and faecal corticosterone as indicators of stress in cows. Veterinary Journal. 2008;177(3):439–441. doi: 10.1016/j.tvjl.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Saeb M, Baghshani H, Nazifi S, Saeb S. Physiological response of dromedary camels to road transportation in relation to circulating levels of cortisol, thyroid hormones and some serum biochemical parameters. Tropical Animal Health and Production. 2010;42(1):55–63. doi: 10.1007/s11250-009-9385-9. [DOI] [PubMed] [Google Scholar]
- Sonne C, Dietz R, Kirkegaard M, Letcher RJ, Shahmiri S, Andersen S, Møller P, Olsen AK, Jensen AL. Effects of organohalogen pollutants on haematological and urine clinical-chemical parameters in Greenland sledge dogs (Canis familiaris) Ecotoxicology and Environmental Safety. 2008;69:381–390. doi: 10.1016/j.ecoenv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Tryland M, Brun E, Derocher AE, Arnemo JM, Kierulf P, Ølberg RA, Wiig Ø. Plasma biochemical values from apparently healthy free-ranging polar bears from Svalbard. Journal of Wildlife Diseases. 2002;38(3):566–575. doi: 10.7589/0090-3558-38.3.566. [DOI] [PubMed] [Google Scholar]
- Van der Staay FJ, De Groot J, Van Reenen CG, Hoving-Bolink AH, Schuurman T, Schmidt BH. Effects of Butafosfan on salivary cortisol and behavioral response to social stress in piglets. Journal of Veterinary Pharmacology and Therapeutics. 2007;30(5):410–416. doi: 10.1111/j.1365-2885.2007.00884.x. [DOI] [PubMed] [Google Scholar]